Bordetella pertussis in Canada: Difference between revisions
No edit summary |
|||
| (61 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
==Introduction== | ==Introduction== | ||
[[Bordetella pertussis]] is the bacterial microbe responsible for the disease "Pertussis" (commonly referred to as 'Whooping Cough'). In this site, we will explore the physical attributes of the microbe, as well as the various toxins produced which facilitate both the infection and proliferation of the disease. Specifically, we will analyze the history of disease in Canada, the steps taken to eradicate disease, and potential treatments for the prevention of future outbreaks. | |||
== | ==Description of the microbe== | ||
= | [[Image:Coqueluche1.jpg|50 px |frame|alt=Puzzle globe logo|Figure 1: Bordetella pertussis (23)]] | ||
The bacterium Bordetella Pertussis is the causative agent of Whooping Cough. This small bacterium (about .8um in length and .4um by width) cannot survive in the open environment, and therefore must reside in a host, and is considered pathogenic. This host is almost always a human, where its “natural” environment is said to be the mucus in the human respiratory tract. The optimum temperature of growth for B.pertussis is about 35-37C, which is the temperature inside a living human host. | The bacterium [[Bordetella Pertussis]] is the causative agent of Whooping Cough. This small bacterium (about .8um in length and .4um by width) cannot survive in the open environment, and therefore must reside in a host, and is considered pathogenic. This host is almost always a human, where its “natural” environment is said to be the mucus in the human respiratory tract. The optimum temperature of growth for B.pertussis is about 35-37C, which is the temperature inside a living human host. | ||
B.pertussis is an aerobic gram negative bacterium that does not produce spores. Due to its lack of flagella, it is also immotile. It resides in the phylum Proteobacteria, class Betaproteobacteria, order Burkholderiales, and family Alcaligenaceae. Because it is an aerobe, it utilizes aerobic respiration. Therefore, the bacterium consists of an electron transport chain on its membrane, and is considered a chemoheterotroph. Like other gram negative bacteria, it possesses an inner and outer membrane, with a thin peptidoglycan cell wall in between. This cell wall is attached to the outer membrane via lipoproteins. Like other gram negatives, the outer membrane of B.pertussis is coated with LPS (lipopolysachharides), which are long sugar linked and lipid-anchored polysaccharides coating the outer membrane. LPS are endotoxins, meaning that they are toxic to host (or potentially other bacteria) cells when detached from the bacteria. However, B.pertussis consists of very unusual LPS compared to other gram negatives. Namely, it contains two forms of LPS that have a different phosphate composition than standard Lipid A form LPS. | B.pertussis is an aerobic gram negative bacterium that does not produce spores. Due to its lack of flagella, it is also immotile. It resides in the phylum [[Proteobacteria]], class Betaproteobacteria, order Burkholderiales, and family Alcaligenaceae. Because it is an aerobe, it utilizes aerobic respiration. Therefore, the bacterium consists of an electron transport chain on its membrane, and is considered a chemoheterotroph. Like other gram negative bacteria, it possesses an inner and outer membrane, with a thin peptidoglycan cell wall in between. This cell wall is attached to the outer membrane via lipoproteins. Like other gram negatives, the outer membrane of B.pertussis is coated with LPS (lipopolysachharides), which are long sugar linked and lipid-anchored polysaccharides coating the outer membrane. LPS are endotoxins, meaning that they are toxic to host (or potentially other bacteria) cells when detached from the bacteria(2). However, B.pertussis consists of very unusual LPS compared to other gram negatives. Namely, it contains two forms of LPS that have a different phosphate composition than standard Lipid A form LPS. | ||
Bordetella pertussis does not necessarily cause whooping cough by itself, but rather produces toxins that do so. Among producing pertussis toxin, filamentous hemagglutinin, and hemolysin, B.pertussis produces the adenylate cyclase toxin (CyaA) – a direct causative agent of Whooping Cough | [[Bordetella pertussis]] does not necessarily cause whooping cough by itself, but rather produces toxins that do so. Among producing pertussis toxin, filamentous hemagglutinin, and hemolysin, B.pertussis produces the adenylate cyclase toxin (CyaA) – a direct causative agent of Whooping Cough (1). B.pertussis attaches to host cells via the virulence factor P.69 pertactin, which is an extracellularly exposed domain of a membrane protein expressed by B.pertussis – and targets the adhesion to mammalian cells. In addition to this, the filamentous hemagglutinin (FHA) also helps mediate cell adhesion, and contains similar motifs as P.69 pertactin (2). Once attached, B.pertussis is able to transfer CyaA to the target host cell. | ||
The pertussis toxin (PT), also produced by B.pertussis, is known to also be an important factor in whooping cough respiratory infections. | The pertussis toxin (PT), also produced by B.pertussis, is known to also be an important factor in whooping cough respiratory infections. The resultant coughing is painful, and also is what spreads the highly contagious B.pertussis to others (3). | ||
The Tahoma 1 strain of B.pertussis has its entire genome sequenced – it is 1 circular chromosome 4,086,189 nucleotides long (3867 genes), of which about 67% of the entire genome is GC rich. | The Tahoma 1 strain of B.pertussis has its entire genome sequenced – it is 1 circular chromosome 4,086,189 nucleotides long (3867 genes), of which about 67% of the entire genome is GC rich (4). | ||
| Line 25: | Line 26: | ||
http://microbewiki.kenyon.edu/index.php/Bordetella_pertussis | http://microbewiki.kenyon.edu/index.php/Bordetella_pertussis | ||
