Ralstonia eutropha: Difference between revisions
No edit summary |
|||
| (36 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | |||
{{Biorealm Genus}} | {{Biorealm Genus}} | ||
==Classification== | ==Classification== | ||
===Higher order taxa=== | ===Higher order taxa=== | ||
| Line 17: | Line 19: | ||
Genus: Cupriavidus/Ralstonia/Wautersia | Genus: Cupriavidus/Ralstonia/Wautersia | ||
===Species=== | ===Genus and Species=== | ||
Synonyms and strains: | |||
1. Cupriavidus necator | 1. ''Cupriavidus necator'' | ||
2. Wautersia eutropha | 2. ''Wautersia eutropha'' | ||
3. Ralstonia eutropha | 3. ''Ralstonia eutropha'' | ||
a. Ralstonia eutropha JMP 134 | a. ''Ralstonia eutropha JMP 134'' | ||
b. Ralstonia eutropha H850 | b. ''Ralstonia eutropha H850'' | ||
c. Ralstonia eutropha H16 '' | c. ''Ralstonia eutropha H16 '' | ||
| Line 42: | Line 44: | ||
==Description and significance== | ==Description and significance== | ||
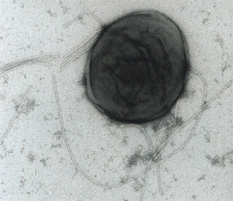
[[Image:Reut_72.jpg|frame|left|Photo courtesy of Dr. Bernardo Gonzalez and Danilo Perez-PantojaFrom The Regents of the University of California]] | |||
''Ralstonia eutropha'' can be found in both soil and water. This bacterium has great potential for use in bioremediation as it is able to degrade a great number of chlorinated aromatic (chloroaromatic) compounds and chemically related pollutants. ''R. eutropha H16'' was formerly known as ''Alcaligenes eutrophus'' and was originally isolated from sludge. ''R. eutropha JMP 134'' and other strains belonging to this species are models for studying microbial production of “polyhydroxyalkanoates (polyesters produced in nature by bacterial fermentation of sugar or lipids), and chemolithoautotrophic (organisms utilize inorganic compounds as energy sources) metabolism in aerobic heterotrophs.(JGI)”[1] | |||
''R. eutropha'' strains can utilize hydrogen, carbon dioxide and organic compounds for development and is a model organism for hydrogen oxidation because it can nurture on hydrogen as the sole energy source. ''R. eutropha JMP 134'' was isolated due to its ability to degrade the herbicide 2,4-dichlorophenoxyacetic acid. The degradation abilities are encoded on a plasmid. | |||
''R. eutropha'' is Gram-negative bacterium and is non-spore forming. (Gram-negative means they have two membranes and have red or pink stain where as Gram-positive have only one membrane and have blue stain.) Many of the Gram-negative bacteria are pathogenic but this bacterium is not. They have motility and are facultative aerobes which can live in both aerobic and anaerobic environments. ''R. eutropha'' has two flagella and two membranes and are usually rod shape. ''R. eutropha JMP 134'' have multiple habitats and ''Ralstonia eutropha H16'' have a specialized habitat but both requires non-halophilic (not salty) environment. The optimal temperature is 30 degrees Celsius. | |||
==Genome structure== | ==Genome structure== | ||
''R. eutropha JMP 134'' has one megaplasmid, two chromosomes and plasmid pJP4. Megaplasmid has 60.6% of G-C (guanine and cytosine) content (a way to characterize genes), 512 proteins and one RNA at the length of 634917 bp (base pair). Chromosome 1 has 64.7% G-C content, 3439 proteins and 66 RNA at the length of 380653 bp. Chromosome 2 has 65.0% of G-C content, 2407 proteins and 20 RNA at the length of 272615 bp. Plasmid pJP4 has 64.7% G-C content, 88 proteins and no RNA found so far at the length of 87688 bp. [3] | |||
''R. eutropha H16'' has one megaplasmid pHG1 and two chromosomes. Megaplasmid pHG1 had 62.3% G-C content and 429 proteins at the length of 452156 bp, this is where essential genetic information for the “facultatively lithoautrotrophic” and anaerobic existence of its host. Related functional genes form loose clusters into groups in the megaplasmid. Lithoautotrophy-related genes are the largest functional group, which is made up of 41 genes that are responsible for the biosynthesis of four different hydrogenases. There is another cluster that is in charge of denitrification. During sequencing several enzymes involved in the “mineralization of aromatic compounds” were discovered. Chromosome 1 has 66.5% G-C content, 3651 proteins and 60 RNA at the length of 405203 bp. Chromosome 2 has 66.8% G-C content, 2555 proteins and 13 RNA at the length of 290000 bp. [2] | |||
==Cell structure and metabolism== | ==Cell structure and metabolism== | ||
