Encephalitozoon hellem: Difference between revisions
No edit summary |
|||
| (82 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
{{Biorealm Genus}} | {{Biorealm Genus}} | ||
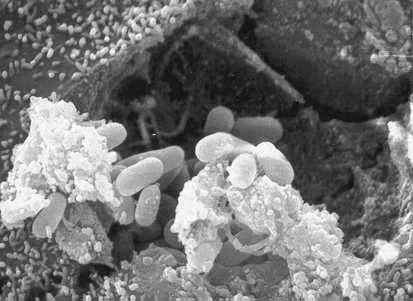
[[Image: Micrsp_devStageSEM.jpg|frame|center|''Encephalitozoon hellem''; Scanning electron micrograph showing an eukaryotic cell bursting and releasing spores of Encephalitozoon hellem to the extracellular medium. | [[Image: Micrsp_devStageSEM.jpg|frame|center|''Encephalitozoon hellem''; Scanning electron micrograph showing an eukaryotic cell bursting and releasing spores of Encephalitozoon hellem to the extracellular medium. | ||
| Line 7: | Line 8: | ||
===Higher order taxa=== | ===Higher order taxa=== | ||
<b>Superkingdom, Kingdom, Phylum, Suborder, Family, Genus: </b> | <b><font color=blue>Superkingdom, Kingdom, Phylum, Suborder, Family, Genus:</font></b> | ||
Eukaryota, Fungi, Microsporidia, Apansporoblastina, Unikaryonidae, Encephalitozoon | Eukaryota, Fungi, Microsporidia, Apansporoblastina, Unikaryonidae, Encephalitozoon | ||
===Species=== | ===Species=== | ||
| Line 17: | Line 17: | ||
==Description and significance== | ==Description and significance== | ||
<i>Encephalitozoon hellem</i> was initially dectected and isolated in 1991 from three AIDS patients who were suffering from keratoconjunctivitis among other ailments. Conjunctival scrapings and corneal tissue samples were taken from each of the three patients and were tested using SDS-PAGE analysis and Western Blotting [http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=1995733&ordinalpos=151&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum=abtract 4]. All three isolates had identical banding patterns and appeared similar to another species of Encephalitozoon, E. cuniculi, and the new AIDS related microsporidian was then given its name, <i>Encephaliozoon hellem</i>. | <i>Encephalitozoon hellem</i> was initially dectected and isolated in 1991 from three AIDS patients who were suffering from keratoconjunctivitis, among other ailments. Conjunctival scrapings and corneal tissue samples were taken from each of the three patients and were tested using SDS-PAGE analysis and Western Blotting ([http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=1995733&ordinalpos=151&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum=abtract 4]). All three isolates had identical banding patterns and appeared similar to another species of Encephalitozoon, E. cuniculi, and the new AIDS related microsporidian was then given its name, <i>Encephaliozoon hellem</i>. | ||
In | In 1994, another AIDS patient experiencing a foreign body sensation in his left eye had a conjunctival swab tested using a calcofluor stain and a fluorescent-antibody stain with murein antiserum raised against <i>E. hellem</i>. A cross-reaction occured with another species of Encephalitozoon, so a PCR test was performed. It was found that the amplified rRNA generated digestion patterns by restriction endonuclease FokI that were indentical to the digestion patterns of <i>E. hellem</i> ([http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=228923=abstract 3]). These observations allowed researchers to conclude that <i>Encephalitozoon hellem</i> causes keratoconjunctivitis in addition to other disseminated infections in immunosuppressed patients. Doctors and researchers soon found that patients treated with albendazole and topical fumagillin responded rapidly with no opthalmologic signs ([http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=228923=abstract 3]). | ||
<i>Encephalitozoon hellem</i> is a unicellular microsporidian that is thought to | <i>Encephalitozoon hellem</i> is a unicellular microsporidian that is thought to be fecal borne. It is a spore forming parasite whose spores measure approximately 1x1.5-2.0 microns in diameter ([http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=1995733&ordinalpos=151&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum=abtract 4]). <i>E. hellem</i> has been named one of the four most common human microsporidian parasites, with its human hosts consisting mainly of immunodificient patients. The genotyping of this microsporidian has been helpful in understanding other forms of human microsporidosis and their transmission ([http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=88110=abstract 5]). Microsporidia such as <i>E. hellem</i> is culturable from affected tissues and useful for species-species diagnosis. While the culturing of these microbes is time-consuming and requires expert skill, species-species identification of microsporidia is important in order to study the various levels of drug responses of the differing species of Encephalitozoon([http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=228923=abstract 3]). | ||
==Genome structure== | ==Genome structure== | ||
