User:Joeho604: Difference between revisions
| (17 intermediate revisions by the same user not shown) | |||
| Line 12: | Line 12: | ||
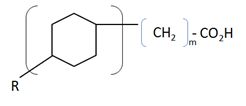
[[Image: Naphthenic_Acids.JPG |thumb|300px|left| Monocyclic naphthenic acid.]] | [[Image: Naphthenic_Acids.JPG |thumb|300px|left| Monocyclic naphthenic acid.]] | ||
Along with the 20-30 wt% of solids (sands and clays) and slightly alkaline water (pH>7.5), mature tailings consist of 1-3 wt% of residual bitumen and naphtha, comprising of a mixture [http://en.wikipedia.org/wiki/Paraffin paraffins] (n-alkanes), [http://en.wikipedia.org/wiki/Isoparaffin iso-paraffins] (branched alkanes),[http://en.wikipedia.org/wiki/Olefins olefins] (alkenes), [http://en.wikipedia.org/wiki/Napthene naphthenes] (cycloalkanes), naphthenic acids (cyclopentyl and cyclohexyl carboxylic acids), and monoaromatics (benzene, toluene, ethylbenzene, and xylenes, or BTEX) [[#References |[2]]][[#References |[3]]]. Other minor elements include trace metals (Cr, Mn, Co, Ni, Cu, Zn, As, Sr, Mo, Ba), and ions (HCO3-, PO43-, NO3-, SO42-, Na+, K+, Mg2+, Ca2+, Cl-) [[#References |[4]]]. | Along with the 20-30 wt% of solids (sands and clays) and slightly alkaline water (pH>7.5), mature tailings consist of 1-3 wt% of residual bitumen and naphtha, comprising of a mixture [http://en.wikipedia.org/wiki/Paraffin paraffins] (n-alkanes), [http://en.wikipedia.org/wiki/Isoparaffin iso-paraffins] (branched alkanes),[http://en.wikipedia.org/wiki/Olefins olefins] (alkenes), [http://en.wikipedia.org/wiki/Napthene naphthenes] (cycloalkanes), [http://en.wikipedia.org/wiki/Olefins olefins], [http://en.wikipedia.org/wiki/Naphthenic_acid naphthenic acids] (cyclopentyl and cyclohexyl carboxylic acids), and monoaromatics (benzene, toluene, ethylbenzene, and xylenes, or [http://en.wikipedia.org/wiki/BTEX BTEX] ) [[#References |[2]]][[#References |[3]]]. Other minor elements include trace metals (Cr, Mn, Co, Ni, Cu, Zn, As, Sr, Mo, Ba), and ions (HCO3-, PO43-, NO3-, SO42-, Na+, K+, Mg2+, Ca2+, Cl-) [[#References |[4]]]. | ||
Toxicity of tailings ponds to aquatic organisms is often associated with naphthenic acids, which have surfactant properties that penetrate the cell membrane [[#References |[5]]]. | Toxicity of tailings ponds to aquatic organisms is often associated with naphthenic acids, which have surfactant properties that penetrate the cell membrane [[#References |[5]]]. | ||
| Line 19: | Line 19: | ||
==Active vs. Inactive pond== | ==Active vs. Inactive pond== | ||
In active ponds, wastes from bitumen extraction are continually collected in the basin. This results in continual input of electron donors and acceptors of metal ions into the pond. In active ponds, gypsum is added to aid densification while microbial activities are dominated by anaerobes at lower depths. | In active ponds, wastes from bitumen extraction are continually collected in the basin. This results in continual input of electron donors and acceptors of metal ions into the pond. In active ponds, [http://en.wikipedia.org/wiki/Gypsum gypsum] is added to aid densification while microbial activities are dominated by anaerobes at lower depths. In inactive ponds, wastes from bitumen extractions are no longer collected. In this situation, there are no inputs of electron donor and acceptors while microbial activities are confined to the upper depths with lower anaerobic activities. Therefore, the status of the pond influences the microbial community found [[#References |[9]]]. | ||
