West Nile Virus: Difference between revisions
No edit summary |
|||
| (19 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
==Introduction== | ==Introduction== | ||
The West Nile Virus (WNV) is a neurotropic human pathogen. This virus belongs to the flavivirus genus which is in the Flaviviridae family along with the dengue virus and the yellow fever virus. The West Nile Virus is a mosquito-borne virus and affects many other animals besides humans, such as birds, reptiles and other mammals. The WNV is mainly found in tropical and temperate regions around the world and was first discovered in Uganda in 1937. The first major outbreak of the disease occurred in 1994 in Algeria and it finally hit the western hemisphere in New York in 1999. Many humans who do become infected with the disease are subclinical where they do not show any symptoms however transmission of the WNV can result in the West Nile Fever. The West Nile Fever, not always, but can affect the central nervous system and lead to a neurological disease. | The West Nile Virus (WNV) is a [http://en.wikipedia.org/wiki/Neurotropic_virus neurotropic] human pathogen. This virus belongs to the flavivirus genus which is in the Flaviviridae family along with the [http://en.wikipedia.org/wiki/Dengue_virus dengue virus] and the [http://en.wikipedia.org/wiki/Yellow_fever yellow fever virus]. The West Nile Virus is a mosquito-borne virus and affects many other animals besides humans, such as birds, reptiles and other mammals. The WNV is mainly found in tropical and temperate regions around the world and was first discovered in Uganda in 1937[21]. The first major outbreak of the disease occurred in 1994 in Algeria and it finally hit the western hemisphere in New York in 1999 [21]. Many humans who do become infected with the disease are subclinical where they do not show any symptoms however transmission of the WNV can result in the West Nile Fever. The West Nile Fever, not always, but can affect the central nervous system and lead to a neurological disease. | ||
==Structure and Function== | ==Structure and Function== | ||
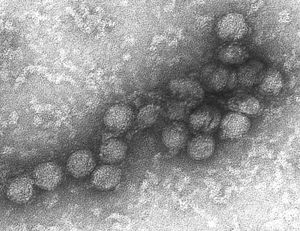
[[File:West Nile virus EM PHIL 2290 lores.jpg|thumb|An electron micrograph of the West Nile Virus. This image was provided by the Centers for Disease Control and Prevention, part of the United States Department of Health and Human Services. It is a part of the public domain.]] | |||
====RNA and Proteins==== | ====RNA and Proteins==== | ||
The West Nile Virus is and enveloped virion that contains a positive single stranded genome. It is in Group IV ((+)ssRNA). The genome is about 11 kb of a single open reading frame [10]. Through the use of cryoelectron microscopy it has been discovered that the virus has icosahedral symmetry and is ∼500 Å in diameter. The virus has no surface projections or spikes [9]. The virus has | The West Nile Virus is and enveloped virion that contains a positive single stranded genome. It is in Group IV ((+)ssRNA). The genome is about 11 kb of a single open reading frame [10]. Through the use of cryoelectron microscopy it has been discovered that the virus has icosahedral symmetry and is ∼500 Å in diameter. The virus has no surface projections or spikes [9]. The virus has multi-layer organization where the outermost layer corresponds to the E and M transmembrane proteins with a very high density. The core nucleocapsid consists of copies of the genome RNA and the capsid proteins [9]. The viral RNA is translated to produce 3 structural and 7 nonstructural proteins. The structural proteins are capsid, envelope, and premembrane proteins and the nonstructural proteins include NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5. The structural proteins are encoded at the 5’ end of the RNA strand and serve the function of virus entry and encapsulation of the genome [10]. The nonstructural proteins serve multiple functions due to the limited number of proteins present in the genome. NS1 does not have an exact role in viron assembly however it has been speculated to help in the replication process [2]. NS2A, NS2B, NS4A, and NS4B inhibit the inate immune response against viral infection in the body [3]. NS3 is the protease that cleaves other nonstructural proteins from the polyprotein and encodes enzyme activities [11]. The NS5 protein is essential for viral replication due to the fact that it is the polymerase that encodes a methltransferase [12]. | ||
====Life Cycle==== | ====Life Cycle==== | ||
