Anthrax in the United States: Difference between revisions
| Line 35: | Line 35: | ||
*''Bacillus anthracis'' is also capable of forming biofilms which allow it to be resistant to the host immune system and antibiotics [13]. | *''Bacillus anthracis'' is also capable of forming biofilms which allow it to be resistant to the host immune system and antibiotics [13]. | ||
===Virulence=== | ===Virulence=== | ||
Revision as of 21:00, 27 August 2009
Introduction
Anthrax, which means 'coal' in Greek, is a severe disease caused by the bacteria Bacillus anthracis, which stays alive in its surroundings by sporulating. Because of this protective measure, the disease itself is fairly old and can be found naturally on all continents, including Asia, southern Europe, sub-Sahelian Africa and Australia [1]. Anthrax can be contracted by humans and herbivorous animals, though other infected mammals and birds have been found [2].
Besides being a threat to the human population, anthrax has also been used in acts of biological warfare. The spores are easily transmitted, and the disease itself (especially inhalational anthrax) has a high mortality rate, making it an effective weapon to spread through a human population.
Description of Anthrax
Anthrax is a zoonotic disease that can infect both humans and animals [3]. It is generally known to be a seasonal disease, simply because temperature can affect how and when an animal may become infected from contact with the spores [4]. The spores can survive for long periods of time, living in the soil until it comes into contact with something. Although human contact does not spread the disease, humans can get infected from touching or inhaling spores from contaminated animal products. Even eating rare meat from an infected animal is enough to cause anthrax. Most human cases occur due to contact with sheep, goat, and cattle, as well as wool and hides. Fortunately, anthrax is not a major health concern in today's society, although outbreaks have been know to occur. The largest known epidemic took place in Zimbabwe and lasted for 6 consecutive years (1979-1985). Infected cattle led to an estimated 10,000 human cases [3].
Symptoms of the anthrax will generally appear within 7 days after infection [5]. The infection can affect the skin, respiratory tract, and gastrointestinal tract.
Cutaneous Anthrax
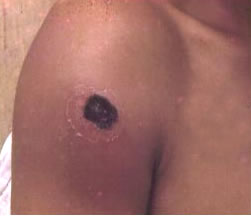
The most common type of anthrax, cutaneous (skin) anthrax, happens when a person has a cut of some type on the skin, allowing the bacteria to enter. An itchy lesion usually forms at this site, but after 1-2 days, it turns into a vesicle, and later, an ulcer. The surrounding lymph nodes are may also be subject to swelling. The ulcer, generally 1-3cm in diameter, contains a black necrotic (dead) center and is typically painless. However, if left untreated, approximately 20% of cutaneous anthrax cases can lead to death [6].
Respiratory (Inhalational/Pulmonary) Anthrax
Respiratory anthrax, also known as inhalational or pulmonary anthrax, is contracted by breathing in the anthrax spores. It has similar symptoms to the cold and flu for the first few days – a sore throat, muscle aches, and fever are often common. However, the disease then deviates, resulting in acute breathing difficulty and shock, which is often fatal. Untreated cases have a 100% mortality rate [3].
Gastrointestinal Anthrax
Gastrointestinal anthrax usually results from eating infected meat. The infection severely inflames the intestines, causing severe diarrhea and vomiting of blood, initially preceded by nausea, decreased appetite, and fever. If it goes untreated for long enough, toxaemia and shock will occur, leading to death [7]. Gastrointestinal anthrax can also occur in the oropharyngeal – the symptoms then become a sore throat, difficulty in swallowing, fever, swollen lymph nodes, and toxaemia. Even with treatment, about 50% of oropharyngeal anthrax infections are fatal [9].
The Bacillus anthracis Microbe
Microbewiki Link: Bacillus anthracis
Discovery
In 1887, Robert Koch showed that Bacillus anthracis could form endospores, and caused anthrax when injected into animals. He developed a method of purifying blood samples of Bacillus anthracis to grow cultures. The bacteria was unable to survive by itself for very long, but the endospores it formed were much more resilient. It was also the first organism used to develop a vaccine with a weakened strain [10].
Structure
- Gram-positive cells share cell walls made of peptidoglycan, teichoic acids, lipoteichoic acids, capsular polysaccharides, and S-layer crystalline proteins for their cell structure, but Bacillus anthracis differs by being surrounded by its poly-D-glutamate capsule instead of a polysaccharide capsule, having no teichoic acid, and having non-glycosylated S-layer proteins. Cell wall polysaccharides act to anchor the S-layer proteins to the cell wall [12].
