Sporothrix schenckii: Difference between revisions
| Line 156: | Line 156: | ||
Enter summaries of the most recent research here--at least three required | Enter summaries of the most recent research here--at least three required | ||
S. schenckii is ever-changing and new isolates are constantly being discovered and analyzed. Here are some of the most recent research projects being conducted on Sporothrix Schenckii: | |||
Revision as of 00:17, 28 August 2007
A Microbial Biorealm page on the genus Sporothrix schenckii
When drinking wine amongst the roses Or guzzling beer while throwing bricks Or playing games in bales of hay Where lurks the tricky sporothrix, Beware, the price you pay for play, When you get struck by dread mycoses -S. Vaisrub

Classification(1)
Higher order taxa
cellular organisms; Eukaryota; KINGDOM: Fungi/Metazoa group; Fungi; Dikarya; PHYLUM: Ascomycota; SUBDIVISION: Pezizomycotina; CLASS: Sordariomycetes; SUBCLASS: Sordariomycetidae; ORDER: Ophiostomatales; FAMILY: Ophiostomataceae; mitosporic Ophiostomataceae; GENUS: Sporothrix
Species
|
NCBI: Taxonomy |
Sporothrix schenckii
Description and significance
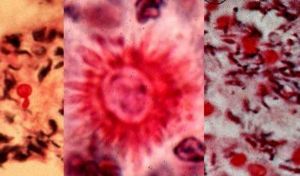
Sporothrix is a thermally dimorphic fungus which is distributed worldwide and isolated from soil, living and decomposing plants, woods, and peat moss. The genus Sporothrix contains one active species, Sporothrix schenckii Sporothrix schenckii is an occasional cause of human infections. Sporothrix schenckii is the pathogenic species. Macroscopic and microscopic features of these species are different from each other. The nonpathogenic Sporothrix sp. may occasionally convert to a yeast phase at 37°C (2). In mammalian tissues, S. schenckii is yeast-like, appearing as spherical or cigar-shaped bodies measuring 4 to 6 μm in length that reproduce by budding. (3)
The primary habitats for S. schenckii are the soil and plants. The ecology of S. schenckii determines the epidemiology of sporotrichosis. The conditions for the survival of S. schenckii in nature are aspects such as temperature, humidity, and necessary nutrients, as well as the conditions favoring human infection. S. schenckii is isolated most often from soil, live plants, or plant debris, wood, and straw, but it can also be found in insects, hair, water, air, and a variety of other sources. Several domestic animals and rodents are carriers of this fungus.
Isolates from nature have different characteristics. Strains isolated from
rotten palm tree trunks, dry grass from armnadillo
and rodent holes or nests, and soil covered by
mosses formned oval dark-pigmented radulaspores
and numerous conidia. Multiple spicules
were observed on the hyphae after conidial detachment.
These strains grew at 37 degrees C and were
pathogenic in mice. However, strains isolated from rat dung, wood, and soil did
not grow at 37 degrees C, formed spherical, dark-pigmented
conidia which could not be detached
easily from hyphae, and were nonpathogenic.
Whereas the
strain from a human was in the yeast phase at
37 degrees C and was pathogenic for species like golden hamsters
and mice, the presumed wild-type strain isolated
from the soil around Aechmea grew faster at 30
than at 37 degrees C, converted only partially to the
yeast phase, and was nonpathogenic in animals(4).