The West Nile Virus enters the cell through receptor-mediated endocytosis. DC-SIGNR is a major receptor for this endocytosis [13]. Once the virus is internalized in the cell, the virus matures as the pH drops to become slightly acidic within the envelope. Once the endosome has matured the envelope protein undergoes conformational changes where the endocytic membrane fuses with the viral lipid membrane. This causes the release of the viral RNA genome into the cytoplasm of the cell [14]. Once released into the cell the single RNA is replicated and translated into proteins. The viral RNA is replicated by both cellular and viral proteins. The 3 structural proteins expressed from the viral RNA assemble onto the membranes in the endoplasmic reticulum and bud into the cytoplasm by the Golgi network. The virus exits the cell in a lipid envelope through exocytosis when cellular enzymes cleave the prM [4]. | The West Nile Virus enters the cell through receptor-mediated endocytosis. DC-SIGNR is a major receptor for this form of endocytosis [13]. Once the virus is internalized in the cell, the virus matures as the pH drops to become slightly acidic within the envelope. Once the endosome has matured the envelope protein undergoes conformational changes where the endocytic membrane fuses with the viral lipid membrane. This causes the release of the viral RNA genome into the cytoplasm of the cell [14]. Once released into the cell the single RNA is replicated and translated into proteins. The viral RNA is replicated by both cellular and viral proteins. The 3 structural proteins expressed from the viral RNA assemble onto the membranes in the endoplasmic reticulum and bud into the cytoplasm by the Golgi network. The virus exits the cell in a lipid envelope through exocytosis when cellular enzymes cleave the prM [4]. | ||
==Pathogenesis== | ==Pathogenesis== | ||
====Vectors==== | ====Vectors==== | ||
Mosquitoes are the main vectors of the West Nile virus. They acquire the virus after ingesting the blood from a viremic animal. The virus infects the cells of the mosquito by replicating in the cells of the midgut while the blood is being digested[15]. The mosquitoes are not affected by the virus due to various types of chitin and proteins present in the midgut as well as acquiring a high tolerance for the virus. The midgut contains a peritrophic matrix which reduces pathogen invasion to the epithelial cells[16]. Once replicated, the virus travels through the hemolymph to the salivary glands. Given enough time this will lead to a high level of viremia in the saliva of the mosquito which can then be transmitted to other organisms when they are feeding [15]. | Mosquitoes are the main vectors of the West Nile virus. They acquire the virus after ingesting the blood from a viremic animal. The virus infects the cells of the mosquito by replicating in the cells of the midgut while the blood is being digested[15]. The mosquitoes are not affected by the virus due to various types of chitin and proteins present in the midgut as well as acquiring a high tolerance for the virus. The midgut contains a peritrophic matrix which reduces pathogen invasion to the epithelial cells[16]. Once replicated, the virus travels through the hemolymph to the salivary glands. Given enough time this will lead to a high level of viremia in the saliva of the mosquito, which can then be transmitted to other organisms when they are feeding [15]. | ||
====Host Reservoirs==== | ====Host Reservoirs==== | ||
Many different types of mosquito species can carry the West Nile Virus. The most common mosquito vector of the United States is C. tarsali.[1]. | Many different types of mosquito species can carry the West Nile Virus. The most common mosquito vector of the United States is C. <i> tarsali </i>.[1]. Mosquitoes feed on a variety of organisms which makes it quite easy to spread the disease. Birds are the most common host reservoir since many species can have the disease yet show no signs or symptoms. The West Nile virus is maintained in the environment through this constant cycle between animal hosts and mosquitoes. Birds are also a prime target as a host reservoir because they can carry high levels of viremia in their blood whereas humans cannot and are considered a dead end hosts [1]. | ||
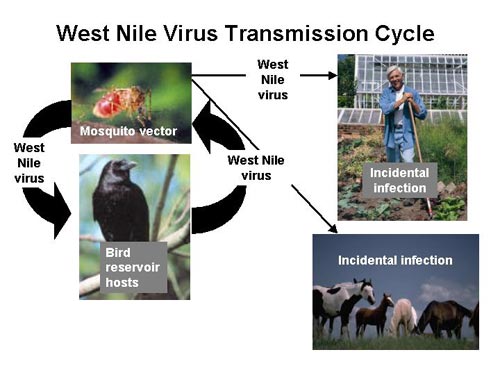
[[File:West_Nile_virus_transmission_cycle.jpg|thumb|500x375px|right|Flow chart of the West Nile Virus. Provided by the Centers for Disease Control and Prevention and is a part of the public domain.]] | [[File:West_Nile_virus_transmission_cycle.jpg|thumb|500x375px|right|Flow chart of the West Nile Virus. Provided by the Centers for Disease Control and Prevention and is a part of the public domain.]] | ||