''R. eutropha'' are Gram-negative bacteria and usually have a rod shape. ''R. eutropha JMP134'' has flagella present and two membranes. ''R. eutropha H16'' forms two types of pili. ''R. eutropha H16'' is a soil residing betaproteobacterium. [2] | |||
''R. eutropha'' is able to degrade a large list of chloroaromatic compounds and chemically related pollutants. This bacteria can be used for the production of biodegradable thermoplastic by the characteristics of “polyhydroxyalkanoates (PHAs), and chemolithoautotrophic metabolism in aerobic heterotrophs.” Polyhydroxyalkanoates are a broad type of biodegrable polymer that can be used for biodegrable plastic. [1] ''R. eutropha'' is able to develop only with hydrogen and carbon dioxide as its only energy source and carbon source. When oxygen is not present it can use different metabolism to grow. It can obtain energy by denitrification anaerobically. As mentioned above this is why ''R. eutropha'' serves as a model organism for genetics and control of autotrophic carbon dioxide fixation and hydrogen fixation. ''R. eutropha'' also can metabolize heavy metals which makes it good candidate for studies into polymer production. | |||
==Ecology== | ==Ecology== | ||
''R. eutropha'' in general can be found in both soil and water. ''R. eutropha'' JMP134 lives in multiple habitats that are non-halophilic environments where as ''R. eutropha'' H16 lives in specialized habitat that is non-halophilic. ''R. eutropha'' does not require oxygen. Optimal temperature is 30 degree Celsius. ''R. eutropha'' thrives most successfully in the presence of millimolar concentrations of several heavy metal including Zinc, Cadmium, Cobalt, Lead, Mercury, Nickel and Chromium. | |||
==Pathology== | ==Pathology== | ||
There is no known pathogen among different strains of Ralstonia eutropha. | There is no known pathogen among different strains of ''Ralstonia eutropha''. | ||
==Application to Biotechnology== | ==Application to Biotechnology== | ||
This bacterium is able to degrade a large list of chloroaromatic compounds and chemically related pollutants. These species are models for study microbial production of polyhydroxyalkanoates and chemolithoautotrophic metabolism. Polyhydroxyalkanoates are a broad type of biodegrable polymer that can be used for biodegrable plastic. There is currently research about developing biodegrable plastics using ''R. eutropha'' H16’s capability to store large percent of poly[R-(–)-3-hydroxybutyrate] and other polyesters. There is another on-going research about protein purification using ''R. eutropha'' because it is about to produce its own “affinity matrix” [Research 4]. Its capability to develop on hydrogen only might help the future hydrogen based biotechnology for production of diverse, commercially, environmentally and medically valuable compounds, for example metabolites and polymers. | |||
==Current Research== | ==Current Research== | ||
8.1 Integrated recombinant protein expression and purification platform based on ''Ralstonia eutropha'' - - Protein purification of recombinant proteins is very costly to do. Various ways are being develop to make it more efficient and less costly. This research paper talks about how protein purification can be obtained by using ''R. eutropha''. In single step pure, active beta-galactosidase was obtained. This purification uses the advantage of “specific interaction of phasin proteins with granules of the intracellular polymer polyhydrosybutyrate (PHB)” in ''R. eutropha''. | |||
8.2 The ''R. eutropha H16'' phasin PhaP1 is targeted to intracellular triacylglycerol inclusions in ''Rhodococcus opacus PD630'' and ''Mycobacterium smegmatis mc2155'', and provides an anchor to target other proteins - - The major phasin that binds to the surface of polyhydroxyalkanoates (PHA) is ''R. eutropha H16'' phasin PhaP1. In this research they realized that PhaP1 was mainly located on the surface of intracellular triacylgycerol (TAG) inclusions and some at the plasma membrane. This is first reported incident where PhaP1 binding to lipid inclusions as well we PHA inclusions was demonstrated. And since this PhaP1 is non-specific, it can be utilized to mark other proteins. | |||