Genome structure
The genomic sequence of S. schenckii has not yet been sequenced and recorded; however, this dimorphic fungus is a candidate for fungal sequencing by the Broad Institute(9). This organization is known for completion and sequencing of other eukaryotic fungi including Histoplasma capsulatum, Candida albicans, Saccharomyces cerevisiae (RM11-1a), and many more. Although various research projects have been conducted, little is known about the biology and population structure of S. schenckii, although recent molecular and phenotypic data seem to demonstrate that different genetic lineages exist within this species(10). S. schenckii has its natural habitat in soil and plants, although it has been isolated from a variety of other sources. Recent molecular studies have demonstrated the existence of a high level of intraspecific variability and that isolates are mainly grouped according to their geographical origin. Nevertheless, it is specifically because of its clinical siginficance that S. schenckii should be given more consideration and dissection. Its virulence and pathogenicity should make it a viable candidate for complete sequencing and further analysis
Cell structure and metabolism
Capsular stains prepared by the Muir method indicate the presence of what is interpreted as a distinct capsular-slime layer about the cells. The cell wall measures from approximately 100 to 300 nm in total thickness and appears to be present in two distinct electron-dense layers. Multiple storage granules are noted scattered throughout the cytoplasm along with cell membrane- associated intracytoplasmic membranes. These membranes consist of circular, three-layered, electron-dense structures which clearly communicate with the cell membrane. Seen over the entire outer surface of the cell wall are electron-dense microfibrils which are intimately associated with the cell wall. These microfibrils are long and intertwining. The outer limit of the cytoplasm is the plasma membrane which has the characteristics of a "unit membrane". Microfibrils and the layering effect of the cell wall are prominent. The cell wall is thinner in the mycelial phase than in the yeastlike phase, and was never observed to show the electron-dense microfibrils characteristically seen in yeastlike phase preparations. (5)
The lipid compound of the cell wall plays an important role in the pathogenesis of this mycosis and was found to inhibit the phagocytic process and to induce high liberation of nitric oxide. S. schenckii form some polysaccharides which are firmly bound to the cell wall and some polysaccharides which are released into the medium. These polysaccharides are usually associated with peptide components in the form of covalent complexes in which the carbohydrate part represents about 85% of the total. Soluble and insoluble polysaccharides and their complexes participate in building the structural complex of the cell wall. (4)
METABOLISM
Sporothrix schenckii fungi are free living. They do not contain chlorophyll and cannot synthesize macromolecules from carbon dioxide and energy derived from light rays. Therefore Sporothrix schenckii is a heterotroph, living on preformed organic matter. The important aspects of Sporothrix schenckii are:
a. The synthesis of chitin, a polymer of N-acetyl glucosamine, and other compounds, for use in forming the cell wall. These induce immune hypersensitivity.
b. The synthesis of ergosterol for incorporation into the plasma membrane. This makes the plasma membrane sensitive to those antimicrobial agents which either block the synthesis of ergosterol or prevent its incorporation into the membrane or bind to it, e.g. amphotericin B.
c. The synthesis of proteins on ribosomes that are different from those found in bacteria. This makes the fungi immune to those antimicrobial agents that are directed against the bacterial ribosome, e.g., chloramphenicol.(6)
Ecology
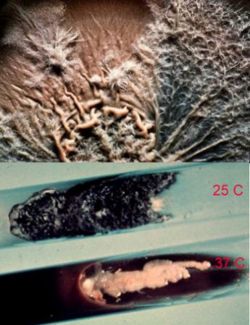
Sporothrix schenckii is a cosmopolitan fungus, isolated from soil and decaying plant materials. Sphagnum moss and rose bushes are well known source of this organism. At 25°C, on Sabouraud’s dextrose agar, colonies were initially white, becoming pinkish tan on the surface. At 37°C, on enriched media, colonies were cream to buff color, creamy in texture. S. schenckii is differentiated from other fungi by its slow growth, initially white colonies turning black, and ovoid conidia produced sympodially (rosette formation). Nonpathogenic Sporothrix species do not convert to yeast phase at 37°C on enriched media. S. schenckii isolates are susceptible to amphotericin B, itraconazole, and ketoconazole, but less susceptible to fluconazole. (2)
As was previously stated, S. schenckii is indeed a thermally dimorphic fungus and the macroscopic morphology varies depending on the temperature of growth. At 25°C, colonies grow moderately rapidly. They are moist, leathery to velvety, and have a finely wrinkled surface. From the front and the reverse, the color is white initially and becomes cream to dark brown in time ("dirty candle-wax" color). At 37°C, colonies grow moderately rapidly. They are yeast-like and creamy. The color is cream to beige. The conversion of the mould form to the yeast form is required for definitive identification of Sporothrix schenckii.