====Transmission to Host Organism==== | ====Transmission to Host Organism==== | ||
The West Nile virus is transmitted from the mosquito to the host organism when the mosquito begins the probing process of blood feeding. During the probing process the proboscis of the mosquito injects the skin of the organism with pharmacological active saliva proteins to locate the blood source. In a study performed by Styler (2011) it was shown that when the West Nile virus is transmitted by mosquitoes the viremia of the host organism is significantly higher than when the virus is transmitted with only a syringe. This suggests that mosquito saliva plays an important role in enhancing the transmission and survival of the West Nile virus in host organisms [6]. Since there is such a great amount of selective pressure upon mosquito saliva from the host immune system it is likely that the West Nile virus co-evolved in order to use saliva proteins to | The West Nile virus is transmitted from the mosquito to the host organism when the mosquito begins the probing process of blood feeding. During the probing process the proboscis of the mosquito injects the skin of the organism with pharmacological active saliva proteins to locate the blood source. In a study performed by Styler (2011), it was shown that when the West Nile virus is transmitted by mosquitoes, the viremia of the host organism is significantly higher than when the virus is transmitted with only a syringe. This suggests that mosquito saliva plays an important role in enhancing the transmission and survival of the West Nile virus in host organisms [6]. Since there is such a great amount of selective pressure upon mosquito saliva from the host immune system it is likely that the West Nile virus co-evolved in order to use saliva proteins to its advantage. More research needs to be done on what exact proteins are responsible for the successful transmittance of the virus [1]. | ||
Saliva from mosquitoes effectively decreases immune response from the host organism. When the West Nile virus is transmitted with mosquito saliva there is a reduction in T cells recruited to the inoculation site compared to when the virus is transmitted alone. The saliva dis-regulates antigen-presenting cells signaling which decreases the amount of T cells recruited. With a lower amount of T cells it is more difficult for the host organism to destroy the virus [18]. | Saliva from mosquitoes effectively decreases immune response from the host organism. When the West Nile virus is transmitted with mosquito saliva there is a reduction in T cells recruited to the inoculation site compared to when the virus is transmitted alone. The saliva dis-regulates antigen-presenting cells signaling which decreases the amount of T cells recruited. With a lower amount of T cells it is more difficult for the host organism to destroy the virus [18]. | ||
The level of viremia is dependent upon | The level of viremia is dependent upon how the virus is transmitted to the host organism; extravascularly or intravascularly. Mosquitoes inoculate lower doses of the virus directly into the blood while feeding compared to when the saliva does not come into contact with the organisms blood. Directly inducting the virus into the blood stream may have an affect on viral tropism. When immediately introduced to the blood there is an earlier development of viremia as well as higher rates of infecting other co-feeding mosquitoes [17]. | ||
==Immune Mechanism== | ==Immune Mechanism== | ||
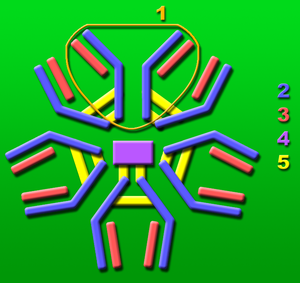
[[File: | [[File:IgM.png|thumb|225 x 240px|left| [http://en.wikipedia.org/wiki/File:IgM.png IgM molecule] responsible for controlling viremia levels of the WNV within the body. Lennert, B. (2005)]] | ||
When the West Nile virus is initially transmitted from a mosquito to another animal the virus initially infects the keratinocytes and Langerhans cells. These cells then migrate to the lymph nodes and initial replication begins [19]. From the lymph nodes the virus is then spread to visceral organs and a second replication occurs [20]. If viremia levels become high enough the virus can cross the blood-brain barrier and cause | When the West Nile virus is initially transmitted from a mosquito to another animal the virus initially infects the keratinocytes and [http://en.wikipedia.org/wiki/Langerhans_cell Langerhans cells]. These cells then migrate to the lymph nodes and initial replication begins [19]. From the lymph nodes the virus is then spread to visceral organs and a second replication occurs [20]. If viremia levels become high enough the virus can cross the blood-brain barrier and cause [http://en.wikipedia.org/wiki/Meningoencephalitis meningoencephalitis]. The envelope glycoprotein of the West Nile virus has been shown to be very influential is the success of the virus to enter the central nervous system. This is due to the domain III of the protein which acts as the receptor binding domain [7]. | ||