=Microbial Processes= | =Microbial Processes= | ||
| Line 28: | Line 28: | ||
[[Image: Methanogeneis.JPG |thumb|600px|right| Anaerobic hydrocarbon degradation via methanogenesis by competing acetoclastic or hydrogenotrophic methanogens.[[#References|[2]]].]] | [[Image: Methanogeneis.JPG |thumb|600px|right| Anaerobic hydrocarbon degradation via methanogenesis by competing acetoclastic or hydrogenotrophic methanogens.[[#References|[2]]].]] | ||
Anaerobic methanogenic hydrocarbon degradation is the source of CH4 emissions in tailings ponds. Metabolism begins with beta-oxidation of naphtha (such as long and short n-alkanes) to acetate and H2 by Syntrophus/Smithella. The acetate produced is coupled to methanogenesis either by acetoclastic methanogens to generate CH4 gas directly from acetate; or by archaeal hydrogenotrophic methanogens to reduce CO2 into CH4 with the utilization of H2 as a reducing agent following acetate oxidation [[#References |[6]]][[#References |[10]]]. Biodegradation of short n-alkanes (C<12) are preferentially selected by order of decreasing length of C-chains: C10>C8>C7>C6. Long n-alkanes (C≥12), however, do not show any preferential order of biodegradation [[#References |[3]]][[#References |[10]]]. Preference for BTEX biodegradation follows the sequence of: toluene>o-xylene>m-plus p xylene>ethylbenzene> benzene [[#References |[2]]]. | Anaerobic methanogenic hydrocarbon degradation is the source of CH4 emissions in tailings ponds. Metabolism begins with beta-oxidation of naphtha (such as long and short n-alkanes) to acetate and H2 by <i>Syntrophus/Smithella</i>. The acetate produced is coupled to methanogenesis either by [http://en.wikipedia.org/wiki/Methanogen acetoclastic methanogens] to generate CH4 gas directly from acetate; or by archaeal [http://en.wikipedia.org/wiki/Methanogen hydrogenotrophic methanogens] to reduce CO2 into CH4 with the utilization of H2 as a reducing agent following acetate oxidation [[#References |[6]]][[#References |[10]]]. Biodegradation of short n-alkanes (C<12) are preferentially selected by order of decreasing length of C-chains: C10>C8>C7>C6. Long n-alkanes (C≥12), however, do not show any preferential order of biodegradation [[#References |[3]]][[#References |[10]]]. Preference for BTEX biodegradation follows the sequence of: toluene>o-xylene>m-plus p xylene>ethylbenzene> benzene [[#References |[2]]]. | ||
==Hydrocarbon Biodegradation and Sulfate/ Nitrate Reduction== | ==Hydrocarbon Biodegradation and Sulfate/ Nitrate Reduction== | ||
Anaerobic biodegradation of hydrocarbons under sulfate reducing and denitrifying conditions, by sulfate-reducing bacteria (SRB) and nitrate-reducing bacteria (NRB) respectively, is known to impede with methanogenesis. The competition for electron donors ultimately determines the predominance of SRB or methanogens under anaerobic conditions. With a slight advantage in thermodynamic and kinetics over methanogens, SRB outcompete methanogens for byproducts from syntrophs following hydrocarbon oxidation. Under high sulfate concentrations, SRB form consortia with syntrophs which allows SRB to utilize its H2, acetate, and other electron donors for sulfate reduction [[#References |[5]]][[#References |[11]]]. | Anaerobic biodegradation of hydrocarbons under sulfate reducing and denitrifying conditions, by [http://en.wikipedia.org/wiki/Sulfate_reducing_bacteria