<i>Encephalitozoon</i> species have the smallest genomes reported to date among all single-celled eukaryotes. It is suggested that this is because of an early divergence from microsporidia that are no longer supported by current phylogenic data. The genome size of <i>E. hellem</i> is approximately 2.39 Mb ([http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=11456324&ordinalpos=47&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum=abstract 7]). Analysis of the Internal Transcribed Spacer (ITS) sequence of the rRNA genes and SSU rRNA sequences of various isolates indentified 3 genotypes of <i>E. hellem</i>, 1A, 1B, and 1C. Each of these genotypes have 1253 bp, 1313 bp, and 1373 bp respectively and all have identical ITS sequences. These three genotypes differ in small insertions or deletions and some point mutations ([http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=193859#r24=abstract 8]). While ITS sequences served as a valuable marker in researching <i>E. hellem</i>, the polar tubule protein (PTP) gene proved to be more valuable as it found an additional genotype, 2B, having 1421 base pairs. Genotype 2B has very different ITS and SSU rRNA sequences and extensive differences in PTP sequences([http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=88110=abstract 5]). | |||
Later studies have revealed two more genotypes, 2A and 2B. While six different genotypes have been discovered, the significance of <i>E. hellem's</i> genetic diversity is unclear. The number of <i>E. hellem</i> isolates is very limited leaving researchers without enough information to compare genotype distribution among humans and other hosts such as birds ([http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=88110=abstract 5]). | |||
The polar tubule protein gene, called PTP1, is the best known target so far for genotyping <i>Encephalitozoon hellem</i>. PTP1 is an intronless gene that exists as a single copy per haploid genome. It is very rich in glycine and proline and contains a hydrophilic central domain of six tandem repeat units. The length of the protein depends on the number of repeats present, but is approximately 43 kDa in size ([http://www.ncbi.nlm.nih.gov/sites/entrez?db=PubMed&cmd=Retrieve&list_uids=11155928=abstract 9]). | |||
Pulsed-field gel electrophoresis (PFGE) is most often used for typing <i>Encephalitozoon hellem</i> and has found two karyotypes A and B with 7 and 8 chromosomal bands respectively. PFGE is currently the most discriminative tool to research the intraspecific diversity of <i>E. hellem</i> ([http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=193859#r24=abstract 8]). | |||
==Cell structure and metabolism== | ==Cell structure and metabolism== | ||
<i>Encephalitozoon hellem</i> is a unicellular, intracellular microsporidian species ([http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=12120990&ordinalpos=40&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum=abstract 1]). It is a parasite whose gram positive spores measure approximately 1x1.5-2.0 microns. Each spore has a unique polar tubule organelle that makes six to eight coils inside the cell. These polar tubules are the ultrastructures of <i>E. hellem</i> ([http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=1995733&ordinalpos=151&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum=abstract 4]). On the opposite end of the polar tubule is a polar vacuole that responds to environmental stimuli such as pH and pressure changes. Other organelles include ribosomes and nuclei, but no mitochondria are present. However, microsporidia such as <i>E. hellem</i> are true eukaryotes because they do possess a nuclear envelope and other intracellular membranes ([http://cmr.asm.org/cgi/reprint/2/2/158?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&titleabstract=microsporidia&searchid=1&FIRSTINDEX=0&resourcetype=HWCIT=abstract 11]). Inside each cell there is a monokaryotic nuclei and thick endospores w/ irregulary shaped exospores ([http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=1995733&ordinalpos=151&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum=abstract 4]). The thick spore wall is composed of an electron dense undercoat and a thick electron dense lucent layer of chitin ([http://cmr.asm.org/cgi/reprint/2/2/158?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&titleabstract=microsporidia&searchid=1&FIRSTINDEX=0&resourcetype=HWCIT=abstract 11]). The cells' nuclear envelope is punctuated by rare nuclear pores about 50-60 nm and does not break down upon nuclear division. Instead, the nuclear envelope elongates into a dumbell shape with the two daughter nuclei at each end linked by a long straight bundle of microtubules ([http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=9627995&ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVBrief=abstract 10]). | |||
There are also amorphous plaques associated with the nuclear envelope close to the poles of the spindle called electron-dense spindle plaques (ESP). These ESPs are the focus of microtubule arrangement. Based on the organization of the cell, it can be assumed that both the spindle and the cytoplasmic microtubules are involved in the nuclear division process ([http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=9627995&ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVBrief=abstract 10]). | |||