8.3 ''R. eutropha H16'' encodes two and possible three intracellular poly [D-(-)-3-hydroxybutyrate] (PHB) depolymerase genes - - In this study the researchers were able to identify PHB depolymerase genes from ''R. eutropha'': phaZ1, phaZ2 and phaZ3. They found that in rich medium phaZ1 is capable of intracellular PHB degradation. With further testing they came to the conclusion that phaZ2 is an intracellular depolymerase. The function of phaZ3 is remains mystery. | |||
8.4 Method for the continuous microbiological production of polyhydroxy butyric acid - - This article talks about the research of creating biodegradable plastic. Polyhydroxybutyric acid (PHB) is the gene that degenerate or degrades toxic objects. However plastics are still present instead of biodegradable plastics because their costs are definitely less. These researchers are trying to make more efficient and inexpensive ways to get PHB. | |||
==References== | ==References== | ||
1. JGI Microbial Genomics | |||
[http://genome.jgi-psf.org/draft_microbes/raleu/raleu.home.html] | |||
2. Complete nucleotide sequence of pHG1: a Ralstonia eutropha H16 megaplasmid encoding key enzymes of H(2)-based ithoautotrophy and anaerobiosis. | |||
[http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=12948488] | |||
3. HAMAP: Ralstonia eutropha (strain JMP134) (Alcaligenes eutrophus) complete proteome | |||
[http://expasy.org/sprot/hamap/RALEJ.html] | |||
4. Genome sequence of the bioplastic-producing "Knallgas" bacterium Ralstonia eutropha H16 | |||
[http://www.nature.com/nbt/journal/v24/n10/abs/nbt1244.html] | |||
5. Ralstonia eutropha JMP134 The Institute for Fenomic Research | |||
[http://rice.tigr.org/tigr-scripts/CMR2/GenomePage3.spl?database=ntre01] | |||
6.Ralstonia eutropha H16 | |||
[http://gib.genes.nig.ac.jp/single/main.php?spid=Reut_H16] | |||
7. Biodegradable Plastic News | |||
[http://biodegradableplastics.bio-tec.biz/] | |||
Research References | |||
1. Ralstonia eutropha H16 Encodes Two and Possibly Three Intracellular | |||
Poly[D-(�)-3-Hydroxybutyrate] Depolymerase Genes | |||
[http://jb.asm.org/cgi/reprint/185/13/3788.pdf] | |||
2. Process for the production of polyhydroxyoctanoate by streptomyces lividans | |||
[http://www.patentstorm.us/patents/6692945-description.html] | |||
3. Method for the continuous microbiological production of polyhydroxy butyric acid | |||
[http://www.patentstorm.us/patents/6395520-description.html] | |||
4. Integrated Recombinant Protein Expression and Purification Platform Based on Ralstonia eutropha | |||
[http://aem.asm.org/cgi/content/abstract/71/10/5735] | |||
5. The Ralstonia eutropha H16 phasin PhaP1 is targeted to intracellular triacylglycerol inclusions in Rhodococcus opacus PD630 and Mycobacterium smegmatis mc2155, and provides an anchor to target other proteins | |||
[http://mic.sgmjournals.org/cgi/content/abstract/152/11/3271] | |||
Edited by student of [mailto:ralarsen@ucsd.edu Rachel Larsen] and Kit Pogliano | Edited by student of [mailto:ralarsen@ucsd.edu Rachel Larsen] and Kit Pogliano | ||
KMG | |||
Latest revision as of 15:24, 2 June 2011
A Microbial Biorealm page on the genus Ralstonia eutropha
Classification
Higher order taxa
Domain:Bacteria
Phylum:Proteobacteria
Class: Betaproteobacteria
Order: Burkholderiales
Family:Burkholderiaceae
Genus: Cupriavidus/Ralstonia/Wautersia
Genus and Species
Synonyms and strains:
1. Cupriavidus necator
2. Wautersia eutropha
3. Ralstonia eutropha
a. Ralstonia eutropha JMP 134
b. Ralstonia eutropha H850
c. Ralstonia eutropha H16
|
NCBI: Taxonomy |
|
NCBI: [1] |
Description and significance
Ralstonia eutropha can be found in both soil and water. This bacterium has great potential for use in bioremediation as it is able to degrade a great number of chlorinated aromatic (chloroaromatic) compounds and chemically related pollutants. R. eutropha H16 was formerly known as Alcaligenes eutrophus and was originally isolated from sludge. R. eutropha JMP 134 and other strains belonging to this species are models for studying microbial production of “polyhydroxyalkanoates (polyesters produced in nature by bacterial fermentation of sugar or lipids), and chemolithoautotrophic (organisms utilize inorganic compounds as energy sources) metabolism in aerobic heterotrophs.(JGI)”[1]
R. eutropha strains can utilize hydrogen, carbon dioxide and organic compounds for development and is a model organism for hydrogen oxidation because it can nurture on hydrogen as the sole energy source. R. eutropha JMP 134 was isolated due to its ability to degrade the herbicide 2,4-dichlorophenoxyacetic acid. The degradation abilities are encoded on a plasmid.