==Description of the disease== | |||
Bortadella Pertussis secretes numerous toxins known to affect cellular homeostasis. The primary disease caused by the microbe is known as “Pertussis” (commonly referred to as 'Whooping Cough'). | Bortadella Pertussis secretes numerous toxins known to affect cellular homeostasis. The primary disease caused by the microbe is known as “Pertussis” (commonly referred to as 'Whooping Cough'). | ||
===General Disease Information:=== | |||
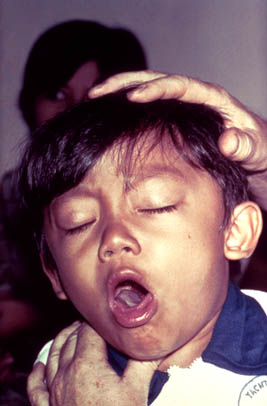
[[Image:Pertussis.jpg|thumb|alt=Puzzle globe logo|Figure 2: One of the symptoms of whooping cough is chronic, ongoing coughs (16)]] | |||
Whooping cough is characterized by uncontrollable, heavy coughing fits which persist for many weeks. Initially the symptoms may mimic that of the common cold, later developing into severe coughing and the potential development of further illness i.e. pneumonia, hypoxia (oxygen deprivation to tissues), apnea (suspension of breathing), seizures, etc. The disease progression can be broken up into several stages, each characterized by different symptoms. Initially the patient enters the “Catarrhal Phase” in which they display symptoms like that of the common cold. This usually develops within the first two weeks of disease. The second major stage is referred to as the “Paroxysmal Stage” and is characterized by uncontrollable fits of coughing followed by high pitched ‘whoop’ sounds upon air uptake. This stage is the most severe and is often the time when a patient will exhibit other disease symptoms. If the patient has the immune strength to combat the illness, they enter the final stage called the “Convalescent Stage”. Here the patient begins recovery from disease and uncontrollable coughing slowly diminishes (6). | |||
===Molecular Mechanisms of Disease:=== | ===Molecular Mechanisms of Disease:=== | ||
| Line 44: | Line 46: | ||
Pertussis toxin directs its activity intracellularly by affecting the inhibitory subunit of G-protein coupled receptors. PT catalyzes the ADP-Ribosylation of the Gi subunit and prevents inhibition of the protein Adenylyl Cyclase (AC). Adeneylyl Cyclase is responsible for the production of intracellular cAMP, a common downstream messenger in many cell signaling pathways. | Pertussis toxin directs its activity intracellularly by affecting the inhibitory subunit of G-protein coupled receptors. PT catalyzes the ADP-Ribosylation of the Gi subunit and prevents inhibition of the protein Adenylyl Cyclase (AC). Adeneylyl Cyclase is responsible for the production of intracellular cAMP, a common downstream messenger in many cell signaling pathways. | ||
In a normal cell, Gi subunits work concurrently with Gs proteins. Gs proteins are responsible for the activation of AC while Gi is responsible for inhibiting AC. When functioning properly, the two proteins regulate intracellular cAMP levels to maintain cellular homeostasis. In a cell affected by PT, Gi is inhibited and cannot act to “shut off” AC. As a result, the AC is not regulated and will constitutively produce cAMP until the Gs protein is removed. These high levels of cAMP have extreme downstream effects on gene expression and the viability of the cell. This destroys the ciliated epithelial cells that normally would sweep away mucus, and without this ciliated action, vigorous coughing is needed to clear the airway. | In a normal cell, Gi subunits work concurrently with Gs proteins. Gs proteins are responsible for the activation of AC while Gi is responsible for inhibiting AC. When functioning properly, the two proteins regulate intracellular cAMP levels to maintain cellular homeostasis (3, 4, 6). In a cell affected by PT, Gi is inhibited and cannot act to “shut off” AC. As a result, the AC is not regulated and will constitutively produce cAMP until the Gs protein is removed. These high levels of cAMP have extreme downstream effects on gene expression and the viability of the cell. This destroys the ciliated epithelial cells that normally would sweep away mucus, and without this ciliated action, vigorous coughing is needed to clear the airway (3, 4, 6). | ||
==== | ====Adenylate Cyclase Toxin (CyaA)==== | ||
CyaA is the primary toxin that contributes the development of 'Whooping cough' symptoms. It predominantly controls the initial localization of microbe to the lung and airway tissues for colonization. B. pertussis adheres to the host cell via P.69 pertactin virulence factor. | CyaA is the primary toxin that contributes the development of 'Whooping cough' symptoms. CyaA has a series of G and D rich polypeptide residues, which is a characteristic repeat for many toxins. It predominantly controls the initial localization of microbe to the lung and airway tissues for colonization. B. pertussis adheres to the host cell via P.69 pertactin virulence factor. It consists of an RGD tripeptide motif which attaches to various sites on mammalian adhesion proteins (some include fibronectin, vitronectin, and fibrinogen) which facilitates interaction between the mammalian host cell and the bacterial microbe (2). CyaA acts on cells in both calcium and temperature-dependent manners where the CyaA catalytic domain is directly moved across the target cells plasma membrane. Specifically, it binds and invades cells by forming transmembrane cation selective channels. These channels can further affect the affected cell by offsetting cellular homeostasis, occasionally resulting in cellular lysis. CyaA may also contribute to the increase in intracellular cAMP levels of the host cell (1,5,6,7). | ||
===Transmission of disease=== | ===Transmission of disease=== | ||
| Line 55: | Line 56: | ||