- It is large and rod shaped and about 1-1.2um wide, and 3-5 um long. It is capable of growing both in aerobic and anaerobic conditions. It is similar to Bacillus cereus and Bacillus thuringiensis both genotypically and phenotypically, and all are around the same size and capable of forming spores [12].
- When faced with starvation, Bacillus anthracis begins endospore formation. The cell splits asymmetrically and forms a mother cell and forespore . The mother cell engulfs the forespore and then lyses to release the spore. These spores are capable of living in soil for many years [17]. Mature spores are capable of resisting heat, cold, dessication, radiation, and disinfectant. This allows the spores to survive in inhospitable environments and germinate when conditions are more favorable [12].
- Bacillus anthracis is also capable of forming biofilms which allow it to be resistant to the host immune system and antibiotics [13].
Virulence
- Bacillus anthracis has the ability to synthesize virulence factors. Virulence factors pXO1, anthrax toxin, and pXO2, capsule gene, are responsible for the pathogenicity. They are both required for the virulence of Bacillus anthracis and distinguish Bacillus anthracis from Bacillus cereus [12].
- The capsule is made up of a poly-D-glutamate polypeptide while most bacteria have polysaccharide capsules. The capsule, encoded by pX02, can be characterized as "smooth" or mucoid colonies or by "rough" colonies which are not virulent . The capsule gene both protects against phagocytosis and mediates the bacterial infection of the bloodstream. Bacillus anthracis capsule and anthrax toxin grow in response to increased atmospheric Co2 [12].
- The anthrax toxin, coded by pX01, consists the edema factor (EF), protective antigen (PA), and the lethal factor (LF). The EF increases fluid build up in the cells and is also known as the inherent adenylate cyclase [12]. The LF promotes cell death and bacterium release. The PA translocates the two toxins into the cytosol [10].
- Combinations of these factors cause these results in experimental animals:
Protective antigen with lethal factor is lethal.
Edema factor with protective antigen causes edema.
Edema factor with lethal factor is inactive.
All three factors together cause edema, necrosis, and is lethal [12].
Transmission of disease
How is it transmitted? Is there a vector (animal/insect)?
Problems with Anthrax in America
Outbreak of anthrax in the United States
Although human anthrax was historically known to be contracted from high exposures to animals and animal products that were infected with Bacillus Anthracis [1], the spread of human anthrax in the United States took on a different approach. Moreover, research into prevention and medication of two types of anthrax, cutaneous and inhalation anthrax, were known and established by the 21st century, but the sudden outbreak came as a shock. As a result of inhalation of anthrax spores and/or coming into contact with contaminated products, the spread of Bacillus anthracis took speed. The problem arises during early October to November in 2001, as the United States of America announced its first ten confirmed cases of human anthrax from bioterrorism [3].
Spread of anthrax in the U.S.
Intentional contaminated letters and packages were mailed to various districts of the U.S., targeted victims remained helpless and vulnerable to the Bacillus anthracis spores upon opening and handling. The packages held within them various infective powders and materials anticipated to be delivered to abortion clinics, reproductive health clinics, and other destinations such as government offices [2]. As a result from this event, the U.S. postal office was drastically effected as all of the initial patients were either employees of the postal office, mail handlers and sorters [3].
Effects of anthrax on the U.S. citizens
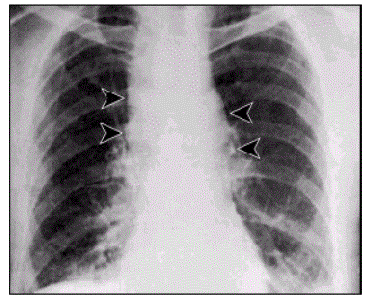
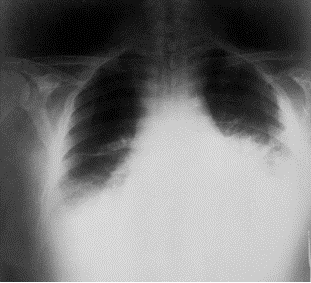
The problem caused by this hazardous disease in America results in various health issues along with social disruption and an economic crisis [2]. Victims experienced chills, a fever, difficulty coughing, constant sweats, fatigue, and typical feelings of morning sickness; nausea and vomiting. As an outcome of the ten initially infected citizens, all displayed abnormal chest x-rays; infiltrates, mediastinal widening, and pleural effusion [3]. With efforts to restore health to the ten patients, methods of multiple drug antibiotics were used along with guidance and supportive care, however only 60% of the initial ten patients survived. Overall, an alarming 22 cases were suspected to be infected from the anthrax contaminated mails . Among the twenty-two infected, five were announced dead, and of those five all were infected with inhalation anthrax [2]. This event not only stimulated a massive public health alarm in the United States, but in international countries as well. The 2001 anthrax attacks organized by bioterrorists proved its ability to cause not only destruction to the citizens of U.S., but also its ability to interfere with social and economic status.
