Antibacterial Surfaces: Difference between revisions
| Line 17: | Line 17: | ||
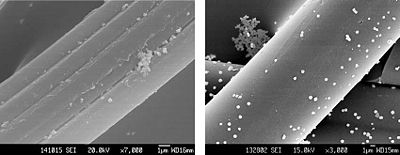
[[Image:Chen ShuiXiaAgACF.JPG|thumb|400px|right|Distribution of silver particles on activated carbon fibers. [http://www.denniskunkel.com/product_info.php?products_id=531 Dennis Kunkel Microscopy, Inc.]]] | [[Image:Chen ShuiXiaAgACF.JPG|thumb|400px|right|Distribution of silver particles on activated carbon fibers. [http://www.denniskunkel.com/product_info.php?products_id=531 Dennis Kunkel Microscopy, Inc.]]] | ||
<br>Nano-silver is an antimicrobial substance that is composed of silver particles that are on the nanometer scale. Partially oxidized nano-Ag can carry Ag+, which forms on the surface of the nano-particles [6 Lok]. It can catalyze oxidation reactions, which in turn can disrupt proteins and cell membranes of bacteria. | <br>Nano-silver is an antimicrobial substance that is composed of silver particles that are on the nanometer scale. Partially oxidized nano-Ag can carry Ag+, which forms on the surface of the nano-particles [6 Lok]. It can catalyze oxidation reactions, which in turn can disrupt proteins and cell membranes of bacteria.<br> | ||
<br>When loaded with Ag through adsoption, Nano-SiO¬2 gains antibacterial properties. According to Wang et al., this antibacterial agent has a “nearly spherical structure with average particle size about 60 nm.” [2] When grafted to wool, Ag-loaded nano-SiO¬2 can destroy up to 84% of E. coli and 94% of S. aureus. [2] This manner of antibacterial surface is limited by the weak bonding between the composite particles and the wool surface. Despite this, “even after washed for 20 times, the grafted wool gave an antibacterial ratio of around 70%,” indicating that this bonding is strong enough to keep a majority of the agent associated with the surface. [2] This type of method is proposed as a way for “fabrics such as medical clothes, protective garments, and hygienic textiles” to become bio-protective [2]. <br> | |||
<br>Nano-SiO¬2 is a resilient structure that can also carry other inorganic antibacterial elements, such as zinc [4]. Zeolite, phosphates, apatite, and titanium oxides have also been successfully adhered to nano-SiO2. Depending on the amount of metal present, the contact time needed to kill bacteria range from 2 to 10 hours [4].<br> | |||
<br>In the treatment of water, activated carbon fiber (ACF) has been used to remove pollutants via adsorption. These fibers encompass a very large surface area, and contain many pores that “collect” pollutants suspended in water. The activity of these fibers can be hampered by the growth of bacteria that adhere to the micropores. The fusing of silver with ACF represents a countermeasure against this bacterial incursion. Chen et al. examined the antibacterial activity of silver supporting activated carbon fibers (ACF-Ag), finding it extremely effective against E. coli and S. aureus [3]. Antibacterial activity was determined to be directly related to the amount of silver adhered to ACF. Interestingly, water treatment was aided by leaving enough of the ACF specific surface area available for bacterial adsorption, bringing said bacteria into closer contact with the silver. After being employed for bacterial destruction, the activity of ACF-Ag was regenerated by washing it with deionized water. After 5 cycles of washing, no reduction in ACF-Ag antibacterial activity was observed [3]. A potential concern with this method of water purification is over-exposure to suspended silver. Under the conditions of Chen et al., whereby 50 mg of ACF-Ag complex was soaked and shaken in 50 ml water for several hours, the maximum amount of silver released was around 10 ppm, meeting “the quality standard of drinking water” [3]. Most water treatment does not use this much ACF per amount of water, so it is surmised that the concentration of released silver will be even lower. Since silver can become permanently deposited in various human body tissues, it is important to keep the concentration of released silver low.<br> | |||
<br>Ag-loaded nano-SiO2 also presents a potential health hazard, due to the high rate of dissociation from wool when washed. While silver is a fairly harmless element for people, nano-SiO2 structures could potentially build up in our bodies after constant exposure. | |||
<br> | <br> | ||
Revision as of 02:48, 14 April 2009
Overview of Antibacterial Surfaces
Outside of the body, bacterial control has traditionally been conducted by applying temporary agents like bleach, or through controlled temperature/pressure. The study of antibacterial surfaces investigates the viability of surfaces that intrinsically destroy bacteria and hamper future growth. A number of novel approaches are being employed in the search of effective antibacterial activity. Most of these approaches involve an adhesive coating on benign objects such as glass and polymers. This technology has great potential for medical, commercial, and home use.