Bordetella pertussis cannot live in the outside environment. As a result, it has evolved to become a highly contagious microbe which is most commonly spread airborne. B. pertussis has negative effects on the airway tissues resulting in the loss of cilia mucus sweeping functions. The affected individual must now cough rigorously to clear the airway of excess mucus. The strong, uncontrollable coughing provides a mechanism for B. pertussis to leave the internal host environment and infect other individuals. | Bordetella pertussis cannot live in the outside environment. As a result, it has evolved to become a highly contagious microbe which is most commonly spread airborne. B. pertussis has negative effects on the airway tissues resulting in the loss of cilia mucus sweeping functions. The affected individual must now cough rigorously to clear the airway of excess mucus. The strong, uncontrollable coughing provides a mechanism for B. pertussis to leave the internal host environment and infect other individuals. | ||
=== | ==How/why is pertussis a problem in Canada== | ||
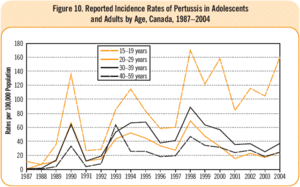
[[Image:Vpd-pert2-cig2006-lrg e.gif|thumb|alt=Puzzle globe logo|Graph 1: Incidence rates of adolescence and adults in Canada 1987-2004(16)]] | |||
In Canada, the number of reported infected individuals of pertussis cases has fluctuated throughout the years, due to outbreaks and despite new advances in vaccination technology; reported infected individuals has ranged from 2,165 to 10,151 within this period of time. These numbers are predicted to be under-representations of the true values due to incomplete diagnosis or misreporting (8). The number of individuals affected per 100,000 of population has been increasing for adults through the time period of 1987-2004. However, this disease has been reported to be more common in infants than in older children and adults. The percentage of reported infected adolescents (defined to be less than 15 years of age) and adults has been “increasing from 9.6% in 1995 to 16.4%, 21.2%, and 31.3% in 1998, 2001 and 2004 respectively” (8). | |||
===Use of whole cell pertussis vaccine=== | |||
In the 1940s, a whole cell pertussis fluid vaccine was introduced in Canada and was then replaced by an absorbed whole cell prtussis fluid vaccine in the 1980s. The absorbed whole cell pertussis fluid vaccine is made by suspending killed B. pertussis cells with an addition of other agents. These agents included in the vaccine are either absorbed diphtheria and tetanus toxoids (DPT), absorbed diphtheria and tetanus toxoids plus inactivated polio vaccine (DPT-Polio), or DPT or DPT-Polio combined with Haemophilus influenzae type b (Hib) conjugate vaccine (DPT-Hib, DPT-Polio-Hib) (9). The combined vaccines trigger an increase antibody production to B. pertussis, as well as diphtheria and tetanus antigens (9). | |||
There have been adverse reactions towards the whole-cell pertussis vaccines. There have been reports of minor local reactions, which result in redness, swelling, as well as pain, in 50% to 75% of vaccine patients. It was proposed that a new type of vaccine was needed since the whole cell pertussis vaccine did not seem protected, and the polymorphism mutations of B. pertussis rendered the vaccine less effective (10). This low efficacy resulted in high numbers of reported cases of B. pertussis, and thus became a outbreak. | |||
The efficacy of the whole-cell pertussis vaccines was 70%-90% in 1940s and 1950s. However, its efficacy has dimished through the last four decades, with efficacy percentages ranging from 40-100%, due to the vaccination increase and thus increasing the immunity against the vaccine (11). | |||
===Use of polymerase chain reaction as detection=== | |||
One of the culprits behind few of the pertussis outbreaks could be the use of nucleic acid amplification tests, such as polymerase chain reaction (PCR), as diagnosis of pertussis. | |||
The PCR method, for testing for pertussis, is carried out by culturing the sample and adding florescent antibody conjugates and PCR. Then, after the sample is cutured, swabs are elided and lysed. The PCR test are carried out on lysate using primers and then the product is detected through gel electrophoresis methods and stained with ethium bromide (14). | |||
PCR is a very sensitive method of the detection of B. pertussis and the test can remain positive for a longer period of time compared to other methods. The sensitivity also allows for contamination to occur, which may often lead to false positives (15). This could have led to the documented outbreaks of pertussis, if many tests were conducted in the same laboratory. Also, until recently, the Canadian policy of diagnosis of pertussis stated that a positive test on a PCR was enough to diagnose an individual with pertussis, even without a clinical confirmation (15). | |||
On the other hand, PCR could be a helpful tool in detecting certain cases of pertussis that would have been undetectable by standard culture, which could include milder or partially treated cases (11). | |||
===The cohort effect of pertussis=== | |||
In the 1990s, Canada experienced a significant increase of infected, infant pertussis individuals. The cause of this outbreak was not due to unvaccination, as 95% of the infected infants have had three or more vaccinations (3). Once vaccinated, an individual may be protected from B. pertussis, but that protection may decrease through evolution of the bacteria, and thus there was a waning of the vaccination. As that protection decreases, the infect population that was once protected may be vulnerable. Therefore, that population can be infected again and the average age group that is infected increases as it ages (3). | |||
The low efficacy of the whole cell pertussis vaccination may have led to a whole infant population that was vulnerable to B. pertussis. Despite the vaccination advances, there have still be a large group of individuals that have stemmed from the 1985 outbreak that continues to be effected (3). | |||
The assumption that pertussis is more common in infants is a trend that has been noticed throughout the years of research on B. pertussis. This has led to more attention given to infants and are increasingly tested for pertussis. However, this results in misdiagnosis of pertussis in older individuals (3). Also, it is generally known that sensitivity of diagnosis is higher in infants than in older individuals, which makes testing more convenient and easier for infants. There may be many cases of undocumented pertussis cases due to the lack of attention given to pertussis symptoms in the older population (2). | |||