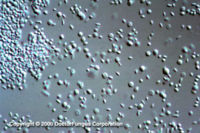
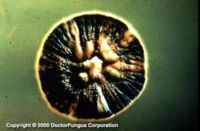
Similar to its macroscopic morphology, microscopic features of Sporothrix schenckii also vary depending on the temperature of growth. At 25°C, septate hyaline hyphae, conidiophores, and conidia are observed. Conidiophores are sympodial and appear weakly differentiated from the vegetative hyphae. They often have an inflated base and arise at right angles from the hyphae. Conidia have two types. The first type are unicellular, hyaline to brown, oval, thin-walled, and are typically arranged in rosette-like clusters at the tips of the conidiophores. The second type of conidia are brown (dematiaceous),oval or triangular, thick-walled, cessile, and are attached directly to the sides of the hyphae. The latter type of conidia are typically present only in freshly isolated strains. At 37°C, Sporothrix schenckii produces oval to cigar-shaped (also called "cigar bodies") yeast cells. Single or multiple buds may be produced by a single yeast cell.
Pathology
Despite the existence of the fungus worldwide, infections due to Sporothrix schenckii are more common at certain geographical areas. Peru is an area known for many instances of Sporothrix schenckii infections. Sporothrix schenckii is the causative agent of sporotrichosis also known as "rose handler's disease". Sporotrichosis is a subcutaneous infection with a common chronic and a rare progressive course. The infection starts following entry of the infecting fungus through the skin via a minor trauma and may affect an otherwise healthy individual. Following entry, the infection may spread via the lymphatic route. Nodular lymphangitis may develop. Patients infected with Sporothrix schenckii may be misdiagnosed as pyoderma gangrenosum due to the large ulcerations observed during the course of sporotrichosis.
More specifically, Sporotrichosis is primarily a chronic mycotic infection of the cutaneous or subcutaneous tissues and adjacent lymphatics characterized by nodular lesions which may suppurate and ulcerate. Infections are caused by the traumatic implantation of the fungus into the skin, or very rarely, by inhalation into the lungs. Secondary spread to articular surfaces, bone and muscle is not infrequent, and the infection may also occasionally involve the central nervous system, lungs or genitourinary tract. S. schenckii, the fungus causing sporotrichosis, enters the body through scratches or cuts in the skin. Therefore, people who handle plants with sharp thorns or needles, like roses, barberry, or pines, are more likely to get sporotrichosis. Sporotrichosis is not passed directly from person to person, so it is not possible to catch sporotrichosis from another person who has it.
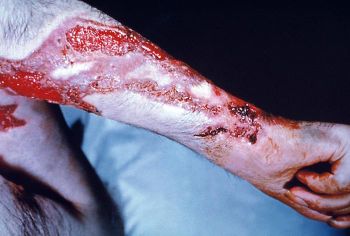
The first signs of sporotrichosis are painless pink, red, or purple bumps usually on the finger, hand, or arm where the fungus entered the body. These bumps may appear anywhere from one to 12 weeks after infection, but usually appear within three weeks. Unlike many other fungal infections sporotrichosis does not cause fever or any feelings of general ill health. The reddish bumps eventually expand and fester, creating skin ulcers that do not heal. In addition, the infection often moves to nearby lymph nodes. Although most cases of sporotrichosis are limited to the skin and lymph channels, occasionally the joints, lungs, and central nervous system become infected. In rare cases, death may result.
People who have weakened immune systems, either from a disease such as acquired immune deficiency Syndrome (AIDS) or leukemia, or as the result of medications they take (corticosteroids, chemotherapy drugs), are more likely to get sporotrichosis and are more at risk for the disease to spread to the internal organs. Alcoholics and people with diabetes mellitus or a pre-existing lung disease are also more likely to become infected. Although sporotrichosis is painless, it is important for people with symptoms to see a doctor and receive treatment.
When sporotrichosis is limited to the skin and lymph system, it is usually treated with a saturated solution of potassium iodine that the patient dilutes with water or juice and drinks several times a day. The iodine solution can only be prescribed by a physician. This treatment must be continued for many weeks. Skin ulcers should be treated like any open wound and covered with a clean bandage to prevent a secondary bacterial infection. The drug itraconazol (Sporanox), taken orally, is also available to treat sporotrichosis. In serious cases of sporotrichosis, when the internal organs are infected, the preferred treatment is the drug amphotericin B. Amphotericin B is a strong anti-fungal drug with potentially severe toxic side effects. It is given intravenously, so hospitalization is required for treatment. The patient may also receive other drugs to minimize the side effects of the amphotericin B. (8)
Application to Biotechnology
Sporothrix schenckii is a pathogenic eukaryotic fungus. For this reason it is not believed to have any meaningful application to biotechnology. S. schenckii has not been found to produce any useful compounds or enzymes. However, as discussed in "Pathogens" section, it has been found to produce a disease known as Sporotrichosis. For this reason, S. schenckii is being researched in order to assess the pathogenicity of this fungus, in hopes of discovering more efficient ways of detecting infection immediately. Please refer to Section "A" of Current Research for further discussion.