Researchers have found that <i>E. hellem</i> cells synthesize single cytosolic neutral aminopeptidase activites that preferentially cleave leucine and arginine. This activity is associated with the microbes sporoplasm being passed down the polar tubule for host cell invasion and also for the regulation of parasite development. The maximal activity occurs at a pH of 7.2. The aminopeptidase has been estimated by direct fluorogenic analysis to have a molecular mass of 72 kDa and based on other studies they have been found to be potential targets for chemotherapeutic agents in parasitic diseases ([http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&dopt=AbstractPlus&list_uids=12435118=abstract 13]). | |||
==Ecology== | ==Ecology== | ||
Microsporidia, such as <i>E. hellem</i>, naturally infect birds. From a study of 570 birds from environments ranging from captive to free-range, 20 of those birds were found to shed <i>Encephalitozoon hellem</i> spores. Often species of parrots are the most infected. Of the eleven species that carried these spores, eight of them were aquatic birds. It is plausible then that <i>E. hellem</i> may originate not only from livestock birds as previously studied, but also from aquatic birds ([http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1489349=abstract 14]). Of these aquatic birds, <i>E. hellem</i> is most prevalent in birds who come into frequent contact with surface water indicating the microbes' ability to survive in water, and even longer in waters at lower temperatures. Studies have shown that a single waterfowl can introduce into the water approximately 910,000,000 microsporidia spores that infect humans, majority of which are <i>E. hellem</i>. This introduction is all via fecal matter where the <i>E. hellem</i> spores flourish until they come into contact with host cells. <i>Encephalitozoon hellem</i> is not known to live in any other type of environment and has thus only been found as fecal borne ([http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1489349=abstract 14]). | |||
==Pathology== | ==Pathology== | ||
<i>E. hellem</i> was first isloated in | <i>E. hellem</i> was first isloated in HIV patients, but has also been found to infect mice, birds, and even bats. When isolating <i>E. hellem</i> in the HIV patient, there were 24 monoclonal antibodies that were used against not only <i>E. hellem</i>, but also two other species of Encephalitozoon, E. intestinalis and E. cuniculi. The antibodies did not react with either of these two, indicating antibody specificity across the three species. Antigenic diversity of the different karyotypes of <i>E. hellem</i> was demonstrated as well when two monoclonal antibodies reacted with karyotype B but not with karyotype A ([http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=12120990&ordinalpos=40&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum=abstract 1]). | ||
Experiments exposing chickens to <i>E. hellem</i> found that the microbe was detectable in the feces of the animals | Experiments exposing chickens to <i>E. hellem</i> found that the microbe was detectable in the feces of the animals for up to 19 days, showing that <i>E. hellem</i> is potentially fecal borne and that chickens are a very likely host. While a definite route of infection is not well documented, it has been assumed that transmission of <i>E. hellem</i> most likely occurs by direct contact or by the ingestion of contaminated food or water ([http://www.ars.usda.gov/research/publications/publications.htm?SEQ_NO_115=148413=abstract 2]). | ||
Once a host has been found, <i>E. hellem</i>, like other microsporidia, act as spore forming parasites that use their unique polar tubule as a means of infecting their hosts. A mature, resistant spore will extrude the polar tubule and through this, the sporoplasm will be injected into the host cell. The sporoplasm is then able to multiply inside of the host cell via binary fission or multiple fission. Like the photo above illustrates, the spores will continue to multiply until the host cell cytoplasm is filled, causing it to burst and release more spores into the surroundings that will mature and carry on the infection to other cells ([http://www.dpd.cdc.gov/DPDx/HTML/Microsporidiosis.htm=abstract 6]). | |||
Studies have found that the glycosylation of the ultrastructure gene PTP1, can be modified by O-linked mannosylation indicating that mannose pretreatment of host cells may decrease infection by <i>E. hellem</i>. This suggests that the O-mannosylation of PTP1 may have a functional significance for the ability of <i>E. hellem</i> to invade their hosts ([http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=15501763&ordinalpos=22&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum=abstract 12]). | |||
The clinical manifestations of <i>E. hellem</i> include keratoconjunctivitis, infection of respiratory and genitourinary tract, and disseminated infection. With <i>E. hellem</i> being one of the four most common human microsporidian parasites, hosts are usually HIV patients but infections have also been reported to infect immunodificient patients that do not have HIV ([http://www.dpd.cdc.gov/DPDx/HTML/Microsporidiosis.htm=abstract 6]). | |||
==Application to Biotechnology== | ==Application to Biotechnology== | ||
