Rhodospirillum rubrum: Difference between revisions
Cozzolino.m (talk | contribs) |
Cozzolino.m (talk | contribs) |
||
| Line 15: | Line 15: | ||
===Species=== | ===Species=== | ||
''Rhodospirillum rubrum'' | ''Rhodospirillum rubrum'' | ||
Revision as of 03:31, 27 October 2011
A Microbial Biorealm page on the genus Rhodospirillum rubrum
Classification
Higher order taxa
Kingdom: Bacteria
Phylum: Proteobacteria
Class: Alphaproteobacteria
Order: Rhodospirillales
Family: Rhodospirillaceae
Genus: Rhodospirillum
Species
Rhodospirillum rubrum
NEUF2011
Description and significance
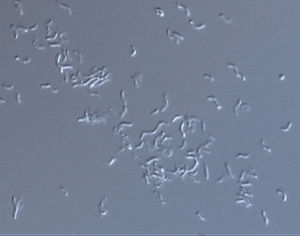
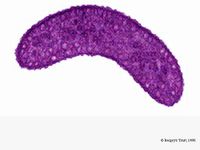
Rhodospirillum rubrum is a Gram-negative, mesophilicproteobacteria. Its optimal growth temperature is 25-30 degrees Celsius. It has multi-layered outer envelopes, which contain mostly unsaturated, but some saturated fats in its cell wall. R. rubrum is a spirilla, meaning it has a spiral-shape. It is polarly flagellated, and therefore motile. Its length is 3-10 um, with a width of 08-1.0 um.
R. rubrum is a facultative anaerobe. Depending on the presence of oxygen, it can undergo alcoholic fermentation or aerobic respiration. It also is capable of photosynthesis and contains carotenoid and bateriochlorophyll in its chromatophore particles. These molecules help to absorb light and convert it to energy and also give it its distinct purple-red color under anaerobic conditions. R. rubrum is colorless under aerobic conditions.
Although photosynthesis is active under aerobic conditions, it is generally suppressed in the presence of O2. Sulfur is a major byproduct of photosynthesis, not O2. R. rubrum can grow heterotrophically or autotrophically when photosynthetic. Unlike many plants, R. rubrum contains no chlorophyll a (absorption spectra 430-662 nm). However, it does contain chlorophyll b (absorption spectra 660-680 nm) and bacteriochlorophylls (800-925 nm). This allows it to utilize more energy from the electromagnetic spectra. R. rubrum also oxidizes carbon monoxide (CO) with hydrogen gas as the pathway’s end product, and can use sulfide at low concentrations as an electron donor in carbon dioxide reduction. Additionally, it is a nitrogen fixing bacteria; it uses nitrogenase to convert atmospheric nitrogen gas to ammonia.
There are several applications of R. rubrum in the field of biotechnology. It is a model system of light to chemical energy conversion and for its nitrogen fixing pathways. It is also the subject of radiation resistance studies. It can be used in several ways for consumption, as well. The proteobacteria may be a source of animal food and agricultural fertilizer. Another important role in research includes the production of vitamins. It is also being researched for its production of biological plastic from precursors of poly-hydroxy-butric-acid. R. rubrum may also be a contributor in biological hydrogen fuels, mainly through its evolution of the enzyme nitrogenase.
Genome structure
The genome's sequencing is finished. It consists of a circular chromosome with 4,352,825 base pairs, 65% of which are guanine-cystosine pairs. There is also a plasmid with 53,732 base pairs with 60% guanine-cytosine. In total, there are 3,850 protein coding genes and 83 RNA genes. Of these genes, 6.9% are transcription-related; 4.6% translation, ribosome structure, and biosynthesis; 4.0% replication, recombination and repair; 7.9% signal transduction mechanisms; 5.9% cell wall and membrane biogenesis; 6.6% energy production and conversion; 5.0% carbohydrate transport and metabolism; 9.9% amino acid transport and metabolism; 4.7% coenzyme transport and metabolism; 3.7% lipid transport and metabolism; and 6.5% inorganic ion transport and metabolism.