Covalent Modification: Alkylated Polethylenimine
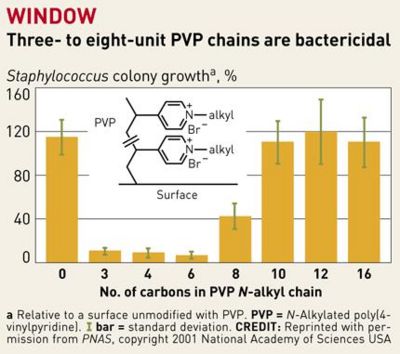
The covalent attachment of long-chained hydrophobic cations to glass and plastic surfaces can render the modified surface capable of killing bacteria on contact. This method does not involve the release of antiseptics – the long-chained cations intrinsically destroy bacteria upon contact, by virtue of its structure. Unlike other surface treatments, no regeneration of the active material is required, aside from clearing debris from the hydrophobic cations, which can simply be done by washing with detergent. A wide variety of surfaces and textiles can be rendered bactericidal this way. As an added benefit, it appears that bacteria will have little opportunity to adapt against this threat.
In a study by Lin et al., poly(vinyl-N-hexylpyridinium) chains were found “equally lethal against wild-type and mutant, including antibiotic-resistant, strains of the ubiquitous pathogenic bacterium Staphylococcus aureus,” killing between 90-99% of the S. aureus bacteria. [1] These hexyl-PVP chains kill a wide variety of both Gram-positive and Gram-negative bacteria, and the activity of the surface can be reset simply by washing it with detergent [1]. A wide variety of multidrug resistant (MDR) bacterial strains were also destroyed by exposure to hexy-PVP. It is proposed that the chains penetrate the bacterial cell wall/ membrane, and cause lysis by severing the structures at many points. This novel antibacterial agent has such an overarching, destructive effect on bacteria that it will be very difficult for them to adapt. Only one form of resistance against these molecular “daggers” is known right now, and that is conferred by MDR pumps. The NorA MDR pump of S. aureus protects the cell by expelling amphipathic cations [1]. Mutants with a knockout in the norA gene were compared to their “pump-competent parents,” exhibiting a small difference in killing efficiency of hexyl-PVP: ~99% of airborne/waterborne of pump-lacking mutants were killed, while ~95% of airborne and ~97% of waterborne pump-competent cells were killed [1]. It is believed that the extracellular protrusion of MDR pumps aid in keeping the bacteria at “an arms length” away from the hexyl-PVP antiseptic, rather than specifically binding and expelling hexyl-PVP. It therefore seems unlikely that resistance to surface hexyl-PVP will come to fruition through MDR pumps. Surfaces such as plastic and glass could be made permanently antibacterial after being covalently bound to hexyl-PVP. This presents a wide set of possibilities for limiting bacterial growth in indoor environments. Hypothetically, even floors in high-traffic areas such as hospitals could be given this treatment, radically decreasing the amount of bacteria in the hospital. Furthermore, all manner of intracutaneous devices can be treated this way to prevent post-surgery sepsis – many deaths occur from bacteria embedded on such devices during insertion into the human body.