<i>Encephalitozoon hellem</i> is not known to produce any enzymes that are beneficial to the environment or its inhabitants. However, studies performed on <i>E. hellem</i> have shown potential to be beneficial to clinical and biological research. For example, culturing of the microbe is useful for species-species diagnosis and studying the different drug responses of each ([http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=228923=abstract 3]). It is also believed that different sources of <i>E. hellem</i> isolates could be important to finding new variants and genotyping other isolates may be important to evaluating the actual variability in the species ([http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=193859#r24=abstract 8]). Also, the genotyping of this microsporidian has been helpful in understanding other forms of human microsporidosis and their transmission ([http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=88110=abstract 5]). | |||
==Current Research== | ==Current Research== | ||
A recent study diagnosed a free-ranging European brown hare with chronic interstitial nephritis. The hare was infected with both <i>E. hellem</i> and E. intestinalis. Researchers noted wedge shaped lesions on the hare's kidneys, a symptom characterized by interstitial nephritis, most commonly associated with E. cuniculi infection. Other symptoms of the hare included dehydration, atrophic muscles, and it was severly thin. This results of this study were the first documented description of <i>E. hellem</i> and E. intestinalis infection in a European brown hare, this being an instance of this microsporidic infection in a non-human besides a bird. The presence of chronic interstitial nephritis in the hare suggested that <i>E. hellem</i> and E. intestinalis together could cause E. cuniculi-like lesions. Thoughout the experiment <i>E. hellem</i> seemed to be more pathogenic than E. intestinalis ([http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame=abstract 15]). | |||
Another study implemented retrospective analysis of 110 formalin-stored diarrheic stool samples from AIDS patients who suffered from intestinal microsporidiosis. These samples were taken between 1992 and 2003, and of these 2.1% had <i>E. hellem</i> spores present. Samples that were older than 10 years did not have as distinguished results and were deemed unsuitable for retrospective analysis ([http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame=abstract 16]). The identification of microsporidian spores using multiplex FISH assay proved to be more sensitive and give more clear results that chromotropo-2R and Calcofluor White M2R stains. Besides <i>E. hellem</i>, other microsporidia such as E. intestinalis were also present in the patients' samples. These results were able to show that micropsoridian co-infection was not uncommon in HIV/AIDS patients and may have contributed to a large amount of illnesses and infections that may have not been present had <i>E. hellem</i> existed in the body alone. Furthermore, this study found that the identification of <i>E. hellem</i> among other Encephalitozoon species was a useful tool for assessing spore shedding intensity in intestinal microsporidiosis and can also be used for epidemiological investigations in addition to clincal studies ([http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame=abstract 16]). | |||
The immune response to <i>E. hellem</i> of both the innate and and adaptive immune systems has also been recently studied. In this experiment two-day old chickens were inoculated perorally, through the mouth, and intraperitoneally, directly into the abdominal cavity, with <i>E. hellem</i> spores. ELISA tests were used to determine the anti-E. hellem IgY, IgA, and IgM responses in both sera and fecal samples. In the sera, the IgY was the only one measured in significant levels whereas in the fecal samples all three antibodies were present, but still with IgY being the most prevalent ([http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame=abstract 17]). More antibodies formed in the chickens that were inoculated intraperitoneally. It is possible that this occured because of the unnatural route of infection and the spores being able to directly contact the immune system and not go through intestinal barriers. This study was able to show that an <i>E. hellem</i> infection is asymptomatic and that spore-shedding is dependent on concurrent infection. Fecal samples of asymptomatic birds supported the assumption that the bird droppings are a potential source of infection for other hosts, such as humans. Also, based on the results on the antibody testing, the results show that immunodeficient humans infected with microsporidiosis, such as <i>E. hellem</i> infection, may develop parasite specific antibodies. This research could potentially help with future pharmacological advances against microsporidiosis ([http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame=abstract 17]). | |||
==References== | ==References== | ||
| Line 56: | Line 75: | ||
5. Xiao, L. et. al [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=88110=abstract Genotyping Encephalitozoon hellem Isolates by Analysis of the Polar Tube Protein Gene]J Clinical Microbiology 39, 2191–2196 (2001) | 5. Xiao, L. et. al [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=88110=abstract Genotyping Encephalitozoon hellem Isolates by Analysis of the Polar Tube Protein Gene]J Clinical Microbiology 39, 2191–2196 (2001) | ||