The elderly and individuals who are immune compromised are more susceptible to contracting the West Nile virus developing fatal encephalitis. It is speculated that decreased amounts of immunoglobulin M (IgM), CD4+, and CD8+ T cells are the cause of this. In one study it was found that viremia levels were lower in mice that were inducted with a neutralizing IgM response early in the infection of the West Nile virus [8]. | The elderly and individuals who are immune compromised are more susceptible to contracting the West Nile virus developing fatal encephalitis. It is speculated that decreased amounts of immunoglobulin M (IgM), CD4+, and CD8+ T cells are the cause of this. In one study it was found that viremia levels were lower in mice that were inducted with a neutralizing IgM response early in the infection of the West Nile virus [8]. This leads researchers to believe that the IgM molecule plays a large role in recruiting antibodies to limit viremia levels and decreases the likelihood of the virus spreading to the central nervous system [8]. | ||
==Conclusion== | ==Conclusion== | ||
The West Nile Virus is a is a neurotropic human pathogen that consists of a positive single stranded RNA genome. The virus is primarily transmitted via mosquitoes. Mosquitoes become infected with the virus by host organisms while feeding. Once viremia levels are high enough in the saliva of the infected mosquito it can then transmit the virus to another organism. Mosquito saliva not only plays a role in transmitting the virus, but it also suppresses the immunity of the targeted organism allowing for the virus to successfully replicate in the new environment. | The West Nile Virus is a is a neurotropic human pathogen that consists of a positive single stranded RNA genome. The virus is primarily transmitted via mosquitoes. Mosquitoes become infected with the virus by host organisms while feeding. Once viremia levels are high enough in the saliva of the infected mosquito it can then transmit the virus to another organism. Mosquito saliva not only plays a role in transmitting the virus, but it also suppresses the immunity of the targeted organism, allowing for the virus to successfully replicate in the new environment. | ||
There is no vaccine for this virus suitable for humans. There is a vaccine that can be given to horses however it has been | There is no vaccine for this virus suitable for humans. There is a vaccine that can be given to horses however it has not been tested in humans. Scientists are currently researching this topic for a cure to this virus. The Centers for Disease Control and Prevention (CDC) are taking precautions and various steps to control this virus. They are developing faster ways to detect and diagnose WNV in addition to developing and improving mosquito prevention and control programs [21]. | ||
==References== | ==References== | ||
| Line 48: | Line 50: | ||
2. Westaway E.G., Mackenzie J.M., Khromykh A.A. 2002. [http://link.springer.com/chapter/10.1007/978-3-642-59403-8_16 "Replication and gene function in Kunjin virus." ]Current Topics in Microbiology and Immunology Volume 267, 2002, pp 323-351. | 2. Westaway E.G., Mackenzie J.M., Khromykh A.A. 2002. [http://link.springer.com/chapter/10.1007/978-3-642-59403-8_16 "Replication and gene function in Kunjin virus." ]Current Topics in Microbiology and Immunology Volume 267, 2002, pp 323-351. | ||
3. Liu | 3. Liu W.J., Chen H.B., Wang X.J., Huang H., Khromykh A.A. 2004. [http://www.ncbi.nlm.nih.gov/pubmed/15507609"Analysis of adaptive mutations in Kunjin virus replicon RNA reveals a novel role for the flavivirus nonstructural protein NS2A in inhibition of beta interferon promoter-driven transcription."] J. Virol. 78:12225–12235 | ||
4. Lindenbach DR, CM. 2007. [http://www.med.yale.edu/micropath/pdf/Lindenbach2007.pdf "Flaviviridae: the viruses and their replication."]Fields Virology, 5th Edition. pp 991–1041. | 4. Lindenbach DR, CM. 2007. [http://www.med.yale.edu/micropath/pdf/Lindenbach2007.pdf "Flaviviridae: the viruses and their replication."]Fields Virology, 5th Edition. pp 991–1041. | ||
5. Styer | 5. Styer L.M., Lim P., Louie K.L., Albright R.G., Krammer L.D., Bernard K.A. 2011. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3028906/ "Mosquito saliva causes enhancement of West Nile virus infection in mice."] J. Virol. 85:1517-1527. | ||