Current Research
Enter summaries of the most recent research here--at least three required
S. schenckii is ever-changing and new isolates are constantly being discovered and analyzed. Here are some of the most recent research projects being conducted on Sporothrix Schenckii:
A. This report contains the results of a molecular phylogenetic
analysis of the S. schenckii species complex inferred from DNA
sequence data from three different loci. One of the most interesting
results is the discovery that the isolates of S. schenckii,
practically all of clinical origin, were grouped into six putative
phylogenetic species. These cryptic species were further subdivided
into a number of smaller groups that appear to be
reproductively isolated in nature. This suggests not only that
the existing S. schenckii populations are in the process of divergence
but also that all of the resulting lineages are undergoing
separation into distinct taxa. Our results were validated
by the recent study of Neyra et al., who analyzed the
genetic diversity of the same Peruvian isolates that we tested,
by using amplified fragment length polymorphism analysis and
ITS2 sequencing. In that study, the isolates were grouped into
two clades that were similar to those obtained in our phylogenetic
trees.
Another interesting aspect of our analysis was the finding
that each of the main groups exhibited a degree of geographical
specificity, which agrees with previous reports. The
possible existence of different species within S. schenckii In conclusion, S. schenckii appears to be a complex of species,
some prevailing in certain geographical regions. An accurate
knowledge of species limits could be of high medical
interest, as they may show different clinical patterns and respond
differently to therapy. For instance, the Brazilian isolates
present a distinctive clinical picture with immune manifestations
(erythema multiforme), disseminated cutaneous
lesions, and atypical forms (10).
References
(1) NCBI: Sporothrix schenckii, Accessed August 15, 2007, <http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=29908&lvl=3&lin=f&keep=1&srchmode=1&unlock>
(2) Doctor Fungus: Sporothrix Species, Accessed August 19, 2007, <http://www.doctorfungus.org/thefungi/Sporothrix.htm>
(3) Sindy Hu,1 Wen-Hung Chung,1 Shuen-Iu Hung,2 Hsin-Chun Ho,1 Zen-Whe Wang,1 Chien-Hsun Chen,1 Shu-Chuan Lu,3 Tseng-tong Kuo,3 and Hong-Shang Hong. "Detection of Sporothrix schenckii in Clinical Samples by a Nested PCR Assay". J Clin Microbiol. 2003
(4) Travassos LR, Lloyd KO. "Sporothrix schenckii and related species of Ceratocystis". Microbiol Rev. 1980 Dec;44(4):683-721.
(5) Lane JW, Grrison RG, Field MF. "Ultrastructural studies on the yeastlike and mycelial phases of Sporotrichum schenckii". J Bacteriol. 1969 Nov;100(2):1010-9
(6) The Fungi, Accessed August 23, 2007, <http://www.kcom.edu/faculty/chamberlain/Website/Lects/Fungi.htm#classif>
(7) Omar Lupi, MD, PhD,a Stephen K. Tyring, MD, PhD, MBA,b and Michael R. McGinnis. )"Tropical dermatology: Fungal tropical diseases". <http://www.botany.utoronto.ca/courses/bot405/notes/Lupi%20et%20al.,%202005%20tropical%20fungal%20diseases.pdf>
(8) Medical Dictionary, Accessed August 25, 2007, <http://www.cureresearch.com/medical/sporothrix_schenckii.htm>
(9) Broad Institute: Fungal Genome Intiative, Accessed August 21, 2007, <http://www.broad.mit.edu/annotation/fungi/fgi/nominated.html>
(10) Rita Marimon,1 Josepa Gene´,1* Josep Cano,1 Luciana Trilles,2 Ma´rcia Dos Santos Laze´ra,2 and Josep Guarro1. "Molecular Phylogeny of Sporothrix schenckii". JOURNAL OF CLINICAL MICROBIOLOGY, Sept. 2006, p. 3251–3256.
Edited by Whitney Johnson-Courtright of Rachel Larsen