==What is being done to address this problem== | ==What is being done to address this problem== | ||
==What | ===Use of acellular pertussis vaccine=== | ||
[[Image:Vaccinated 84 pertussis.jpg |frame|alt=Puzzle globe logo|Figure 3: Vaccinations are often performed on adolescents (22)]] | |||
In 1996, PMC Canada released the "Five Component Pertussis" vaccine as part of the DTaP (Diptheria, Tetanus, acellular Pertussis) vaccine. This new vaccine updated the original that was developed in 1930's, replacing the old whole-cell design with new acellular components comprising of FHA (filamentous hemaglutinin), 69K (69KDA) protein, or FIM (fimbriae) (17). | |||
By not using the whole cell, the DTaP vaccine proves to be a safe alternative. The acellular pertussis vaccine produces a longer lasting immunity, reduced side effects, reduced number or required injections. However, due to the nature of the vaccine, it cannot be combined for injection with Haemophilus Influenzae type B or polio vaccinations (18). | |||
The vaccination is given on an immunization schedule of 2 months, 4 months, 6 months. Additional booster doses are administered at 18 months of age, and the final shot is given around 4-6 years of age (19). | |||
===Use of Antibiotics=== | |||
A variety of antibiotics may be used to combat pertussis, including erythromycin estolate, erythromycin ethylsuccinate, azithromycin, clarithromycin. They may be applied before the paroxysmal phase, or given as a preventative to individuals in close contact with a person affected by pertussis. The standard antibiotic treatment for pertussis is a 14 day course of 15-20 mg/kg of erythromycin per day. Alternative antibiotics are offered in substitution of erythromycin for patients unable to handle erythromycin. | |||
If applied before the paroxysmal stage, antibiotics often reduce the severity and duration of the symptoms. If applied after the paroxysmal stage, the antibiotics are effective at eradicating the disease, but clinical studies offer varying opinions of its efficacy against the symptoms. Some studies report a reduction in symptoms while others state the antibiotics have no discernible effect. | |||
Erythromycin cannot be used for children under the age of 14 days. Clinical studies have shown that the use of erythromycin in infants can be associated with pyloric stenosis (21). | |||
==What could be done to improve the situation?== | |||
===Immunization=== | |||
With the advent of the acellular pertussis vaccine, pertussis can be prevented in individuals that have been immunized with the vaccine. Pertussis still poses a problem for infants as the vaccine cannot be given until 2 months of age. That 2 month window can be slightly ameliorated with the use of antibiotics as a preventative, but antibiotics cannot be used before the age of 2 weeks. This phase before the vaccine can be used is the window of opportunity for pertussis. Future advances in pertussis prevention should be directed towards counteracting the vulnerability of infants. | |||
===Detection of pertussis=== | |||
As testing with PCR has led to possibilities of misreporting of the number of cases of pertussis in Canada, a more conclusive way of determining the true number of reported cases of pertussis must be applied. Until recently, the governmental policy for determining the effect of pertussis was the PCR testing, even without clinical testing (8). Now that clinical testing is required to report a case of pertussis, it would be even more ideal to have other methods of testing to guarantee that the individual is suffering from pertussis to accurately describe its virulence upon the population. | |||
==Summary== | |||
As the disease “whooping cough” could be attributed to the bacterium Bordetella pertussis, it is actually the toxins, rather than the presence of the bacteria, that accounts for the various symptoms associated with whooping cough. The effect that it has had on Canadians has been ongoing, despite vaccination development. | |||
The research done on B. pertussis can be quite complicated, as the problem of polymorphism has been associated with all bacteria. However, the research for better vaccinations or other methods of resistance must not ever halt. At times, the disease may seem eradicated, but it may sporadically become a nuance once again. Research on methods of detection, vaccination, or antibiotics can always be improved and should never be ignored. | |||
==References== | ==References== | ||
1. R Benz, E Maier, D Ladant, A Ullmann and P Sebo; Adenylate cyclase toxin (CyaA) of Bordetella pertussis. Evidence for the formation of small ion-permeable channels and comparison with HlyA of Escherichia coli. | |||
http://www.jbc.org/cgi/content/abstract/269/44/27231 | |||
2. Char, Ian; Chatfield, Steven; Douce, Gillian; Everest, Paul; Li, Jingli; “Role of the Bordetella pertussis P.69lpertactin protein and the P.69/pertactin RGD motif in the adherence to and invasion of mammalian cells”; http://mic.sgmjournals.org/cgi/reprint/142/11/326. | |||
3. Cox, Michael M.; Nelson, David L.; Principles of Biochemistry. W.H. Freeman and Company, New York, NY. | |||
4. Fricks IP, Carter RL, Lazarowski ER, Harden TK. Gi-dependent cell signaling responses of the human P2Y14 receptor in model cell systems. | |||
http://www.ncbi.nlm.nih.gov/pubmed/19339661?ordinalpos=9&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum | |||
5. Guermonprez P,et al. The Adenylate Cyclase Toxin of Bordetella pertussis Binds to Target Cells via the aMb2Integrin (CD11b/CD18); | |||
http://jem.rupress.org/cgi/reprint/193/9/1035 | |||
6. N/A; Pertussis; http://www.cdc.gov/ncidod/dbmd/diseaseinfo/pertussis_t.htm | |||