6.Schneider | 6.Schneider B.S., Soong L., Coffey L.L., Stevenson H.L., McGee C.E., Higgs, S. 2010. [http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0011704 "Aedes aegypti saliva alters leukocyte recruitment and cytokine signaling by antigen-presenting cells during West Nile virus infection."] PLoS ONE 5(7) | ||
7. Lim | 7. Lim S.M., Koraka P., Osterhaus A., Martina B. 2011. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3185772/ "West Nile Virus: Immunity and Pathogenesis."] Viruses 3(6). pp 811-828. | ||
8. Diamond MS, Sitati EM, Friend LD, Higgs S, Shrestha B, Engle M. 2003. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2194144/ "A Critical Role for Induced IgM in the Protection against West Nile Virus Infection".] J Exp Med. pp 1853–1862. | 8. Diamond MS, Sitati EM, Friend LD, Higgs S, Shrestha B, Engle M. 2003. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2194144/ "A Critical Role for Induced IgM in the Protection against West Nile Virus Infection".] J Exp Med. pp 1853–1862. | ||
| Line 62: | Line 64: | ||
9. Mukhopadhyay, S., Kim, B., Chipman, P., Rossmann, M., and Kuhn, R. 2003. [http://www.sciencemag.org/content/302/5643/248.short#ref-4 "Structure of West Nile Virus."] Science 302(5643). pp 248. | 9. Mukhopadhyay, S., Kim, B., Chipman, P., Rossmann, M., and Kuhn, R. 2003. [http://www.sciencemag.org/content/302/5643/248.short#ref-4 "Structure of West Nile Virus."] Science 302(5643). pp 248. | ||
10. Khromykh | 10. Khromykh A.A., Meka H., Guyatt K.J., Westaway E.G. 2001. [http://jvi.asm.org/content/75/14/6719.abstract?ijkey=380db7e0a2a5eac8f608958aeb9cccd687f926be&keytype2=tf_ipsecsha "Essential role of cyclization sequences in flavivirus RNA replication."] J. Virol. 75:6719–6728. | ||
11. Bazan J.F., Fletterick R.J. 1989. [http://www.ncbi.nlm.nih.gov/pubmed/2548336 "Detection of a trypsin-like serine proteasedomain in flaviviruses and pestiviruses."] Virology 1989;171:637–639 | |||
12. Koonin E.V. 1993. [http://www.ncbi.nlm.nih.gov/pubmed/8385698 "Computer-assisted identification of a putative methyltransferase domain in NS5 protein of flaviviruses and lambda 2 protein of reovirus."] J Gen Virol 1993;74(Pt 4):733–740. | |||
13. Davis C.W., Nguyen H., Hanna H.L., Sanchez M.D., Doms R.W., Pierson, T.C. 2006. [http://cmr.asm.org/cgi/ijlink?linkType=ABST&journalCode=jvi&resid=80/3/1290 "West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection."] J. Virol. 80:1290–1301. | |||
14. Modis Y., Ogata S., Clements D., Harrison S.C. 2004. [http://ry6af4uu9w.search.serialssolutions.com/?rft.jtitle=Nature;%20Physical%20Science%20(London)&rft.stitle=Nature;%20Physical%20Science%20(London)&rft.aulast=Modis&rft.auinit1=Y.&rft.volume=427&rft.issue=6972&rft.spage=313&rft.epage=319&rft.atitle=Structure%20of%20the%20dengue%20virus%20envelope%20protein%20after%20membrane%20fusion.&rft_id=info:doi/10.1038/nature02165&rft_id=info:pmid/14737159&rft.genre=article&rft_val_fmt=info:ofi/fmt:kev:mtx:journal&ctx_ver=Z39.88-2004&url_ver=Z39.88-2004&url_ctx_fmt=info:ofi/fmt:kev:mtx:ctx&rfr_id=info:sid/cmr.asm.org&ctx_tim=2013-04-18T04:41:37.81-07:00 "Structure of the dengue virus envelope protein after membrane fusion."] Nature 427:313–319. | |||
15. Girard Y.A., Popov V., Wen J., Han V., Higgs S. 2005. [http://www.bioone.org/doi/full/10.1603/0022-2585%282005%29042%5B0429%3AUSOWNV%5D2.0.CO%3B2 "Ultrastructural study of West Nile virus pathogenesis in Culex pipiens quinquefasciatus (Diptera: Culicidae)"]. J. Med. Entomol. 42:429–444. | |||
16. Kato N. 2008. [http://ry6af4uu9w.search.serialssolutions.com/?rft.jtitle=Vector%20borne%20and%20zoonotic%20diseases%20(Larchmont,%20N.Y.)&rft.stitle=Vector%20Borne%20Zoonotic%20Dis&rft.aulast=Kato&rft.auinit1=N.&rft.volume=8&rft.issue=5&rft.spage=701&rft.epage=712&rft.atitle=Evaluation%20of%20the%20function%20of%20a%20type%20I%20peritrophic%20matrix%20as%20a%20physical%20barrier%20for%20midgut%20epithelium%20invasion%20by%20mosquito-borne%20pathogens%20in%20Aedes%20aegypti.&rft_id=info:doi/10.1089/vbz.2007.0270&rft_id=info:pmid/18627241&rft.genre=article&rft_val_fmt=info:ofi/fmt:kev:mtx:journal&ctx_ver=Z39.88-2004&url_ver=Z39.88-2004&url_ctx_fmt=info:ofi/fmt:kev:mtx:ctx&rfr_id=info:sid/cmr.asm.org&ctx_tim=2013-04-18T05:34:00.007-07:00 "Evaluation of the function of a type I peritrophic matrix as a physical barrier for midgut epithelium invasion by mosquito-borne pathogens in Aedes aegypti."] Vector Borne Zoonotic Dis. 8:701–712. | |||