Ecology
Rhodospirillum Rubrum is found in many natural aquatic environments such as ponds, lakes, streams, and standing water. R. rubrum is also often found in mud and sewage. Due to the fact that R. rubrum can grow both aerobically and anaerobically it is capable of inhabiting a wide variety of conditions. Studies have shown that R. rubrum makes large changes in its chemical composition to adapt to different environments. R. rubrum prefers habitats with a pH of 6.8-7.2 and a temperature of 25-30 degrees Celcuis.
Cell structure and metabolism
Interesting features of cell structure; how it gains energy; what important molecules it produces.
Basic Metabolism Versatile organism that can obtain energy through alternative mechanism.
Two main distinctive metabolisms that Rhodospirillum Rubrum can carry out: Competition between respiratory and photosynthetic process is determine by the present of light and energy.
Rhodospirillum can grow in dark chemo-tropical environment with the presence of O2 or they can grow in a photo-tropical environment without O2.
Phototrophically grown Rhodospirillum contain photosynthetic electron transport and ATP synthesis enzymes in their membrane and contain a membrane bound pyrophosphatase.
Photosynthetic process – Donation of a Hydrogen from an organic substrate to an oxidizing substance
Entire Complement of photoreactive pigments ( bacteriochlorophyll and carotenoids) in the photosynthetic organism ……. was bound to large particles known as chromatophores. In (Schachman, Pardee and Stanier, 1952)
Chromatophores: flattened disks about 110mu in diameter. (Schachman 1952)
Chromatophores are not present in cells from young cultures (Hickman and Frenkel 1959)
Chromatophores contain Choline phospholipids and are rich in cardiolipin (diphosphatidyl glycerol) and galactosyl diglyerides which are major components of chloroplast lipids (Benson, Wintermans, and Wiser 1959 and Benson 1961)
Chromatophores are complete with respect to components of the electron transport chain containing various cytochromes, flavin and pyridine nucleotide (Newton and Newton 1957, Hulcher and Conti, 1960, Kamen 1961)
Anaerobic light dependent phosphorylation of adenosine diphosphate (ADP) to (ATP) so the entire activity is catalysed by the chromatophores (Frenkel 1956)
Dark: Anabolic Processes - in which oxidation by O2 from an organic substrate provides the energy needed.
Chemotrophically dark grown Rhodospirillum contain low concentration of bacteriochlorophyII and carotenoids and has no bound pyrophosphatase in their membrane.
Anaerobic Respiration Mixed Acid fermentation of sugars Ferment fructose without the addition of accessory oxidants but required the initial presence of bicarbonate before fermentative growth Major products of fermentation are succinate, acetate, propionate, formate, hydrogen, carbon dioxide Nonfermentable substrates, such as succinate, malate, or acetate, supported growth only in the presence of electron acceptor such as dimethyl sulfoxide or trimethylamine oxide In this case major products are carbon dioxide, and dimethyl sulfide Dimethyl sulfoxide or Trimethylamine oxide as a terminal oxidant is approximately 33 to 41% as efficient as O2 (aerobic respiration) in covserving energy through electron transport linked respiration (J.E Schultz and P.F weaver 1982) Pyruvate can also be fermented; major products are acetate, formate, carbon dioxide, and hydrogen (1973)
Aerobic Respiration Aerobic respiration proceeds with a traditional electron transport chain with NADH as an electron transporter (Keister and Minton, 1969) and oxygen as a terminal electron acceptor. Cytochrome C428 is part of the respiratory system (Chances and Smite 1955)
Photosynthesis inhibit aerobic respiration but the actual complicity of respiratory increases and becomes dominant when the photosynthetic apparatus become diluted (Oelze and Weaver 1971)
Nitrogen Fixation Grow under dark condition with fructose which in turns is regulated by NH4+ (NITROGENASE). N2 fixation occurs only in which energy and reductant for growth are both supplied by a strict (nonoxidant dependent) fermentative process
Carbon Monoxide Carbon Monoxide Dehydrogenase (CODH) is an enzyme that is a key component of metabolic processes that interconvert single carbon units essentially allowing growth in a CO-dependent manner in the dark. CODH oxidizes carbon monoxide coupled to the reduction of a hydrogen cation by a hydrogenase resulting in H2 gas