7. Tang WJ, Guo Q; The adenylyl cyclase activity of anthrax edema factor. | |||
http://www.ncbi.nlm.nih.gov/pubmed/19560485?ordinalpos=11&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum | |||
8. "Statement on Pertussis Vaccine." Public Health Agency of Canada: Canada Communicable Disease Report Vol. 23 (ACS-3). 15 July 1997. 19 Aug 2009 < http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/97vol23/23sup/acs3.html>. | |||
9. Ewanowich C, Chui L, Paranchych M et al. Major Outbreak of Pertussis in Northern ALberts, Canada: Analysis of Discrepant Direct Fluorescent-Antibody and Cuture Results by Using Polymerase Chain Reaction Methology. Journal of Clinical Microbiology. July 1993. | |||
10.Ntzeayabo B, De Serres G, Duval B et al. Pertussis resurgence in Canada largely caused by a cohort effect. Social and Community Medicine. Social and Community Medicine. | |||
11.Lambert HJ. Epidemiology of a small pertussis outbreak in Kent County, Michigan. Public Health Rep 1965 | |||
12. Decker MD, Edwards KM, Steinhoff MC et al. Comparison of 13 acellular pertussis vaccines: adverse reactions. Pediatrics 1995. | |||
13.Gustafsson L, Hallander HO, Olin P et al. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med 1996. | |||
14.Skowronsky D, De Serres G, MacDonald D et al. The Changing Age and Seasonal Profile of PErtussis in Canada. J Infectious Disease 2002. | |||
15.Waters V, Jamieson F, Richardson S et al. Outbreak of Astypical Pertussis Detected by Polymerase Chain Reaction in Immunized Preschool-Aged Children. J Infectious Disease. | |||
16.“Canadian Immunization Guide. Seventh Edition – 2006.” Public Healthy Agency of Canada. 19 Aug 2009. <http://www.phac-aspc.gc.ca/publicat/cig-gci/index-eng.ph> . | |||
17. "Connaught and The History of Modern Pertussis Vaccines" Article originally published in Conntact, Vol. 9 #5 (November 1996), p. 4 | |||
http://www.healthheritageresearch.com/Pertussis/Pertussis-CONN-9611.pdf | |||
18. Halperin SA: Canadian Journal Infectious Diseases 7: 6; 359-60, Dec. 1996. | |||
http://www.utoronto.ca/kids/acelular.html | |||
19. Canada Communicable Disease Report Volume 29, ACS-5 1 September 2003 “Prevention of Pertussis in Adolescents and Adults” | |||
20. http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/03vol29/acs-dcc-5-6/acs5.html | |||
21. The Pediatric Infectious Disease Journal Vol 24 Number 5 may 2005 Use of Antibiotics in the Prevention and Treatment of Pertussis Carl-Heinz Wirsing von Koenig, MD | |||
22. "Pertussis vaccination." Online image. Department of Health and Human Services: Center for Disease Control and Prevention. 27 Aug 2009. <http://www.cdc.gov/vaccines/vpd-vac/pertussis/default.htm>. | |||
23. "Bordetella pertussis." Online image. DPT - Difteria/Tetano/Coquelunche. 27 Aug 2009. <http://www.virtual.epm.br/material/tis/curr-bio/trab2001/grupo5/triplice.htm>. | |||
Edited by Bryan Dieffenbach, Gorjan Hrustanovic, Patricia Lee and Andrew Chen, students of [mailto:ralarsen@ucsd.edu Rachel Larsen] | Edited by Bryan Dieffenbach, Gorjan Hrustanovic, Patricia Lee and Andrew Chen, students of [mailto:ralarsen@ucsd.edu Rachel Larsen] | ||
Latest revision as of 20:47, 10 September 2010
Introduction
Bordetella pertussis is the bacterial microbe responsible for the disease "Pertussis" (commonly referred to as 'Whooping Cough'). In this site, we will explore the physical attributes of the microbe, as well as the various toxins produced which facilitate both the infection and proliferation of the disease. Specifically, we will analyze the history of disease in Canada, the steps taken to eradicate disease, and potential treatments for the prevention of future outbreaks.
Description of the microbe
The bacterium Bordetella Pertussis is the causative agent of Whooping Cough. This small bacterium (about .8um in length and .4um by width) cannot survive in the open environment, and therefore must reside in a host, and is considered pathogenic. This host is almost always a human, where its “natural” environment is said to be the mucus in the human respiratory tract. The optimum temperature of growth for B.pertussis is about 35-37C, which is the temperature inside a living human host.
B.pertussis is an aerobic gram negative bacterium that does not produce spores. Due to its lack of flagella, it is also immotile. It resides in the phylum Proteobacteria, class Betaproteobacteria, order Burkholderiales, and family Alcaligenaceae. Because it is an aerobe, it utilizes aerobic respiration. Therefore, the bacterium consists of an electron transport chain on its membrane, and is considered a chemoheterotroph. Like other gram negative bacteria, it possesses an inner and outer membrane, with a thin peptidoglycan cell wall in between. This cell wall is attached to the outer membrane via lipoproteins. Like other gram negatives, the outer membrane of B.pertussis is coated with LPS (lipopolysachharides), which are long sugar linked and lipid-anchored polysaccharides coating the outer membrane. LPS are endotoxins, meaning that they are toxic to host (or potentially other bacteria) cells when detached from the bacteria(2). However, B.pertussis consists of very unusual LPS compared to other gram negatives. Namely, it contains two forms of LPS that have a different phosphate composition than standard Lipid A form LPS.
Bordetella pertussis does not necessarily cause whooping cough by itself, but rather produces toxins that do so. Among producing pertussis toxin, filamentous hemagglutinin, and hemolysin, B.pertussis produces the adenylate cyclase toxin (CyaA) – a direct causative agent of Whooping Cough (1). B.pertussis attaches to host cells via the virulence factor P.69 pertactin, which is an extracellularly exposed domain of a membrane protein expressed by B.pertussis – and targets the adhesion to mammalian cells. In addition to this, the filamentous hemagglutinin (FHA) also helps mediate cell adhesion, and contains similar motifs as P.69 pertactin (2). Once attached, B.pertussis is able to transfer CyaA to the target host cell.