sulfate-reducing bacteria] (SRB) and [http://en.wikipedia.org/wiki/Nitrate_reducing_bacteria nitrate-reducing bacteria] (NRB) respectively, is known to impede with methanogenesis. The competition for electron donors ultimately determines the predominance of SRB or methanogens under anaerobic conditions. With a slight advantage in thermodynamic and kinetics over methanogens, SRB outcompete methanogens for byproducts from syntrophs following hydrocarbon oxidation. Under high sulfate concentrations, SRB form consortia with syntrophs which allows SRB to utilize its H2, acetate, and other electron donors for sulfate reduction [[#References |[5]]][[#References |[11]]]. | ||
[[Image: Sulphate_Reduction.JPG |thumb|400px|right| Sulfate reduction by sulfate-reducing bacteria with the utilization of acetate and hydrogen ions.]] | [[Image: Sulphate_Reduction.JPG |thumb|400px|right| Sulfate reduction by sulfate-reducing bacteria with the utilization of acetate and hydrogen ions.]] | ||
==Naphthenic acid degradation== | ==Naphthenic acid degradation== | ||
Naphthenic acid degradation is performed by a wide range of aerobes through degradation of anteiso-alkyl substituted aliphatic chains by Acinetobacter cerificans, Pseudomonoas citronellonis, and Mycobacterium austrafri; or degradation of ternary substituted structures outside of the B-position to the carboxylic group by Mycobacterium austroafricanum, Mycobacterium fortuitum, and Brevibacterium erthyrogenes; or by methyl substitution on the cyclic ring of alicyclic acids. Modification of the branch and cyclic ring of naphthenic acids make naphthenic acids less refractory [[#References |[5]]]. Generally, this involves aerobic α-oxidation and B-oxidation pathways [[#References |[12]]]. | Naphthenic acid degradation is performed by a wide range of aerobes through degradation of anteiso-alkyl substituted aliphatic chains by <i>Acinetobacter cerificans, Pseudomonoas citronellonis</i>, and <i>Mycobacterium austrafri</i>; or degradation of ternary substituted structures outside of the B-position to the carboxylic group by <i>Mycobacterium austroafricanum, Mycobacterium fortuitum</i>, and <i>Brevibacterium erthyrogenes</i>; or by methyl substitution on the cyclic ring of alicyclic acids. Modification of the branch and cyclic ring of naphthenic acids make naphthenic acids less refractory [[#References |[5]]]. Generally, this involves aerobic α-oxidation and [http://en.wikipedia.org/wiki/B-oxidation B-oxidation pathways] [[#References |[12]]]. | ||
=Microbial Communities:= | =Microbial Communities:= | ||
| Line 47: | Line 47: | ||
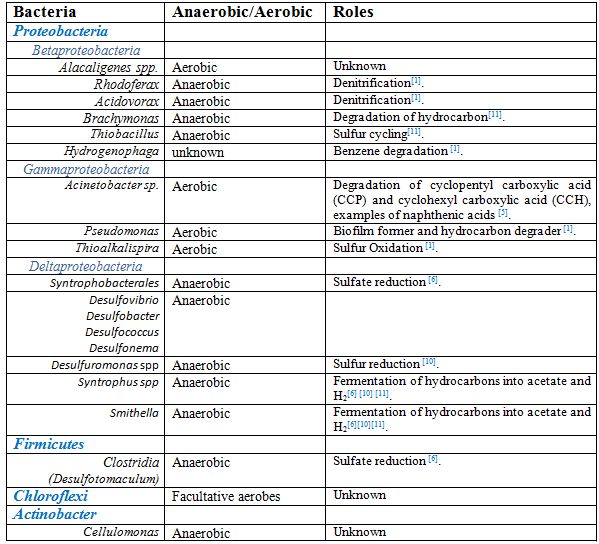
==Aerobic Microbial Communities== | ==Aerobic Microbial Communities== | ||
Pseudomonas, Acinetobacter, Alcaligens, Nocardia, Kurthia, and Xanthomonas species make up the aerobic populations of tailing ponds. Acinobacter spp. or Alcaligenes spp. dominate these depth of tailings, in which oxygen is present at the upper 1-2 metres of tailings ponds [[#References |[1]]][[#References |[5]]][[#References |[9]]]. | <i>Pseudomonas, Acinetobacter, Alcaligens, Nocardia, Kurthia</i>, and <i>Xanthomonas</i> species make up the aerobic populations of tailing ponds. <i>Acinobacter spp.</i> or <i>Alcaligenes spp.</i> dominate these depth of tailings, in which oxygen is present at the upper 1-2 metres of tailings ponds [[#References |[1]]][[#References |[5]]][[#References |[9]]]. | ||