17. Styer L.M., Kent K.A., Albright R.G., Bennett C.J., Krammer L.D., Bernard, K.A. 2007. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1976553/ "Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts."] PLoS Pathog. 3:1262–1270. | |||
18. Schneider B.S., Soong L., Coffey L.L., Stevenson H.L., McGee C.E., Higgs, S. 2010. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2908538/ "Aedes aegypti saliva alters leukocyte recruitment and cytokine signaling by antigen-presenting cells during West Nile virus infection."] PLoS One 5:e11704. doi:10.1371/journal.pone.0011704. | |||
19. Lim P.Y., Behr M.J., Chadwick C.M., Shi P.Y., Bernard K.A. [http://www.ncbi.nlm.nih.gov/pubmed/21367890/ "Keratinocytes are cell targets of West Nile virus in vivo"] J Virol. 2011 May; 85(10):5197-201. | |||
20. Rios M., Zhang M.J., Grinev A., Srinivasan K., Daniel S., Wood O., Hewlett I.K., Dayton A.I. [http://www.ncbi.nlm.nih.gov/pubmed/16584445/ "Monocytes-macrophages are a potential target in human infection with West Nile virus through blood transfusion."] Transfusion. 2006;46:659–667. | |||
21. Centers for Disease Control and Prevention. 2011. [http://www.cdc.gov/ncidod/dvbid/westnile/background.htm "West Nile Virus."] retrieved april 28 2013. | |||
Latest revision as of 19:33, 28 August 2013
Introduction
The West Nile Virus (WNV) is a neurotropic human pathogen. This virus belongs to the flavivirus genus which is in the Flaviviridae family along with the dengue virus and the yellow fever virus. The West Nile Virus is a mosquito-borne virus and affects many other animals besides humans, such as birds, reptiles and other mammals. The WNV is mainly found in tropical and temperate regions around the world and was first discovered in Uganda in 1937[21]. The first major outbreak of the disease occurred in 1994 in Algeria and it finally hit the western hemisphere in New York in 1999 [21]. Many humans who do become infected with the disease are subclinical where they do not show any symptoms however transmission of the WNV can result in the West Nile Fever. The West Nile Fever, not always, but can affect the central nervous system and lead to a neurological disease.
Structure and Function
RNA and Proteins
The West Nile Virus is and enveloped virion that contains a positive single stranded genome. It is in Group IV ((+)ssRNA). The genome is about 11 kb of a single open reading frame [10]. Through the use of cryoelectron microscopy it has been discovered that the virus has icosahedral symmetry and is ∼500 Å in diameter. The virus has no surface projections or spikes [9]. The virus has multi-layer organization where the outermost layer corresponds to the E and M transmembrane proteins with a very high density. The core nucleocapsid consists of copies of the genome RNA and the capsid proteins [9]. The viral RNA is translated to produce 3 structural and 7 nonstructural proteins. The structural proteins are capsid, envelope, and premembrane proteins and the nonstructural proteins include NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5. The structural proteins are encoded at the 5’ end of the RNA strand and serve the function of virus entry and encapsulation of the genome [10]. The nonstructural proteins serve multiple functions due to the limited number of proteins present in the genome. NS1 does not have an exact role in viron assembly however it has been speculated to help in the replication process [2]. NS2A, NS2B, NS4A, and NS4B inhibit the inate immune response against viral infection in the body [3]. NS3 is the protease that cleaves other nonstructural proteins from the polyprotein and encodes enzyme activities [11]. The NS5 protein is essential for viral replication due to the fact that it is the polymerase that encodes a methltransferase [12].
Life Cycle
The West Nile Virus enters the cell through receptor-mediated endocytosis. DC-SIGNR is a major receptor for this form of endocytosis [13]. Once the virus is internalized in the cell, the virus matures as the pH drops to become slightly acidic within the envelope. Once the endosome has matured the envelope protein undergoes conformational changes where the endocytic membrane fuses with the viral lipid membrane. This causes the release of the viral RNA genome into the cytoplasm of the cell [14]. Once released into the cell the single RNA is replicated and translated into proteins. The viral RNA is replicated by both cellular and viral proteins. The 3 structural proteins expressed from the viral RNA assemble onto the membranes in the endoplasmic reticulum and bud into the cytoplasm by the Golgi network. The virus exits the cell in a lipid envelope through exocytosis when cellular enzymes cleave the prM [4].