Prevention
In the United States, the primary way to reduce the risk of exposure is through controlling the livestock. Proper disposal of anthrax infected carcasses and vaccination of at risk herds can help reduce exposure. The most effective and proper way of eliminating the exposure is through incineration of the contaminated soil. Patients that have died from anthrax should be isolated and contaminated materials should be properly disposed of through incineration. It is important that following the first detection of an anthrax infection in a herd, the surrounding animals should be removed and isolated from the field. The animals will then need to be monitored for a period of time and all the animals should be vaccinated if needed.[16]
Biosensors
One of the ways of identifying anthrax is through the use of biosensors, instruments used to determine the particular structures of the material being analyzed. One method utilizes Raman spectroscopy in conjunction with localized surface plasmon resonance sensor (LSPR) to detect anthrax. In Raman spectroscopy, photons from light are directed through a substance, producing photons that scatter differently. Photons scatter differently due to the fact that the molecule undergoes changes in its energy level (ie: vibration), which cause the photons to lose/gain energy. These Raman scatterings can be used in identifying unknown molecules mainly because changes in a molecule’s energy level is distinct to that molecule and so produce distinct photon scatterings. LSPR can be used with Raman spectroscopy to greatly improve the ability of identifying molecules. The combined use of Raman spectroscopy and LSPR is called surface-enhanced Raman spectroscopy (SERS)[18].
Application
SERS has been used to identify Calcium dipicolinate (CaDPA), a key feature of Bacillus anthracis’s endospore. And so, research and test products have been made with the goal of utilizing Raman spectroscopy and LSPR in creating a portable tool for detecting the potential presence of anthrax (and other dangerous pathogens) in the surrounding area[19].
Vaccines
Vaccines can be used to prevent the disease in humans and animals. In the United States however, only high-risk groups such as military personnel are given the vaccine. Vaccine is not recommended for public use in the United States as anthrax cases are very rare and potential adverse side effects have been reported in some patients[16].
Anthrax Vaccine Adsorbed(AVA)
Anthrax Vaccine Adsorbed (AVA) is the only licensed human vaccine in the United States. The goal of the vaccine is to cause the immune system to produce antibodies against B. anthracis's protective antigen(PA), responsible for the uptake of the toxins, lethal factor (LF) and edema factor (EF) into the cell. In a controlled study in which 379 employees received the vaccines, 414 received placebo, and 340 received neither vaccines or placebo, the study documented a vaccine efficacy of 92.5% for protection against anthrax (cutaneous and pulmonary). The duration of this protection is unknown in humans but in animals it has been known to have an effect from one to two years after two doses[15].
Cons of Anthrax Vaccine Adsorbed
- The vaccine calls for six injections administered in a period of 18 months with yearly shots afterward.(Considered to be an extensive duration of time)
- It is temperature sensitive(small temperature range), and thus needs to be stored in a temperature controlled environment.
- There are also adverse effects when taking AVA which range from the mild to serious[21].
- Tenderness in area of inoculation
- Head ache
- Flu symptoms
- Respiratory failure
Recombinant Protective Antigen (rPA)
Recombinant Protective Antigen is a new antigen that is currently being developed in the United States. The goal of rPA is to require fewer doses of the vaccine, be able to be mass produced, and be given to individuals with compromised immune systems[14].
Treatment
Antibiotics
Antibiotics can be used before and after the patient has come in contact with the disease. This therapy is shown to result in a substantial recovery in individuals and animals infected with anthrax if taken immediately. Antibiotics can also be used for prevention and treatment in asymptomatic patients that may have been in exposed to the anthrax but have shown to have less of an effect on the spore form of the disease[16]. It is important to note that antibiotics can kill the bacteria, but do not have an effect on anthrax toxins so late treatment of the infection can be detrimental[14].