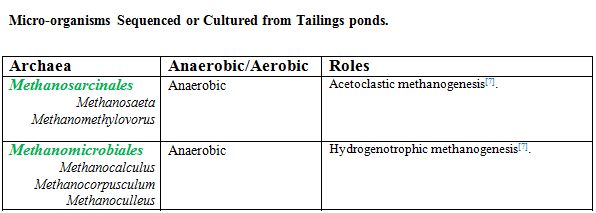
==Anaerobic Microbial Communities== | ==Anaerobic Microbial Communities== | ||
Acetoclastic methanogens, such as Methananosaeta spp, dominate the archaeal population in deep depths of tailings. Hydrogenenotrophic methanogens from Methanomicrobiales are less prevalent because of their disadvantage to compete with acetogens for H2 at lower temperatures [[#References |[7]]]. However, when partnered with syntrophic bacteria, hydrogenotrophic methanogens dominate in CH4 production [[#References |[6]]]. These methanogens together play a key role in CH4 and CO2 emissions. | Acetoclastic methanogens, such as <i>Methananosaeta spp</i>, dominate the archaeal population in deep depths of tailings. Hydrogenenotrophic methanogens from <i>Methanomicrobiales</i> are less prevalent because of their disadvantage to compete with acetogens for H2 at lower temperatures [[#References |[7]]]. However, when partnered with syntrophic bacteria, hydrogenotrophic methanogens dominate in CH4 production [[#References |[6]]]. These methanogens together play a key role in CH4 and CO2 emissions. | ||
Equally important, proteobacteria, such as syntrophs (Pelotomaculum, Syntrophus, and Smithella spp) and sulfate reducing bacteria (Desulfocapsa and Desulfurivibrio), dominate the anaerobic layers [[#References |[7]]]. These bacteria play a key role in biodegradation of naphtha and also influence methanogenesis by methanogens. | Equally important, proteobacteria, such as syntrophs (<i>Pelotomaculum</i>, <i>Syntrophus</i>, and <i>Smithella spp</i>) and sulfate reducing bacteria (<i>Desulfocapsa</i> and <i>Desulfurivibrio</i>), dominate the anaerobic layers [[#References |[7]]]. These bacteria play a key role in biodegradation of naphtha and also influence methanogenesis by methanogens. | ||
| Line 63: | Line 63: | ||
==In Situ Bioremediation of Naphthenic Acids== | ==In Situ Bioremediation of Naphthenic Acids== | ||
Toxicity of refractory naphthenic acids (NAs) is another challenge faced in bioremediation. Plausible options to help degrade these refractory molecules involve: 1) bioaugmentating foreign bacteria in tailings which are capable of enhancing the degradation of NAs; 2) increasing the bioavailability of NAs by using attachment materials such as clays or introducing algae to bioaccumulate hydrophobic NAs for bacterial degradation; 3) biostimulating microbial growth with aeration, nutrients, and organic materials to enhance metabolism. These methods can be applied with wetlands and wet landscape approaches aimed at reclaiming tailings as a land surface or a wetland [[#References |[5]]]. | Toxicity of refractory naphthenic acids (NAs) is another challenge faced in bioremediation. Plausible options to help degrade these refractory molecules involve: 1) bioaugmentating foreign bacteria in tailings which are capable of enhancing the degradation of NAs; 2) increasing the bioavailability of NAs by using attachment materials such as clays or introducing algae to bioaccumulate hydrophobic NAs for bacterial degradation; 3) biostimulating microbial growth with aeration, nutrients, and organic materials to enhance metabolism. These methods can be applied with [http://www.ncbi.nlm.nih.gov/pubmed/15756978 wetlands and wet landscape approaches] aimed at reclaiming tailings as a land surface or a wetland [[#References |[5]]]. | ||
==References== | ==References== | ||