Pathogenesis
Vectors
Mosquitoes are the main vectors of the West Nile virus. They acquire the virus after ingesting the blood from a viremic animal. The virus infects the cells of the mosquito by replicating in the cells of the midgut while the blood is being digested[15]. The mosquitoes are not affected by the virus due to various types of chitin and proteins present in the midgut as well as acquiring a high tolerance for the virus. The midgut contains a peritrophic matrix which reduces pathogen invasion to the epithelial cells[16]. Once replicated, the virus travels through the hemolymph to the salivary glands. Given enough time this will lead to a high level of viremia in the saliva of the mosquito, which can then be transmitted to other organisms when they are feeding [15].
Host Reservoirs
Many different types of mosquito species can carry the West Nile Virus. The most common mosquito vector of the United States is C. tarsali .[1]. Mosquitoes feed on a variety of organisms which makes it quite easy to spread the disease. Birds are the most common host reservoir since many species can have the disease yet show no signs or symptoms. The West Nile virus is maintained in the environment through this constant cycle between animal hosts and mosquitoes. Birds are also a prime target as a host reservoir because they can carry high levels of viremia in their blood whereas humans cannot and are considered a dead end hosts [1].
Transmission to Host Organism
The West Nile virus is transmitted from the mosquito to the host organism when the mosquito begins the probing process of blood feeding. During the probing process the proboscis of the mosquito injects the skin of the organism with pharmacological active saliva proteins to locate the blood source. In a study performed by Styler (2011), it was shown that when the West Nile virus is transmitted by mosquitoes, the viremia of the host organism is significantly higher than when the virus is transmitted with only a syringe. This suggests that mosquito saliva plays an important role in enhancing the transmission and survival of the West Nile virus in host organisms [6]. Since there is such a great amount of selective pressure upon mosquito saliva from the host immune system it is likely that the West Nile virus co-evolved in order to use saliva proteins to its advantage. More research needs to be done on what exact proteins are responsible for the successful transmittance of the virus [1].
Saliva from mosquitoes effectively decreases immune response from the host organism. When the West Nile virus is transmitted with mosquito saliva there is a reduction in T cells recruited to the inoculation site compared to when the virus is transmitted alone. The saliva dis-regulates antigen-presenting cells signaling which decreases the amount of T cells recruited. With a lower amount of T cells it is more difficult for the host organism to destroy the virus [18].
The level of viremia is dependent upon how the virus is transmitted to the host organism; extravascularly or intravascularly. Mosquitoes inoculate lower doses of the virus directly into the blood while feeding compared to when the saliva does not come into contact with the organisms blood. Directly inducting the virus into the blood stream may have an affect on viral tropism. When immediately introduced to the blood there is an earlier development of viremia as well as higher rates of infecting other co-feeding mosquitoes [17].
Immune Mechanism
When the West Nile virus is initially transmitted from a mosquito to another animal the virus initially infects the keratinocytes and Langerhans cells. These cells then migrate to the lymph nodes and initial replication begins [19]. From the lymph nodes the virus is then spread to visceral organs and a second replication occurs [20]. If viremia levels become high enough the virus can cross the blood-brain barrier and cause meningoencephalitis. The envelope glycoprotein of the West Nile virus has been shown to be very influential is the success of the virus to enter the central nervous system. This is due to the domain III of the protein which acts as the receptor binding domain [7].
The elderly and individuals who are immune compromised are more susceptible to contracting the West Nile virus developing fatal encephalitis. It is speculated that decreased amounts of immunoglobulin M (IgM), CD4+, and CD8+ T cells are the cause of this. In one study it was found that viremia levels were lower in mice that were inducted with a neutralizing IgM response early in the infection of the West Nile virus [8]. This leads researchers to believe that the IgM molecule plays a large role in recruiting antibodies to limit viremia levels and decreases the likelihood of the virus spreading to the central nervous system [8].
Conclusion
The West Nile Virus is a is a neurotropic human pathogen that consists of a positive single stranded RNA genome. The virus is primarily transmitted via mosquitoes. Mosquitoes become infected with the virus by host organisms while feeding. Once viremia levels are high enough in the saliva of the infected mosquito it can then transmit the virus to another organism. Mosquito saliva not only plays a role in transmitting the virus, but it also suppresses the immunity of the targeted organism, allowing for the virus to successfully replicate in the new environment.