R. eutropha is Gram-negative bacterium and is non-spore forming. (Gram-negative means they have two membranes and have red or pink stain where as Gram-positive have only one membrane and have blue stain.) Many of the Gram-negative bacteria are pathogenic but this bacterium is not. They have motility and are facultative aerobes which can live in both aerobic and anaerobic environments. R. eutropha has two flagella and two membranes and are usually rod shape. R. eutropha JMP 134 have multiple habitats and Ralstonia eutropha H16 have a specialized habitat but both requires non-halophilic (not salty) environment. The optimal temperature is 30 degrees Celsius.
Genome structure
R. eutropha JMP 134 has one megaplasmid, two chromosomes and plasmid pJP4. Megaplasmid has 60.6% of G-C (guanine and cytosine) content (a way to characterize genes), 512 proteins and one RNA at the length of 634917 bp (base pair). Chromosome 1 has 64.7% G-C content, 3439 proteins and 66 RNA at the length of 380653 bp. Chromosome 2 has 65.0% of G-C content, 2407 proteins and 20 RNA at the length of 272615 bp. Plasmid pJP4 has 64.7% G-C content, 88 proteins and no RNA found so far at the length of 87688 bp. [3]
R. eutropha H16 has one megaplasmid pHG1 and two chromosomes. Megaplasmid pHG1 had 62.3% G-C content and 429 proteins at the length of 452156 bp, this is where essential genetic information for the “facultatively lithoautrotrophic” and anaerobic existence of its host. Related functional genes form loose clusters into groups in the megaplasmid. Lithoautotrophy-related genes are the largest functional group, which is made up of 41 genes that are responsible for the biosynthesis of four different hydrogenases. There is another cluster that is in charge of denitrification. During sequencing several enzymes involved in the “mineralization of aromatic compounds” were discovered. Chromosome 1 has 66.5% G-C content, 3651 proteins and 60 RNA at the length of 405203 bp. Chromosome 2 has 66.8% G-C content, 2555 proteins and 13 RNA at the length of 290000 bp. [2]
Cell structure and metabolism
R. eutropha are Gram-negative bacteria and usually have a rod shape. R. eutropha JMP134 has flagella present and two membranes. R. eutropha H16 forms two types of pili. R. eutropha H16 is a soil residing betaproteobacterium. [2]
R. eutropha is able to degrade a large list of chloroaromatic compounds and chemically related pollutants. This bacteria can be used for the production of biodegradable thermoplastic by the characteristics of “polyhydroxyalkanoates (PHAs), and chemolithoautotrophic metabolism in aerobic heterotrophs.” Polyhydroxyalkanoates are a broad type of biodegrable polymer that can be used for biodegrable plastic. [1] R. eutropha is able to develop only with hydrogen and carbon dioxide as its only energy source and carbon source. When oxygen is not present it can use different metabolism to grow. It can obtain energy by denitrification anaerobically. As mentioned above this is why R. eutropha serves as a model organism for genetics and control of autotrophic carbon dioxide fixation and hydrogen fixation. R. eutropha also can metabolize heavy metals which makes it good candidate for studies into polymer production.
Ecology
R. eutropha in general can be found in both soil and water. R. eutropha JMP134 lives in multiple habitats that are non-halophilic environments where as R. eutropha H16 lives in specialized habitat that is non-halophilic. R. eutropha does not require oxygen. Optimal temperature is 30 degree Celsius. R. eutropha thrives most successfully in the presence of millimolar concentrations of several heavy metal including Zinc, Cadmium, Cobalt, Lead, Mercury, Nickel and Chromium.