1 | [1] Golby, S., Ceri, H., Gieg, LM., Chatterjee, I., Marques, L., and Turner, R. “ Evaluation of microbial biofilm communities from an Alberta oil sands tailings pond.” FEMS Microbiol Ecol., 2012, DOI: 10.1111/j.1574-6941.2011.01212.x | ||
2 | [2] Siddique, T., Fedorak, PM., Mackinnon, MD., and Foght, JM. “Metabolism of BTEX and Naphtha Compounds to Methane in Oil Sands Tailings.” Environ Sci Technol., 2007, DOI: 10.1021/es062852q | ||
3 | [3] Siddique, T., Fedorak, PM., and Foght, JM. “Biodegradation of Short-Chain n-Alkanes in Oil Sands Tailings under Methanogenic Conditions.” Environ Sci Technol., 2006, DOI: 10.1021/es060993m | ||
4 | [4] Mahdavi, H., Ulrich, A., and Liu, Y. “Metal removal from oil sands tailings pond water by indigenous micro-alga.”Chemosphere., 2012, DOI: 10.1016/j.chemosphere.2012.04.041 | ||
5 | [5] Quagraine, EK., Peterson, HG., and Headley, JV “In Situ Bioremediation of Naphthenic Acids Contaminated Tailing Pond Waters in the Athabasca Oil Sands Region-Demonstrated Field Studies and Plausible Options: A Review.”J. Envir Science and Health., 2005, DOI: 10.1081/ESE-200046649 | ||
6 | [6] Siddique, T., Penner, T., Klassen, J., Nesbo, C., and Foght, JM. “Microbial Communites Involved in Methane Production from Hydrocarbons in Oil Sands Tailings” Environ. Science. Technology, 2012, DOI: 10.1021/es302202c | ||
7 | [7] Penner, TJ., and Foght, JM., “Mature fine tailings from oil sands processing harbour diverse methanogenic communities.” Can J. Microbiol., 2010, DOI: 10.1139/W10-029 | ||
8 | [8] Brown, D., Ramos-Padron, E., Gieg, L., and Voorduow, G., “Effect of calcium ions and anerobic microbial activity on sedimentation of oil sands tailings.” International Biodeterioration and biodegradation, 2012, DOI: 10.1016/j.ibiod.2012.07.006 | ||
9 | [9] Gieg, L., “Microbial Activities and Communities in Oil Sands Tailings Ponds.” Retrieved from http://www.ismos-3.org/Presentations/Gieg-ISMOS-3.pdf | ||
10 | [10] Siddique, T., Penner, T., Klassen, J., Nesbo, C., and Foght, JM. “Anaerobic Biodegradation of Longer Chain n-Alkane Coupled to Methane Production in Oil Sands Tailings.” American Chemical Society, 2011, DOI: 10.1021/es200649t | ||
11 | [11] Padron, ER., Bordenave, S., Lin, S., Bhaskar, IM., Dong, X., Sensen, CW, Fournier, J., Voordouw, G., and Gieg, L. “Carbon and Sulfur Cycling by Microbial Communities in Gypsum-Treated Oil Sands Tailings Pond.”Environ Sci Technol., 2011, DOI: 10.1021/es1028487 | ||
12 | [12] Whitby, C., “Microbial Naphthenic Acid Degradation.”Advances in Applied Microbiology., 2010, DOI: http://dx.doi.org/10.1016/S0065-2164(10)70003-4 | ||
Latest revision as of 09:52, 13 December 2012
Introduction

The oil sands in Alberta, Canada, covering over 100000 km2, produce over 1.3 million barrels of bitumin per day [1]. Extraction of bitumen by surface mining of oil sands requires large amount of water and hydrocarbon solvents. The resulting byproduct creates large volumes of water, sands, clays, residual hydrocarbons, heavy metals, naphtha diluents, and naphthenic acids, which are termed as tailings. Due to operational policies preventing zero discharge of any liquids into the environment, these fine tailings are collected and confined into settling basins to create tailings ponds [1]. Despite the toxicity of tailings, microbial communities exist in tailings pond, which aid to accelerate tailings sedimentation while being able to degrade certain compounds in the tailings.