The pertussis toxin (PT), also produced by B.pertussis, is known to also be an important factor in whooping cough respiratory infections. The resultant coughing is painful, and also is what spreads the highly contagious B.pertussis to others (3).
The Tahoma 1 strain of B.pertussis has its entire genome sequenced – it is 1 circular chromosome 4,086,189 nucleotides long (3867 genes), of which about 67% of the entire genome is GC rich (4).
Link to MicrobeWiki Page: Bordetella pertussis
http://microbewiki.kenyon.edu/index.php/Bordetella_pertussis
Description of the disease
Bortadella Pertussis secretes numerous toxins known to affect cellular homeostasis. The primary disease caused by the microbe is known as “Pertussis” (commonly referred to as 'Whooping Cough').
General Disease Information:
Whooping cough is characterized by uncontrollable, heavy coughing fits which persist for many weeks. Initially the symptoms may mimic that of the common cold, later developing into severe coughing and the potential development of further illness i.e. pneumonia, hypoxia (oxygen deprivation to tissues), apnea (suspension of breathing), seizures, etc. The disease progression can be broken up into several stages, each characterized by different symptoms. Initially the patient enters the “Catarrhal Phase” in which they display symptoms like that of the common cold. This usually develops within the first two weeks of disease. The second major stage is referred to as the “Paroxysmal Stage” and is characterized by uncontrollable fits of coughing followed by high pitched ‘whoop’ sounds upon air uptake. This stage is the most severe and is often the time when a patient will exhibit other disease symptoms. If the patient has the immune strength to combat the illness, they enter the final stage called the “Convalescent Stage”. Here the patient begins recovery from disease and uncontrollable coughing slowly diminishes (6).
Molecular Mechanisms of Disease:
Pertussis can act through numerous virulent mechanisms to affect the host organism. Two of the more notable toxins secreted by Bortadella pertussis are Pertussis Toxin (PT) and Adenylyl Cyclase Toxin (CyaA).
Pertussis Toxin (PT)
Pertussis toxin directs its activity intracellularly by affecting the inhibitory subunit of G-protein coupled receptors. PT catalyzes the ADP-Ribosylation of the Gi subunit and prevents inhibition of the protein Adenylyl Cyclase (AC). Adeneylyl Cyclase is responsible for the production of intracellular cAMP, a common downstream messenger in many cell signaling pathways.
In a normal cell, Gi subunits work concurrently with Gs proteins. Gs proteins are responsible for the activation of AC while Gi is responsible for inhibiting AC. When functioning properly, the two proteins regulate intracellular cAMP levels to maintain cellular homeostasis (3, 4, 6). In a cell affected by PT, Gi is inhibited and cannot act to “shut off” AC. As a result, the AC is not regulated and will constitutively produce cAMP until the Gs protein is removed. These high levels of cAMP have extreme downstream effects on gene expression and the viability of the cell. This destroys the ciliated epithelial cells that normally would sweep away mucus, and without this ciliated action, vigorous coughing is needed to clear the airway (3, 4, 6).
Adenylate Cyclase Toxin (CyaA)
CyaA is the primary toxin that contributes the development of 'Whooping cough' symptoms. CyaA has a series of G and D rich polypeptide residues, which is a characteristic repeat for many toxins. It predominantly controls the initial localization of microbe to the lung and airway tissues for colonization. B. pertussis adheres to the host cell via P.69 pertactin virulence factor. It consists of an RGD tripeptide motif which attaches to various sites on mammalian adhesion proteins (some include fibronectin, vitronectin, and fibrinogen) which facilitates interaction between the mammalian host cell and the bacterial microbe (2). CyaA acts on cells in both calcium and temperature-dependent manners where the CyaA catalytic domain is directly moved across the target cells plasma membrane. Specifically, it binds and invades cells by forming transmembrane cation selective channels. These channels can further affect the affected cell by offsetting cellular homeostasis, occasionally resulting in cellular lysis. CyaA may also contribute to the increase in intracellular cAMP levels of the host cell (1,5,6,7).
Transmission of disease
Bordetella pertussis cannot live in the outside environment. As a result, it has evolved to become a highly contagious microbe which is most commonly spread airborne. B. pertussis has negative effects on the airway tissues resulting in the loss of cilia mucus sweeping functions. The affected individual must now cough rigorously to clear the airway of excess mucus. The strong, uncontrollable coughing provides a mechanism for B. pertussis to leave the internal host environment and infect other individuals.
How/why is pertussis a problem in Canada
In Canada, the number of reported infected individuals of pertussis cases has fluctuated throughout the years, due to outbreaks and despite new advances in vaccination technology; reported infected individuals has ranged from 2,165 to 10,151 within this period of time. These numbers are predicted to be under-representations of the true values due to incomplete diagnosis or misreporting (8). The number of individuals affected per 100,000 of population has been increasing for adults through the time period of 1987-2004. However, this disease has been reported to be more common in infants than in older children and adults. The percentage of reported infected adolescents (defined to be less than 15 years of age) and adults has been “increasing from 9.6% in 1995 to 16.4%, 21.2%, and 31.3% in 1998, 2001 and 2004 respectively” (8).
Use of whole cell pertussis vaccine
In the 1940s, a whole cell pertussis fluid vaccine was introduced in Canada and was then replaced by an absorbed whole cell prtussis fluid vaccine in the 1980s. The absorbed whole cell pertussis fluid vaccine is made by suspending killed B. pertussis cells with an addition of other agents. These agents included in the vaccine are either absorbed diphtheria and tetanus toxoids (DPT), absorbed diphtheria and tetanus toxoids plus inactivated polio vaccine (DPT-Polio), or DPT or DPT-Polio combined with Haemophilus influenzae type b (Hib) conjugate vaccine (DPT-Hib, DPT-Polio-Hib) (9). The combined vaccines trigger an increase antibody production to B. pertussis, as well as diphtheria and tetanus antigens (9).