6. [http://www.dpd.cdc.gov/DPDx/HTML/Microsporidiosis.htm=abstract Microsporidiosis] Centers for Disease Control & Prevention | |||
National Center for Infectious Diseases | |||
Division of Parasitic Diseases | |||
7. Delarbre, S. et. al [http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=11456324&ordinalpos=47&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum=abstract Genetic diversity in the microsporidian Encephalitozoon hellem demonstrated by pulsed-field gel electrophoresis] J. Eukaryotic Microbiology 48, 471-474 (2001) | |||
8. Haro, M. et. al [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=193859#r24=abstract Intraspecies Genotype Variability of the Microsporidian Parasite Encephalitozoon hellem] J. Clinical Microbiolgy 41, 4166–4171 (2003) | |||
9. Peuvel, I. et. al [http://www.ncbi.nlm.nih.gov/sites/entrez?db=PubMed&cmd=Retrieve&list_uids=11155928=abstract Polymorphism of the gene encoding a major polar tube protein PTP1 in two microsporidia of the genus Encephalitozoon] Parasitology 121, 581-7 (2000) | |||
10. Bigliardi, E. et. al [http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=9627995&ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVBrief=abstract Mechanisms of microsporidial cell division: ultrastructural study on Encephalitozoon hellem] J Eukaryotic Microbiology 45, 347-51 (1998) | |||
11. Shadduck, J., Greely, E. [http://cmr.asm.org/cgi/reprint/2/2/158?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&titleabstract=microsporidia&searchid=1&FIRSTINDEX=0&resourcetype=HWCIT=abstract Microsporidia and Human Infections] Clinical Microbiology Reviews 2, 158-165 (1989) | |||
12. Xu, Y. et. al, [http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=15501763&ordinalpos=22&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum=abstract Glycosylation of the major polar tube protein of Encephalitozoon hellem, a microsporidian parasite that infects humans] Infect Immun 72,6341-50 (2004) | |||
13. Millership, JJ. et. al [http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&dopt=AbstractPlus&list_uids=12435118=abstract Characterization of aminopeptidase activity from three species of microsporidia: Encephalitozoon cuniculi, Encephalitozoon hellem, and Vittaforma corneae] J Parasitology 88, 843-8 (2002) | |||
14. Slodkowicz-Kowalska, A. et. al [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1489349=abstract Microsporidian Species Known To Infect Humans Are Present in Aquatic Birds: Implications for Transmission via Water] Appl Environ Microbiol 72, 4540–4544 (2006) | |||
15. De Bosschere, H. et. al [http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame=abstract First diagnosis of Encephalitozoon intestinalis and E-hellem in a European brown hare (Lepus europaeus) with kidney lesions] Zoonoses and Public Health 54, 131-134 (2007) | |||
16. Graczyk, TK. et al [http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame=abstract Retrospective species identification of microsporidian spores in diarrheic fecal samples from human immunodeficiency virus/AIDS patients by multiplexed fluorescence in situ hybridization] J Clinical Microbiolgy 45, 1255-1260 (2007) | |||
17. Sakova, K. et. al [http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame=abstract Humoral response of chicken infected with the microsporidium] Parasitology Research 98, 488-492 (2006) | |||
Edited by Tiffany Myer, student of [mailto:ralarsen@ucsd.edu Rachel Larsen] | Edited by Tiffany Myer, student of [mailto:ralarsen@ucsd.edu Rachel Larsen] | ||
Latest revision as of 03:18, 20 August 2010
A Microbial Biorealm page on the genus Encephalitozoon hellem
Classification
Higher order taxa
Superkingdom, Kingdom, Phylum, Suborder, Family, Genus:
Eukaryota, Fungi, Microsporidia, Apansporoblastina, Unikaryonidae, Encephalitozoon
Species
Encephalitozoon hellem
Description and significance
Encephalitozoon hellem was initially dectected and isolated in 1991 from three AIDS patients who were suffering from keratoconjunctivitis, among other ailments. Conjunctival scrapings and corneal tissue samples were taken from each of the three patients and were tested using SDS-PAGE analysis and Western Blotting (4). All three isolates had identical banding patterns and appeared similar to another species of Encephalitozoon, E. cuniculi, and the new AIDS related microsporidian was then given its name, Encephaliozoon hellem.
In 1994, another AIDS patient experiencing a foreign body sensation in his left eye had a conjunctival swab tested using a calcofluor stain and a fluorescent-antibody stain with murein antiserum raised against E. hellem. A cross-reaction occured with another species of Encephalitozoon, so a PCR test was performed. It was found that the amplified rRNA generated digestion patterns by restriction endonuclease FokI that were indentical to the digestion patterns of E. hellem (3). These observations allowed researchers to conclude that Encephalitozoon hellem causes keratoconjunctivitis in addition to other disseminated infections in immunosuppressed patients. Doctors and researchers soon found that patients treated with albendazole and topical fumagillin responded rapidly with no opthalmologic signs (3).