Physical & Chemical Environment
Composition of tailings pond
Along with the 20-30 wt% of solids (sands and clays) and slightly alkaline water (pH>7.5), mature tailings consist of 1-3 wt% of residual bitumen and naphtha, comprising of a mixture paraffins (n-alkanes), iso-paraffins (branched alkanes),olefins (alkenes), naphthenes (cycloalkanes), olefins, naphthenic acids (cyclopentyl and cyclohexyl carboxylic acids), and monoaromatics (benzene, toluene, ethylbenzene, and xylenes, or BTEX ) [2][3]. Other minor elements include trace metals (Cr, Mn, Co, Ni, Cu, Zn, As, Sr, Mo, Ba), and ions (HCO3-, PO43-, NO3-, SO42-, Na+, K+, Mg2+, Ca2+, Cl-) [4]. Toxicity of tailings ponds to aquatic organisms is often associated with naphthenic acids, which have surfactant properties that penetrate the cell membrane [5].
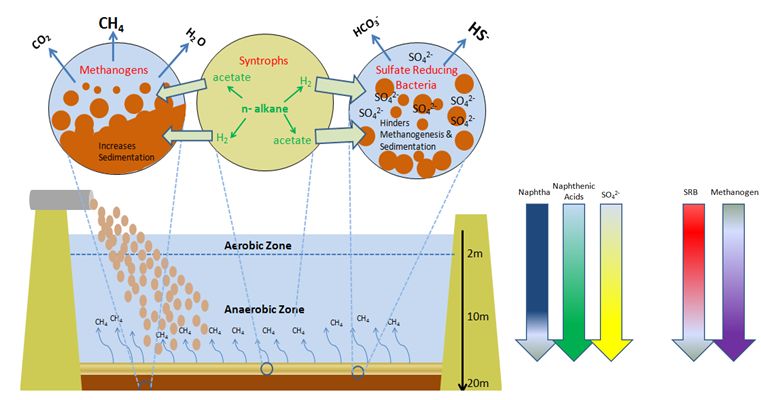
Sedimentation & Gas Emissions
Closer to the inflow, sand settles quickly. However, tailings consolidation of fine clays occurs by gravity at a slow rate over 5-10 years [6]. Stratification therefore results with older and denser fine tailings settled to the bottom and fresh tailings deposited on top. Temperature also increases with depth due to lack of surface cooling and retention of heat at 30-60˚C by deposited tailings [7]. Densification remains an operational challenge, which prevents water from being recycled while delaying the land reclamation process. Anaerobic methanogenic activities by syntrophic bacteria and methanogenic archaea also enhance the rate of tailings sedimentation. Microbe-formed gas channels assist the escape of methane gas bubbles which also aid to drain pore water from deeper tailings. Each day, about 43000m3of methane (CH4) is released. Sulfate and nitrate reduction by sulfate and nitrate-reducing bacteria, however, impeded the actions of methanogenesis, thus slowing the rate of tailings densification. Densification is reached when the volumetric fraction of solids (Fs) increase to 85%(w/w) [8].
Active vs. Inactive pond
In active ponds, wastes from bitumen extraction are continually collected in the basin. This results in continual input of electron donors and acceptors of metal ions into the pond. In active ponds, gypsum is added to aid densification while microbial activities are dominated by anaerobes at lower depths. In inactive ponds, wastes from bitumen extractions are no longer collected. In this situation, there are no inputs of electron donor and acceptors while microbial activities are confined to the upper depths with lower anaerobic activities. Therefore, the status of the pond influences the microbial community found [9].