Pathology
R. rubrum has not been found to infect humans or animals.
Current Research
Radiation resistance
Sewage treatment
Biodegradable plastics
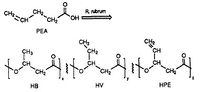
R. rubrum is found to produce a class of biodegradable plastics, poly beta-hydroxyalkanoates (PHAs). The applications of this research can solve many problems caused by synthetic, non-degradable plastics that have adversely impacted the environment. A research experiment performed by Herbert. W. Ulmer et al chronicled the production PHAs containing 3HV units by Rhodospriillum rubrum (1993). Ulmer used 4-pentenoic acid (PEA) as the growth medium for R. rubrum. When the bacteria was placed under "PHA-producing conditions," PHAs were synthesized containing 3 repeating subunits: HB (R = methyl), HV (R = ethyl), HPE (R = vinyl) (See Figure X).
The synthesis of functional, biodegradable plastics by bacteria has the potential to reduce global pollution and fuel expenditure from plastic factories. R. rubrum can be used as a microbiological tool to produce eco-friendly biopolymers.
Renewable energy
The rising concern over environmental pollution from burning fossil fuels has led to an increase in research of Rhodospirillum rubrum. Using R. rubrum as a source for hydrogen to be used in renewable hydrogen fuels addresses several problems created by fossil fuel consumption. Hydrogen oxidizes to water as a combustion product, unlike fossil fuels that create pollutants such as CO, CO2, SOx, and NOx. R. rubrum can produce hydrogen by utilizing the following "water-gas shift reaction" (Najafpour et al, 2004):
CO + H2O → H2 + CO2
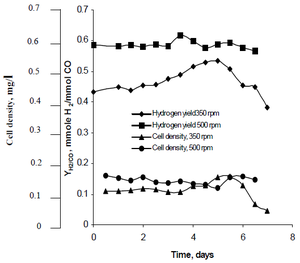
Najafpour's research method observed how agitation, created by sets of turbine impellers set at rates of 350rpm as well as 500 rpm, affected the hydrogen production rates of R. rubrum. His research found that hydrogen has the potential to curb global warming, and provide an eco-friendly technological solution to fuel production and consumption. His study revealed "higher agitation rate yielded higher hydrogen as well as cell density." (See Figure X).
In another microbiological study, performed by Matthew Ross Melnicki, it was found that R. rubrum provides the potential for renewable energy, such as hydrogen fuel, via hydrogen synthesis. Its biological pathways can produce hydrogen levels at high rates when co-cultivated with Chlamydomonas reinhardtii, an algal species which R. rubrum can grow harmoniously with. Melnicki proposed an unconventional co-cultivation approach to synthesizing hydrogen that produced enhanced levels of hydrogen synthesis that may not be achieved if the co-cultivation is uncoupled. C. reinhardtii was favored at higher light intensities, while R. ruburm was more productive under lower light intensities, highlighting the advantages of their co-cultivation.
By using a fed-batch technique for cultivating R. rubrum, it was found that inoculated cultures produced hydrogen during its exponential growth phase, and the synthesis of hydrogen continued to flourish continuously for 70 hours. When growth ceased, Melnicki added succinate, which acts as an electron donor in the metabolic pathway, and hydrogen evolution resumed even though the bacteria now longer grew. However, it is important to note that with continual addition of succinate, reduced evolution rates of hydrogen were found. Once additional growth media was added, rates of hydrogen production increased once again.
Melnicki stated, "while growth is not required for hydrogen production, this work establishes the necessity for cell growth in order to maintain maximal rates, suggesting the industrial suitability of a semi-continuous culture strategy" (Melnicki, 2009).
Add references to reference section.
Cool Factor
Describe something you find "cool" about this microbe.
Possible topics:
Photosynthetic, but does not have light harvesting complex 2 (LHC2), which is commonly found in many photosynthetic bacteria.
High CO2 concentrations inhibit ribulose biphosphate carboxylase expression http://jb.asm.org/cgi/reprint/170/9/4065.pdf
References
Edited by student of Iris Keren