Encephalitozoon hellem is a unicellular microsporidian that is thought to be fecal borne. It is a spore forming parasite whose spores measure approximately 1x1.5-2.0 microns in diameter (4). E. hellem has been named one of the four most common human microsporidian parasites, with its human hosts consisting mainly of immunodificient patients. The genotyping of this microsporidian has been helpful in understanding other forms of human microsporidosis and their transmission (5). Microsporidia such as E. hellem is culturable from affected tissues and useful for species-species diagnosis. While the culturing of these microbes is time-consuming and requires expert skill, species-species identification of microsporidia is important in order to study the various levels of drug responses of the differing species of Encephalitozoon(3).
Genome structure
Encephalitozoon species have the smallest genomes reported to date among all single-celled eukaryotes. It is suggested that this is because of an early divergence from microsporidia that are no longer supported by current phylogenic data. The genome size of E. hellem is approximately 2.39 Mb (7). Analysis of the Internal Transcribed Spacer (ITS) sequence of the rRNA genes and SSU rRNA sequences of various isolates indentified 3 genotypes of E. hellem, 1A, 1B, and 1C. Each of these genotypes have 1253 bp, 1313 bp, and 1373 bp respectively and all have identical ITS sequences. These three genotypes differ in small insertions or deletions and some point mutations (8). While ITS sequences served as a valuable marker in researching E. hellem, the polar tubule protein (PTP) gene proved to be more valuable as it found an additional genotype, 2B, having 1421 base pairs. Genotype 2B has very different ITS and SSU rRNA sequences and extensive differences in PTP sequences(5).
Later studies have revealed two more genotypes, 2A and 2B. While six different genotypes have been discovered, the significance of E. hellem's genetic diversity is unclear. The number of E. hellem isolates is very limited leaving researchers without enough information to compare genotype distribution among humans and other hosts such as birds (5).
The polar tubule protein gene, called PTP1, is the best known target so far for genotyping Encephalitozoon hellem. PTP1 is an intronless gene that exists as a single copy per haploid genome. It is very rich in glycine and proline and contains a hydrophilic central domain of six tandem repeat units. The length of the protein depends on the number of repeats present, but is approximately 43 kDa in size (9).
Pulsed-field gel electrophoresis (PFGE) is most often used for typing Encephalitozoon hellem and has found two karyotypes A and B with 7 and 8 chromosomal bands respectively. PFGE is currently the most discriminative tool to research the intraspecific diversity of E. hellem (8).
Cell structure and metabolism
Encephalitozoon hellem is a unicellular, intracellular microsporidian species (1). It is a parasite whose gram positive spores measure approximately 1x1.5-2.0 microns. Each spore has a unique polar tubule organelle that makes six to eight coils inside the cell. These polar tubules are the ultrastructures of E. hellem (4). On the opposite end of the polar tubule is a polar vacuole that responds to environmental stimuli such as pH and pressure changes. Other organelles include ribosomes and nuclei, but no mitochondria are present. However, microsporidia such as E. hellem are true eukaryotes because they do possess a nuclear envelope and other intracellular membranes (11). Inside each cell there is a monokaryotic nuclei and thick endospores w/ irregulary shaped exospores (4). The thick spore wall is composed of an electron dense undercoat and a thick electron dense lucent layer of chitin (11). The cells' nuclear envelope is punctuated by rare nuclear pores about 50-60 nm and does not break down upon nuclear division. Instead, the nuclear envelope elongates into a dumbell shape with the two daughter nuclei at each end linked by a long straight bundle of microtubules (10).
There are also amorphous plaques associated with the nuclear envelope close to the poles of the spindle called electron-dense spindle plaques (ESP). These ESPs are the focus of microtubule arrangement. Based on the organization of the cell, it can be assumed that both the spindle and the cytoplasmic microtubules are involved in the nuclear division process (10).
Researchers have found that E. hellem cells synthesize single cytosolic neutral aminopeptidase activites that preferentially cleave leucine and arginine. This activity is associated with the microbes sporoplasm being passed down the polar tubule for host cell invasion and also for the regulation of parasite development. The maximal activity occurs at a pH of 7.2. The aminopeptidase has been estimated by direct fluorogenic analysis to have a molecular mass of 72 kDa and based on other studies they have been found to be potential targets for chemotherapeutic agents in parasitic diseases (13).