Microbial Processes
Hydrocarbon Biodegradation and Methanogenesis
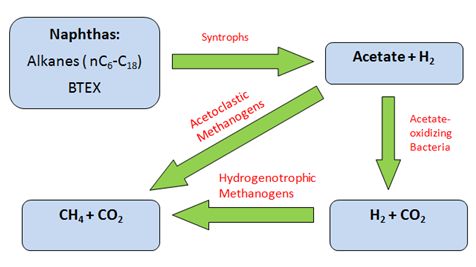
Anaerobic methanogenic hydrocarbon degradation is the source of CH4 emissions in tailings ponds. Metabolism begins with beta-oxidation of naphtha (such as long and short n-alkanes) to acetate and H2 by Syntrophus/Smithella. The acetate produced is coupled to methanogenesis either by acetoclastic methanogens to generate CH4 gas directly from acetate; or by archaeal hydrogenotrophic methanogens to reduce CO2 into CH4 with the utilization of H2 as a reducing agent following acetate oxidation [6][10]. Biodegradation of short n-alkanes (C<12) are preferentially selected by order of decreasing length of C-chains: C10>C8>C7>C6. Long n-alkanes (C≥12), however, do not show any preferential order of biodegradation [3][10]. Preference for BTEX biodegradation follows the sequence of: toluene>o-xylene>m-plus p xylene>ethylbenzene> benzene [2].
Hydrocarbon Biodegradation and Sulfate/ Nitrate Reduction
Anaerobic biodegradation of hydrocarbons under sulfate reducing and denitrifying conditions, by sulfate-reducing bacteria (SRB) and nitrate-reducing bacteria (NRB) respectively, is known to impede with methanogenesis. The competition for electron donors ultimately determines the predominance of SRB or methanogens under anaerobic conditions. With a slight advantage in thermodynamic and kinetics over methanogens, SRB outcompete methanogens for byproducts from syntrophs following hydrocarbon oxidation. Under high sulfate concentrations, SRB form consortia with syntrophs which allows SRB to utilize its H2, acetate, and other electron donors for sulfate reduction [5][11].
Naphthenic acid degradation
Naphthenic acid degradation is performed by a wide range of aerobes through degradation of anteiso-alkyl substituted aliphatic chains by Acinetobacter cerificans, Pseudomonoas citronellonis, and Mycobacterium austrafri; or degradation of ternary substituted structures outside of the B-position to the carboxylic group by Mycobacterium austroafricanum, Mycobacterium fortuitum, and Brevibacterium erthyrogenes; or by methyl substitution on the cyclic ring of alicyclic acids. Modification of the branch and cyclic ring of naphthenic acids make naphthenic acids less refractory [5]. Generally, this involves aerobic α-oxidation and B-oxidation pathways [12].
Microbial Communities:
Microbial communities in tailings pond have high bacterial diversity, much of which changes with the depth of tailings. The cause of such observation is due to the differential stratification of hydrocarbons, metabolites, and electron donors involved in carbon and sulfur cycling. Importantly, methane concentrations varies with depth while sulfate concentrations increases with depth [11]. 16S DNA sequencing reveals individual microorganisms prevalent at different depths of tailings.
Aerobic Microbial Communities
Pseudomonas, Acinetobacter, Alcaligens, Nocardia, Kurthia, and Xanthomonas species make up the aerobic populations of tailing ponds. Acinobacter spp. or Alcaligenes spp. dominate these depth of tailings, in which oxygen is present at the upper 1-2 metres of tailings ponds [1][5][9].
Anaerobic Microbial Communities
Acetoclastic methanogens, such as Methananosaeta spp, dominate the archaeal population in deep depths of tailings. Hydrogenenotrophic methanogens from Methanomicrobiales are less prevalent because of their disadvantage to compete with acetogens for H2 at lower temperatures [7]. However, when partnered with syntrophic bacteria, hydrogenotrophic methanogens dominate in CH4 production [6]. These methanogens together play a key role in CH4 and CO2 emissions. Equally important, proteobacteria, such as syntrophs (Pelotomaculum, Syntrophus, and Smithella spp) and sulfate reducing bacteria (Desulfocapsa and Desulfurivibrio), dominate the anaerobic layers [7]. These bacteria play a key role in biodegradation of naphtha and also influence methanogenesis by methanogens.
Current Research:
Enhanced Sedimentation
The challenges of bioremediation lie on the speed of sedimentation to allow pore water to be recycled for future bitumen extractions so that land recovery may begin. Therefore, understanding the roles of microbial activities and specific micoorganisms involved in degrading residual bitumen and naphtha under methanogenic or sulfate reducing conditions at different depths will provide researchers on how best to conduct bioremediation on tailings pond. Currently, to accelerate sedimentation chemically, Ca2+ cations from gysum are added to help neutralize the negatively charged tailings particle which promotes tailings flocculation for biodegradation [8]. This also has implication on the microbial communities present in tailings.