Alternative Solutions
Vaccine In Progress
Studies and experiments have been made with the hopes of producing an oral vaccine that is free of the side effects of AVA. Current researchers have used Salmonella enterica Serovar Typhi Ty21a strains as the host cell in expressing a lab synthesized protective antigen (PA) gene. The Samonella strain has proven to be useful in vaccines and showed stability in hosting the PA gene. Alterations in the PA gene were made to stabilize the gene in the host cell and to ensure sufficient expression for inducing a strong immune response to PA. The experiment was done on mice which were inoculated with three doses containing the Salmonella strain with PA gene. After the period of vaccination, the mice were then subjected to Bacillus anthracis spores (strain 7702) and observed for their survivability. Results have shown that the vaccination in mice provided the mice with immunity against the anthrax spore. Current research is being made in an attempt to utilize these results in making a vaccine suitable for humans that can be taken orally and in a short amount of time[21].
Medication Kits
The anthrax scare of 2001 and other bio-threats have created attempts at finding methods for providing medication for quick response to an emergency. Some solutions involve possessing medication kits in specific locations for use when the area is exposed to the contaminant. However, ideas like these have been plagued with legal issues revolving around medication that has yet to be approved and licensed by the FDA[23].
Cities Readiness Initiative
Part of this plan offered the idea of sending out members of the Postal Service to contaminated areas at the onset of a biological attack. The servicemen would provide medication to the citizens of the affected area for immediate but temporary relief, in the hopes of inhibiting the spread of the disease while further help arrives. Economic issues hinder this idea because city governments must approve of participation and fund for the initiative which can range from $700,000-$2,000,000. Legal issues also plague this idea concerning the administration of medicine by non-medical personnel[24].
Political Reaction
- The PREP Act
In an attempt to counteract these potential liabilities, the U.S. Government created the Public Readiness and Emergency Preparedness (PREP) Act. Private companys and sectors are covered under this act from liability in their production and distribution of "covered countermeasures"[23]. These "covered countermeasures" can be an assortment of medication intended from the use during a nation-wide emergency that can be legally administered not only from the approval by FDA but also from the approval of Emergency Use Authorization (EUA) or from Investigational New Drug Application (IND). The act also provides restitution for damages or injuries of a person acquired through the use of these covered countermeasures. [25]
- Federal Food, Drug, and Cosmetic Act (FDDCA)
Under section 561, the act states that unapproved drugs granted an Investigational New Drug Application (IND) will be allowed under the authorization of the U.S. Secretary to be administered to the public during a time of emergency. It is an attempt by the Government to allow public access to different types of drugs that have yet to be formally approved by the FDA during a time of need. The drug must[26]:
- be directed for treating an immediate threat disease, like anthrax
- provide proof of the drug's efficacy and safety in humans through clinical trials
- the only drug available for treating that particular disease
- Emergency Use Authorization (EUA)
After a declaration of emergency has been made by the Department of Health and Human Services Secretary, a EUA can be passed allowing selected unapproved drugs to be administered to the public and permitting the use of licensed drugs in ways yet to be legally approved by the FDA. However, like FFDCA's section 561, not all unapproved drugs can be administered. Certain guidelines must be met before the licensed or unlicensed drug can be approved for use even during a national emergency. These guidelines are[23]:
- The disease to be treated is life-threatening
- The drug must have proof of efficacy against the treated disease
- The effects of the drug side more to help the public than to expose the public to possible health risks when administered the drug
- Other drugs are unavailable in treating the disease
References
[1] World Organization for Animal Health, World Health Organization, Food and Agriculture Organization of the United Nations. Anthrax in Humans and Animals. 2008. 4th Edition. P. 3, 36-40]
[2] World Health Organization. The World Health Report 2007 - A Safer Future: Global Public Health Security in the 21st Century. 2007. Ch. 3, New Health threats in the 21st Century. p.35-37]
[3] John A. Jernigan, David S. Stephens, David A. Ashford, Carlos Omenaca, Martin S. Topiel, Mark Galbraith, Michael Tapper, Tamara L. Fisk, Sherif Zaki, Tanja Popovic,* Richard F. Meyer,* Conrad P. Quinn, Scott A. Harper, Scott K. Fridkin, James J. Sejvar, Colin W. Shepard, Michelle McConnell, Jeannette Guarner, Wun-Ju Shieh, Jean M. Malecki, Julie L. Gerberding, James M. Hughes, Bradley A. Perkins, members of the Anthrax Bioterrorism Investigation Team, Centers for Disease Control and Prevention, Atlanta, Georgia, USA; Emory University School of Medicine, Atlanta, Georgia, USA; Cedars Medical Center, Miami, Florida, USA; Virtua Health, Mount Holly, New Jersey, USA; Winchester Medical Center, Winchester, Virginia, USA; Lenox Hill Hospital, New York City, New York, USA; and Palm Beach County Department of Public Health, West Palm Beach, Florida, USA. Bioterrorism-Relation Inhalational Anthrax: The First 10 cases Reported in the United States. 8 November 2001. p. 1-36]
[4] World Health Organization, Global Alert and Response. "Guidance on Anthrax."