Pathology
There is no known pathogen among different strains of Ralstonia eutropha.
Application to Biotechnology
This bacterium is able to degrade a large list of chloroaromatic compounds and chemically related pollutants. These species are models for study microbial production of polyhydroxyalkanoates and chemolithoautotrophic metabolism. Polyhydroxyalkanoates are a broad type of biodegrable polymer that can be used for biodegrable plastic. There is currently research about developing biodegrable plastics using R. eutropha H16’s capability to store large percent of poly[R-(–)-3-hydroxybutyrate] and other polyesters. There is another on-going research about protein purification using R. eutropha because it is about to produce its own “affinity matrix” [Research 4]. Its capability to develop on hydrogen only might help the future hydrogen based biotechnology for production of diverse, commercially, environmentally and medically valuable compounds, for example metabolites and polymers.
Current Research
8.1 Integrated recombinant protein expression and purification platform based on Ralstonia eutropha - - Protein purification of recombinant proteins is very costly to do. Various ways are being develop to make it more efficient and less costly. This research paper talks about how protein purification can be obtained by using R. eutropha. In single step pure, active beta-galactosidase was obtained. This purification uses the advantage of “specific interaction of phasin proteins with granules of the intracellular polymer polyhydrosybutyrate (PHB)” in R. eutropha.
8.2 The R. eutropha H16 phasin PhaP1 is targeted to intracellular triacylglycerol inclusions in Rhodococcus opacus PD630 and Mycobacterium smegmatis mc2155, and provides an anchor to target other proteins - - The major phasin that binds to the surface of polyhydroxyalkanoates (PHA) is R. eutropha H16 phasin PhaP1. In this research they realized that PhaP1 was mainly located on the surface of intracellular triacylgycerol (TAG) inclusions and some at the plasma membrane. This is first reported incident where PhaP1 binding to lipid inclusions as well we PHA inclusions was demonstrated. And since this PhaP1 is non-specific, it can be utilized to mark other proteins.
8.3 R. eutropha H16 encodes two and possible three intracellular poly [D-(-)-3-hydroxybutyrate] (PHB) depolymerase genes - - In this study the researchers were able to identify PHB depolymerase genes from R. eutropha: phaZ1, phaZ2 and phaZ3. They found that in rich medium phaZ1 is capable of intracellular PHB degradation. With further testing they came to the conclusion that phaZ2 is an intracellular depolymerase. The function of phaZ3 is remains mystery.
8.4 Method for the continuous microbiological production of polyhydroxy butyric acid - - This article talks about the research of creating biodegradable plastic. Polyhydroxybutyric acid (PHB) is the gene that degenerate or degrades toxic objects. However plastics are still present instead of biodegradable plastics because their costs are definitely less. These researchers are trying to make more efficient and inexpensive ways to get PHB.
References
1. JGI Microbial Genomics [2]
2. Complete nucleotide sequence of pHG1: a Ralstonia eutropha H16 megaplasmid encoding key enzymes of H(2)-based ithoautotrophy and anaerobiosis. [3]
3. HAMAP: Ralstonia eutropha (strain JMP134) (Alcaligenes eutrophus) complete proteome [4]
4. Genome sequence of the bioplastic-producing "Knallgas" bacterium Ralstonia eutropha H16 [5]
5. Ralstonia eutropha JMP134 The Institute for Fenomic Research [6]
6.Ralstonia eutropha H16 [7]
7. Biodegradable Plastic News [8]
Research References
1. Ralstonia eutropha H16 Encodes Two and Possibly Three Intracellular Poly[D-(�)-3-Hydroxybutyrate] Depolymerase Genes [9]
2. Process for the production of polyhydroxyoctanoate by streptomyces lividans [10]
3. Method for the continuous microbiological production of polyhydroxy butyric acid [11]
4. Integrated Recombinant Protein Expression and Purification Platform Based on Ralstonia eutropha [12]
5. The Ralstonia eutropha H16 phasin PhaP1 is targeted to intracellular triacylglycerol inclusions in Rhodococcus opacus PD630 and Mycobacterium smegmatis mc2155, and provides an anchor to target other proteins [13]
Edited by student of Rachel Larsen and Kit Pogliano
KMG