Ecology
Microsporidia, such as E. hellem, naturally infect birds. From a study of 570 birds from environments ranging from captive to free-range, 20 of those birds were found to shed Encephalitozoon hellem spores. Often species of parrots are the most infected. Of the eleven species that carried these spores, eight of them were aquatic birds. It is plausible then that E. hellem may originate not only from livestock birds as previously studied, but also from aquatic birds (14). Of these aquatic birds, E. hellem is most prevalent in birds who come into frequent contact with surface water indicating the microbes' ability to survive in water, and even longer in waters at lower temperatures. Studies have shown that a single waterfowl can introduce into the water approximately 910,000,000 microsporidia spores that infect humans, majority of which are E. hellem. This introduction is all via fecal matter where the E. hellem spores flourish until they come into contact with host cells. Encephalitozoon hellem is not known to live in any other type of environment and has thus only been found as fecal borne (14).
Pathology
E. hellem was first isloated in HIV patients, but has also been found to infect mice, birds, and even bats. When isolating E. hellem in the HIV patient, there were 24 monoclonal antibodies that were used against not only E. hellem, but also two other species of Encephalitozoon, E. intestinalis and E. cuniculi. The antibodies did not react with either of these two, indicating antibody specificity across the three species. Antigenic diversity of the different karyotypes of E. hellem was demonstrated as well when two monoclonal antibodies reacted with karyotype B but not with karyotype A (1).
Experiments exposing chickens to E. hellem found that the microbe was detectable in the feces of the animals for up to 19 days, showing that E. hellem is potentially fecal borne and that chickens are a very likely host. While a definite route of infection is not well documented, it has been assumed that transmission of E. hellem most likely occurs by direct contact or by the ingestion of contaminated food or water (2).
Once a host has been found, E. hellem, like other microsporidia, act as spore forming parasites that use their unique polar tubule as a means of infecting their hosts. A mature, resistant spore will extrude the polar tubule and through this, the sporoplasm will be injected into the host cell. The sporoplasm is then able to multiply inside of the host cell via binary fission or multiple fission. Like the photo above illustrates, the spores will continue to multiply until the host cell cytoplasm is filled, causing it to burst and release more spores into the surroundings that will mature and carry on the infection to other cells (6).
Studies have found that the glycosylation of the ultrastructure gene PTP1, can be modified by O-linked mannosylation indicating that mannose pretreatment of host cells may decrease infection by E. hellem. This suggests that the O-mannosylation of PTP1 may have a functional significance for the ability of E. hellem to invade their hosts (12).
The clinical manifestations of E. hellem include keratoconjunctivitis, infection of respiratory and genitourinary tract, and disseminated infection. With E. hellem being one of the four most common human microsporidian parasites, hosts are usually HIV patients but infections have also been reported to infect immunodificient patients that do not have HIV (6).
Application to Biotechnology
Encephalitozoon hellem is not known to produce any enzymes that are beneficial to the environment or its inhabitants. However, studies performed on E. hellem have shown potential to be beneficial to clinical and biological research. For example, culturing of the microbe is useful for species-species diagnosis and studying the different drug responses of each (3). It is also believed that different sources of E. hellem isolates could be important to finding new variants and genotyping other isolates may be important to evaluating the actual variability in the species (8). Also, the genotyping of this microsporidian has been helpful in understanding other forms of human microsporidosis and their transmission (5).
Current Research
A recent study diagnosed a free-ranging European brown hare with chronic interstitial nephritis. The hare was infected with both E. hellem and E. intestinalis. Researchers noted wedge shaped lesions on the hare's kidneys, a symptom characterized by interstitial nephritis, most commonly associated with E. cuniculi infection. Other symptoms of the hare included dehydration, atrophic muscles, and it was severly thin. This results of this study were the first documented description of E. hellem and E. intestinalis infection in a European brown hare, this being an instance of this microsporidic infection in a non-human besides a bird. The presence of chronic interstitial nephritis in the hare suggested that E. hellem and E. intestinalis together could cause E. cuniculi-like lesions. Thoughout the experiment E. hellem seemed to be more pathogenic than E. intestinalis (15).
Another study implemented retrospective analysis of 110 formalin-stored diarrheic stool samples from AIDS patients who suffered from intestinal microsporidiosis. These samples were taken between 1992 and 2003, and of these 2.1% had E. hellem spores present. Samples that were older than 10 years did not have as distinguished results and were deemed unsuitable for retrospective analysis (16). The identification of microsporidian spores using multiplex FISH assay proved to be more sensitive and give more clear results that chromotropo-2R and Calcofluor White M2R stains. Besides E. hellem, other microsporidia such as E. intestinalis were also present in the patients' samples. These results were able to show that micropsoridian co-infection was not uncommon in HIV/AIDS patients and may have contributed to a large amount of illnesses and infections that may have not been present had E. hellem existed in the body alone. Furthermore, this study found that the identification of E. hellem among other Encephalitozoon species was a useful tool for assessing spore shedding intensity in intestinal microsporidiosis and can also be used for epidemiological investigations in addition to clincal studies (16).