In Situ Bioremediation of Naphthenic Acids
Toxicity of refractory naphthenic acids (NAs) is another challenge faced in bioremediation. Plausible options to help degrade these refractory molecules involve: 1) bioaugmentating foreign bacteria in tailings which are capable of enhancing the degradation of NAs; 2) increasing the bioavailability of NAs by using attachment materials such as clays or introducing algae to bioaccumulate hydrophobic NAs for bacterial degradation; 3) biostimulating microbial growth with aeration, nutrients, and organic materials to enhance metabolism. These methods can be applied with wetlands and wet landscape approaches aimed at reclaiming tailings as a land surface or a wetland [5].
References
[1] Golby, S., Ceri, H., Gieg, LM., Chatterjee, I., Marques, L., and Turner, R. “ Evaluation of microbial biofilm communities from an Alberta oil sands tailings pond.” FEMS Microbiol Ecol., 2012, DOI: 10.1111/j.1574-6941.2011.01212.x
[2] Siddique, T., Fedorak, PM., Mackinnon, MD., and Foght, JM. “Metabolism of BTEX and Naphtha Compounds to Methane in Oil Sands Tailings.” Environ Sci Technol., 2007, DOI: 10.1021/es062852q
[3] Siddique, T., Fedorak, PM., and Foght, JM. “Biodegradation of Short-Chain n-Alkanes in Oil Sands Tailings under Methanogenic Conditions.” Environ Sci Technol., 2006, DOI: 10.1021/es060993m
[4] Mahdavi, H., Ulrich, A., and Liu, Y. “Metal removal from oil sands tailings pond water by indigenous micro-alga.”Chemosphere., 2012, DOI: 10.1016/j.chemosphere.2012.04.041
[5] Quagraine, EK., Peterson, HG., and Headley, JV “In Situ Bioremediation of Naphthenic Acids Contaminated Tailing Pond Waters in the Athabasca Oil Sands Region-Demonstrated Field Studies and Plausible Options: A Review.”J. Envir Science and Health., 2005, DOI: 10.1081/ESE-200046649
[6] Siddique, T., Penner, T., Klassen, J., Nesbo, C., and Foght, JM. “Microbial Communites Involved in Methane Production from Hydrocarbons in Oil Sands Tailings” Environ. Science. Technology, 2012, DOI: 10.1021/es302202c
[7] Penner, TJ., and Foght, JM., “Mature fine tailings from oil sands processing harbour diverse methanogenic communities.” Can J. Microbiol., 2010, DOI: 10.1139/W10-029
[8] Brown, D., Ramos-Padron, E., Gieg, L., and Voorduow, G., “Effect of calcium ions and anerobic microbial activity on sedimentation of oil sands tailings.” International Biodeterioration and biodegradation, 2012, DOI: 10.1016/j.ibiod.2012.07.006
[9] Gieg, L., “Microbial Activities and Communities in Oil Sands Tailings Ponds.” Retrieved from http://www.ismos-3.org/Presentations/Gieg-ISMOS-3.pdf
[10] Siddique, T., Penner, T., Klassen, J., Nesbo, C., and Foght, JM. “Anaerobic Biodegradation of Longer Chain n-Alkane Coupled to Methane Production in Oil Sands Tailings.” American Chemical Society, 2011, DOI: 10.1021/es200649t
[11] Padron, ER., Bordenave, S., Lin, S., Bhaskar, IM., Dong, X., Sensen, CW, Fournier, J., Voordouw, G., and Gieg, L. “Carbon and Sulfur Cycling by Microbial Communities in Gypsum-Treated Oil Sands Tailings Pond.”Environ Sci Technol., 2011, DOI: 10.1021/es1028487
[12] Whitby, C., “Microbial Naphthenic Acid Degradation.”Advances in Applied Microbiology., 2010, DOI: http://dx.doi.org/10.1016/S0065-2164(10)70003-4