[5] World Health Organization, Fact Sheet. "Anthrax." 2001.
[6] World Health Organization, Initiative for Vaccine Research. "Zoonotic Infections." p.2.
[7] World Health Organization. "Guidelines for the Surveillance and Control of Anthrax in Humans and Animals." 3rd Edition. p 1-20.
[8] Centers for Disease Control and Prevention. "Anthrax: What You Need To Know." 2003.
[9] Centers for Disease Control and Prevention. "Anthrax: Signs and Symptoms." 2003.
[10] NCBI Entrez Genome Project
[11] Choudhury, B., Leoff, C., Saile, E., Wilkins, P., Quinn, C., Kannenberg, E., and Carlson, R. "The Structure of the Major Cell Wall Polysaccharide of Bacillus anthracis Is Species-specific". J. Biol. Chem. Sep 2006. 281: 27932 - 27941.
[12] Todar, K. "Todar's Online Textbook of bacteriology."
[13]
Lee, K., et al. "Phenotypic and functional characterization of Bacillus anthracis biofilms". Microbiology. 2007. 153. 1693-1701.
[14] National Institute of Allergy and Infectious diseases. "Anthrax."
[15] Centers for Disease Control and Prevention. "Use of Anthrax Vaccine in the United States." Advisory Committee on Immunization Practices Membership, October 2000
[16] World Health Organization. "Anthrax." October 2001.
[17] Boydston, J., Yue, L., Kearney, J., and Turnbough, Jr, C. "The ExsY Protein Is Required for Complete Formation of the Exosporium of Bacillus anthracis". J Bacteriol. November 2006. 188(21): 7440–7448.
[18] Anker, J., Hall, P., Lyandres, O., Shah, N., Zhao, J., and Duyne, R. "Biosensing with Plasmonic Nanosensors". Nature Material. 2008. doi:10.1038 (442-453)
[19] Zhang, X., Young, MA., Lyandres, O., and Duyne, R. "Rapid Detection of an Anthrax Biomarker by Surface-enhanced Raman Spectroscopy". PubMed: Journal of the American Chemical Society. March 30 2005. 127(12):4484-9.
[20] NASA image file.
[21] Osorio, M., Wu, Y., Singh, S., Merkel, T., Bhattacharyaa, S., Blake, M., and Kopecko, D. "Anthrax Protective Antigen Delivered by Salmonella enterica Serovar Typhi Ty21a Protects Mice from a Lethal Anthrax Spore Challenge". American Society for Microbiology: Infection and Immunity. Apr 2009. doi:10.1128 (1475-1482).
[22] CDC photo gallery of Salmonella Typhimurium bacteria.
[23] Maher, C., and Lushniak, BD. "Availability of Medical Countermeasures for Bioterrorism Events: US Legal and Regulatory Options". Clinical Pharmacology & Therapeutics. 2009. doi:10.1038 (669-671)
[24] Logan, C. "Cities Readiness Initiative". NGA Center for Best Practices. Jan 2005.
[25] "Division C-Public Readiness and Emergency Preparedness Act". Health Resources and Services Administration. Dec 2005. Public Law 109-148.
[26] "SEC. 561. [21 USC §360bbb] Expanded Access to Unapproved Therapies and Diagnostics". United States Food and Drug Administration. May 2008.
Edited by Kai Kuo, Maggie Chan, Miya Yoshida, Eric Ku, Stephen Chin, students of Rachel Larsen

![Process of Raman Spectroscopy [20]](/images/thumb/9/95/Ramanspectroscopy.jpg/300px-Ramanspectroscopy.jpg)
![Salmonella Typhimurium Bacteria [22]](/images/thumb/f/f3/Salmonella_Typhi.jpg/280px-Salmonella_Typhi.jpg)