The immune response to E. hellem of both the innate and and adaptive immune systems has also been recently studied. In this experiment two-day old chickens were inoculated perorally, through the mouth, and intraperitoneally, directly into the abdominal cavity, with E. hellem spores. ELISA tests were used to determine the anti-E. hellem IgY, IgA, and IgM responses in both sera and fecal samples. In the sera, the IgY was the only one measured in significant levels whereas in the fecal samples all three antibodies were present, but still with IgY being the most prevalent (17). More antibodies formed in the chickens that were inoculated intraperitoneally. It is possible that this occured because of the unnatural route of infection and the spores being able to directly contact the immune system and not go through intestinal barriers. This study was able to show that an E. hellem infection is asymptomatic and that spore-shedding is dependent on concurrent infection. Fecal samples of asymptomatic birds supported the assumption that the bird droppings are a potential source of infection for other hosts, such as humans. Also, based on the results on the antibody testing, the results show that immunodeficient humans infected with microsporidiosis, such as E. hellem infection, may develop parasite specific antibodies. This research could potentially help with future pharmacological advances against microsporidiosis (17).
References
1. Mo L., Drancourt M. Antigenic diversity of Encephalitozoon hellem demonstrated by subspecies-specific monoclonal antibodiesJ. Eukaryotic Microbiology 49, 249-54 (2002)
2. Fayer, R. et. al Detection of Encephalitozoon Hellem in Feces of Experimentally Infected ChickensJ. Eukaryotic Microbiology 50, 5743-575 (2003)
3. Didier, E.S. et. al Diagnosis of disseminated microsporidian Encephalitozoon hellem infection by PCR-Southern analysis and successful treatment with albendazole and fumagillinJ Clinical Microbiology, 34, 947–952 (1996)
4. Didier, E.S. et. al Isolation and characterization of a new human microsporidian, Encephalitozoon hellem (n. sp.), from three AIDS patients with keratoconjunctivitisJ Infect. Dis. 163,617-21 (1991)
5. Xiao, L. et. al Genotyping Encephalitozoon hellem Isolates by Analysis of the Polar Tube Protein GeneJ Clinical Microbiology 39, 2191–2196 (2001)
6. Microsporidiosis Centers for Disease Control & Prevention National Center for Infectious Diseases Division of Parasitic Diseases
7. Delarbre, S. et. al Genetic diversity in the microsporidian Encephalitozoon hellem demonstrated by pulsed-field gel electrophoresis J. Eukaryotic Microbiology 48, 471-474 (2001)
8. Haro, M. et. al Intraspecies Genotype Variability of the Microsporidian Parasite Encephalitozoon hellem J. Clinical Microbiolgy 41, 4166–4171 (2003)
9. Peuvel, I. et. al Polymorphism of the gene encoding a major polar tube protein PTP1 in two microsporidia of the genus Encephalitozoon Parasitology 121, 581-7 (2000)
10. Bigliardi, E. et. al Mechanisms of microsporidial cell division: ultrastructural study on Encephalitozoon hellem J Eukaryotic Microbiology 45, 347-51 (1998)
11. Shadduck, J., Greely, E. Microsporidia and Human Infections Clinical Microbiology Reviews 2, 158-165 (1989)
12. Xu, Y. et. al, Glycosylation of the major polar tube protein of Encephalitozoon hellem, a microsporidian parasite that infects humans Infect Immun 72,6341-50 (2004)
13. Millership, JJ. et. al Characterization of aminopeptidase activity from three species of microsporidia: Encephalitozoon cuniculi, Encephalitozoon hellem, and Vittaforma corneae J Parasitology 88, 843-8 (2002)
14. Slodkowicz-Kowalska, A. et. al Microsporidian Species Known To Infect Humans Are Present in Aquatic Birds: Implications for Transmission via Water Appl Environ Microbiol 72, 4540–4544 (2006)
15. De Bosschere, H. et. al First diagnosis of Encephalitozoon intestinalis and E-hellem in a European brown hare (Lepus europaeus) with kidney lesions Zoonoses and Public Health 54, 131-134 (2007)
16. Graczyk, TK. et al Retrospective species identification of microsporidian spores in diarrheic fecal samples from human immunodeficiency virus/AIDS patients by multiplexed fluorescence in situ hybridization J Clinical Microbiolgy 45, 1255-1260 (2007)
17. Sakova, K. et. al Humoral response of chicken infected with the microsporidium Parasitology Research 98, 488-492 (2006)
Edited by Tiffany Myer, student of Rachel Larsen