There have been adverse reactions towards the whole-cell pertussis vaccines. There have been reports of minor local reactions, which result in redness, swelling, as well as pain, in 50% to 75% of vaccine patients. It was proposed that a new type of vaccine was needed since the whole cell pertussis vaccine did not seem protected, and the polymorphism mutations of B. pertussis rendered the vaccine less effective (10). This low efficacy resulted in high numbers of reported cases of B. pertussis, and thus became a outbreak.
The efficacy of the whole-cell pertussis vaccines was 70%-90% in 1940s and 1950s. However, its efficacy has dimished through the last four decades, with efficacy percentages ranging from 40-100%, due to the vaccination increase and thus increasing the immunity against the vaccine (11).
Use of polymerase chain reaction as detection
One of the culprits behind few of the pertussis outbreaks could be the use of nucleic acid amplification tests, such as polymerase chain reaction (PCR), as diagnosis of pertussis.
The PCR method, for testing for pertussis, is carried out by culturing the sample and adding florescent antibody conjugates and PCR. Then, after the sample is cutured, swabs are elided and lysed. The PCR test are carried out on lysate using primers and then the product is detected through gel electrophoresis methods and stained with ethium bromide (14).
PCR is a very sensitive method of the detection of B. pertussis and the test can remain positive for a longer period of time compared to other methods. The sensitivity also allows for contamination to occur, which may often lead to false positives (15). This could have led to the documented outbreaks of pertussis, if many tests were conducted in the same laboratory. Also, until recently, the Canadian policy of diagnosis of pertussis stated that a positive test on a PCR was enough to diagnose an individual with pertussis, even without a clinical confirmation (15).
On the other hand, PCR could be a helpful tool in detecting certain cases of pertussis that would have been undetectable by standard culture, which could include milder or partially treated cases (11).
The cohort effect of pertussis
In the 1990s, Canada experienced a significant increase of infected, infant pertussis individuals. The cause of this outbreak was not due to unvaccination, as 95% of the infected infants have had three or more vaccinations (3). Once vaccinated, an individual may be protected from B. pertussis, but that protection may decrease through evolution of the bacteria, and thus there was a waning of the vaccination. As that protection decreases, the infect population that was once protected may be vulnerable. Therefore, that population can be infected again and the average age group that is infected increases as it ages (3).
The low efficacy of the whole cell pertussis vaccination may have led to a whole infant population that was vulnerable to B. pertussis. Despite the vaccination advances, there have still be a large group of individuals that have stemmed from the 1985 outbreak that continues to be effected (3).
The assumption that pertussis is more common in infants is a trend that has been noticed throughout the years of research on B. pertussis. This has led to more attention given to infants and are increasingly tested for pertussis. However, this results in misdiagnosis of pertussis in older individuals (3). Also, it is generally known that sensitivity of diagnosis is higher in infants than in older individuals, which makes testing more convenient and easier for infants. There may be many cases of undocumented pertussis cases due to the lack of attention given to pertussis symptoms in the older population (2).
What is being done to address this problem
Use of acellular pertussis vaccine
In 1996, PMC Canada released the "Five Component Pertussis" vaccine as part of the DTaP (Diptheria, Tetanus, acellular Pertussis) vaccine. This new vaccine updated the original that was developed in 1930's, replacing the old whole-cell design with new acellular components comprising of FHA (filamentous hemaglutinin), 69K (69KDA) protein, or FIM (fimbriae) (17). By not using the whole cell, the DTaP vaccine proves to be a safe alternative. The acellular pertussis vaccine produces a longer lasting immunity, reduced side effects, reduced number or required injections. However, due to the nature of the vaccine, it cannot be combined for injection with Haemophilus Influenzae type B or polio vaccinations (18). The vaccination is given on an immunization schedule of 2 months, 4 months, 6 months. Additional booster doses are administered at 18 months of age, and the final shot is given around 4-6 years of age (19).
Use of Antibiotics
A variety of antibiotics may be used to combat pertussis, including erythromycin estolate, erythromycin ethylsuccinate, azithromycin, clarithromycin. They may be applied before the paroxysmal phase, or given as a preventative to individuals in close contact with a person affected by pertussis. The standard antibiotic treatment for pertussis is a 14 day course of 15-20 mg/kg of erythromycin per day. Alternative antibiotics are offered in substitution of erythromycin for patients unable to handle erythromycin. If applied before the paroxysmal stage, antibiotics often reduce the severity and duration of the symptoms. If applied after the paroxysmal stage, the antibiotics are effective at eradicating the disease, but clinical studies offer varying opinions of its efficacy against the symptoms. Some studies report a reduction in symptoms while others state the antibiotics have no discernible effect. Erythromycin cannot be used for children under the age of 14 days. Clinical studies have shown that the use of erythromycin in infants can be associated with pyloric stenosis (21).
What could be done to improve the situation?
Immunization
With the advent of the acellular pertussis vaccine, pertussis can be prevented in individuals that have been immunized with the vaccine. Pertussis still poses a problem for infants as the vaccine cannot be given until 2 months of age. That 2 month window can be slightly ameliorated with the use of antibiotics as a preventative, but antibiotics cannot be used before the age of 2 weeks. This phase before the vaccine can be used is the window of opportunity for pertussis. Future advances in pertussis prevention should be directed towards counteracting the vulnerability of infants.