Hydrophobic polycations can also be covalently bound to porous materials such as cotton, wool, nylon, and polyester. Like hexyl-PVP, N-hexylated+methylated highmolecular-weight polyethylenimine (PEI) has strong antibacterial activity against both Gram-positive and Gram-negative bacteria [8 Lin]. Furthermore, textiles that are covalently bound with N-alkylated PEIs have anti-fungal (airborne) activity. This technique would be particularly useful for producing bactericidal clothes. Hospitals could be rendered bacteria-free by bedsheets and clothing that actively destroy even the most resilient multi-drug resistant bacteria. It could also be rendered a valuable defense against bioterrorism, as surmised by Lin et al. [8].
It has not been determined whether covalently bound long-chained hydrophobic cations disrupt human cells upon contact. This is a potential concern, especially for intracutaneous devices.
Previous attempts to design antibacterial surfaces in this manner were largely unsuccessful, because the “polymer chains weren't sufficiently long and flexible to penetrate bacterial cell walls” [10]. The addition of a linker portion to extend the N-alkylated pyridine groups and a “sweet spot” range of 3-8 PVP chains per unit has fixed this problem. The hydrophobic chains now are produced with enough positive charge so that they repel each other while staying flexible, so that they are guided into bacterial cell walls when in close proximity [10].
Silver Nanoparticles
Nano-silver is an antimicrobial substance that is composed of silver particles that are on the nanometer scale. Partially oxidized nano-Ag can carry Ag+, which forms on the surface of the nano-particles [6 Lok]. It can catalyze oxidation reactions, which in turn can disrupt proteins and cell membranes of bacteria.
When loaded with Ag through adsoption, Nano-SiO¬2 gains antibacterial properties. According to Wang et al., this antibacterial agent has a “nearly spherical structure with average particle size about 60 nm.” [2] When grafted to wool, Ag-loaded nano-SiO¬2 can destroy up to 84% of E. coli and 94% of S. aureus. [2] This manner of antibacterial surface is limited by the weak bonding between the composite particles and the wool surface. Despite this, “even after washed for 20 times, the grafted wool gave an antibacterial ratio of around 70%,” indicating that this bonding is strong enough to keep a majority of the agent associated with the surface. [2] This type of method is proposed as a way for “fabrics such as medical clothes, protective garments, and hygienic textiles” to become bio-protective [2].
Nano-SiO¬2 is a resilient structure that can also carry other inorganic antibacterial elements, such as zinc [4]. Zeolite, phosphates, apatite, and titanium oxides have also been successfully adhered to nano-SiO2. Depending on the amount of metal present, the contact time needed to kill bacteria range from 2 to 10 hours [4].
In the treatment of water, activated carbon fiber (ACF) has been used to remove pollutants via adsorption. These fibers encompass a very large surface area, and contain many pores that “collect” pollutants suspended in water. The activity of these fibers can be hampered by the growth of bacteria that adhere to the micropores. The fusing of silver with ACF represents a countermeasure against this bacterial incursion. Chen et al. examined the antibacterial activity of silver supporting activated carbon fibers (ACF-Ag), finding it extremely effective against E. coli and S. aureus [3]. Antibacterial activity was determined to be directly related to the amount of silver adhered to ACF. Interestingly, water treatment was aided by leaving enough of the ACF specific surface area available for bacterial adsorption, bringing said bacteria into closer contact with the silver. After being employed for bacterial destruction, the activity of ACF-Ag was regenerated by washing it with deionized water. After 5 cycles of washing, no reduction in ACF-Ag antibacterial activity was observed [3]. A potential concern with this method of water purification is over-exposure to suspended silver. Under the conditions of Chen et al., whereby 50 mg of ACF-Ag complex was soaked and shaken in 50 ml water for several hours, the maximum amount of silver released was around 10 ppm, meeting “the quality standard of drinking water” [3]. Most water treatment does not use this much ACF per amount of water, so it is surmised that the concentration of released silver will be even lower. Since silver can become permanently deposited in various human body tissues, it is important to keep the concentration of released silver low.
Ag-loaded nano-SiO2 also presents a potential health hazard, due to the high rate of dissociation from wool when washed. While silver is a fairly harmless element for people, nano-SiO2 structures could potentially build up in our bodies after constant exposure.
Other Methods
Include some current research in each topic, with at least one figure showing data.
Conclusion
Overall paper length should be 3,000 words, with at least 3 figures.
References
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.