Detection of pertussis
As testing with PCR has led to possibilities of misreporting of the number of cases of pertussis in Canada, a more conclusive way of determining the true number of reported cases of pertussis must be applied. Until recently, the governmental policy for determining the effect of pertussis was the PCR testing, even without clinical testing (8). Now that clinical testing is required to report a case of pertussis, it would be even more ideal to have other methods of testing to guarantee that the individual is suffering from pertussis to accurately describe its virulence upon the population.
Summary
As the disease “whooping cough” could be attributed to the bacterium Bordetella pertussis, it is actually the toxins, rather than the presence of the bacteria, that accounts for the various symptoms associated with whooping cough. The effect that it has had on Canadians has been ongoing, despite vaccination development.
The research done on B. pertussis can be quite complicated, as the problem of polymorphism has been associated with all bacteria. However, the research for better vaccinations or other methods of resistance must not ever halt. At times, the disease may seem eradicated, but it may sporadically become a nuance once again. Research on methods of detection, vaccination, or antibiotics can always be improved and should never be ignored.
References
1. R Benz, E Maier, D Ladant, A Ullmann and P Sebo; Adenylate cyclase toxin (CyaA) of Bordetella pertussis. Evidence for the formation of small ion-permeable channels and comparison with HlyA of Escherichia coli. http://www.jbc.org/cgi/content/abstract/269/44/27231
2. Char, Ian; Chatfield, Steven; Douce, Gillian; Everest, Paul; Li, Jingli; “Role of the Bordetella pertussis P.69lpertactin protein and the P.69/pertactin RGD motif in the adherence to and invasion of mammalian cells”; http://mic.sgmjournals.org/cgi/reprint/142/11/326.
3. Cox, Michael M.; Nelson, David L.; Principles of Biochemistry. W.H. Freeman and Company, New York, NY.
4. Fricks IP, Carter RL, Lazarowski ER, Harden TK. Gi-dependent cell signaling responses of the human P2Y14 receptor in model cell systems. http://www.ncbi.nlm.nih.gov/pubmed/19339661?ordinalpos=9&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum
5. Guermonprez P,et al. The Adenylate Cyclase Toxin of Bordetella pertussis Binds to Target Cells via the aMb2Integrin (CD11b/CD18); http://jem.rupress.org/cgi/reprint/193/9/1035
6. N/A; Pertussis; http://www.cdc.gov/ncidod/dbmd/diseaseinfo/pertussis_t.htm
7. Tang WJ, Guo Q; The adenylyl cyclase activity of anthrax edema factor. http://www.ncbi.nlm.nih.gov/pubmed/19560485?ordinalpos=11&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum
8. "Statement on Pertussis Vaccine." Public Health Agency of Canada: Canada Communicable Disease Report Vol. 23 (ACS-3). 15 July 1997. 19 Aug 2009 < http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/97vol23/23sup/acs3.html>.
9. Ewanowich C, Chui L, Paranchych M et al. Major Outbreak of Pertussis in Northern ALberts, Canada: Analysis of Discrepant Direct Fluorescent-Antibody and Cuture Results by Using Polymerase Chain Reaction Methology. Journal of Clinical Microbiology. July 1993.
10.Ntzeayabo B, De Serres G, Duval B et al. Pertussis resurgence in Canada largely caused by a cohort effect. Social and Community Medicine. Social and Community Medicine.
11.Lambert HJ. Epidemiology of a small pertussis outbreak in Kent County, Michigan. Public Health Rep 1965
12. Decker MD, Edwards KM, Steinhoff MC et al. Comparison of 13 acellular pertussis vaccines: adverse reactions. Pediatrics 1995.
13.Gustafsson L, Hallander HO, Olin P et al. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med 1996.
14.Skowronsky D, De Serres G, MacDonald D et al. The Changing Age and Seasonal Profile of PErtussis in Canada. J Infectious Disease 2002.
15.Waters V, Jamieson F, Richardson S et al. Outbreak of Astypical Pertussis Detected by Polymerase Chain Reaction in Immunized Preschool-Aged Children. J Infectious Disease.
16.“Canadian Immunization Guide. Seventh Edition – 2006.” Public Healthy Agency of Canada. 19 Aug 2009. <http://www.phac-aspc.gc.ca/publicat/cig-gci/index-eng.ph> .
17. "Connaught and The History of Modern Pertussis Vaccines" Article originally published in Conntact, Vol. 9 #5 (November 1996), p. 4 http://www.healthheritageresearch.com/Pertussis/Pertussis-CONN-9611.pdf
18. Halperin SA: Canadian Journal Infectious Diseases 7: 6; 359-60, Dec. 1996. http://www.utoronto.ca/kids/acelular.html
19. Canada Communicable Disease Report Volume 29, ACS-5 1 September 2003 “Prevention of Pertussis in Adolescents and Adults”
20. http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/03vol29/acs-dcc-5-6/acs5.html
21. The Pediatric Infectious Disease Journal Vol 24 Number 5 may 2005 Use of Antibiotics in the Prevention and Treatment of Pertussis Carl-Heinz Wirsing von Koenig, MD
22. "Pertussis vaccination." Online image. Department of Health and Human Services: Center for Disease Control and Prevention. 27 Aug 2009. <http://www.cdc.gov/vaccines/vpd-vac/pertussis/default.htm>.
23. "Bordetella pertussis." Online image. DPT - Difteria/Tetano/Coquelunche. 27 Aug 2009. <http://www.virtual.epm.br/material/tis/curr-bio/trab2001/grupo5/triplice.htm>.
Edited by Bryan Dieffenbach, Gorjan Hrustanovic, Patricia Lee and Andrew Chen, students of Rachel Larsen