There is no vaccine for this virus suitable for humans. There is a vaccine that can be given to horses however it has not been tested in humans. Scientists are currently researching this topic for a cure to this virus. The Centers for Disease Control and Prevention (CDC) are taking precautions and various steps to control this virus. They are developing faster ways to detect and diagnose WNV in addition to developing and improving mosquito prevention and control programs [21].
References
1. Colpitts, Tonya M. 2012. "West Nile Virus: Biology, Transmission, and Human Infection." Clinical microbiology reviews 25.pp 635-648
2. Westaway E.G., Mackenzie J.M., Khromykh A.A. 2002. "Replication and gene function in Kunjin virus." Current Topics in Microbiology and Immunology Volume 267, 2002, pp 323-351.
3. Liu W.J., Chen H.B., Wang X.J., Huang H., Khromykh A.A. 2004. "Analysis of adaptive mutations in Kunjin virus replicon RNA reveals a novel role for the flavivirus nonstructural protein NS2A in inhibition of beta interferon promoter-driven transcription." J. Virol. 78:12225–12235
4. Lindenbach DR, CM. 2007. "Flaviviridae: the viruses and their replication."Fields Virology, 5th Edition. pp 991–1041.
5. Styer L.M., Lim P., Louie K.L., Albright R.G., Krammer L.D., Bernard K.A. 2011. "Mosquito saliva causes enhancement of West Nile virus infection in mice." J. Virol. 85:1517-1527.
6.Schneider B.S., Soong L., Coffey L.L., Stevenson H.L., McGee C.E., Higgs, S. 2010. "Aedes aegypti saliva alters leukocyte recruitment and cytokine signaling by antigen-presenting cells during West Nile virus infection." PLoS ONE 5(7)
7. Lim S.M., Koraka P., Osterhaus A., Martina B. 2011. "West Nile Virus: Immunity and Pathogenesis." Viruses 3(6). pp 811-828.
8. Diamond MS, Sitati EM, Friend LD, Higgs S, Shrestha B, Engle M. 2003. "A Critical Role for Induced IgM in the Protection against West Nile Virus Infection". J Exp Med. pp 1853–1862.
9. Mukhopadhyay, S., Kim, B., Chipman, P., Rossmann, M., and Kuhn, R. 2003. "Structure of West Nile Virus." Science 302(5643). pp 248.
10. Khromykh A.A., Meka H., Guyatt K.J., Westaway E.G. 2001. "Essential role of cyclization sequences in flavivirus RNA replication." J. Virol. 75:6719–6728.
11. Bazan J.F., Fletterick R.J. 1989. "Detection of a trypsin-like serine proteasedomain in flaviviruses and pestiviruses." Virology 1989;171:637–639
12. Koonin E.V. 1993. "Computer-assisted identification of a putative methyltransferase domain in NS5 protein of flaviviruses and lambda 2 protein of reovirus." J Gen Virol 1993;74(Pt 4):733–740.
13. Davis C.W., Nguyen H., Hanna H.L., Sanchez M.D., Doms R.W., Pierson, T.C. 2006. "West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection." J. Virol. 80:1290–1301.
14. Modis Y., Ogata S., Clements D., Harrison S.C. 2004. "Structure of the dengue virus envelope protein after membrane fusion." Nature 427:313–319.
15. Girard Y.A., Popov V., Wen J., Han V., Higgs S. 2005. "Ultrastructural study of West Nile virus pathogenesis in Culex pipiens quinquefasciatus (Diptera: Culicidae)". J. Med. Entomol. 42:429–444.
16. Kato N. 2008. "Evaluation of the function of a type I peritrophic matrix as a physical barrier for midgut epithelium invasion by mosquito-borne pathogens in Aedes aegypti." Vector Borne Zoonotic Dis. 8:701–712.
17. Styer L.M., Kent K.A., Albright R.G., Bennett C.J., Krammer L.D., Bernard, K.A. 2007. "Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts." PLoS Pathog. 3:1262–1270.
18. Schneider B.S., Soong L., Coffey L.L., Stevenson H.L., McGee C.E., Higgs, S. 2010. "Aedes aegypti saliva alters leukocyte recruitment and cytokine signaling by antigen-presenting cells during West Nile virus infection." PLoS One 5:e11704. doi:10.1371/journal.pone.0011704.
19. Lim P.Y., Behr M.J., Chadwick C.M., Shi P.Y., Bernard K.A. "Keratinocytes are cell targets of West Nile virus in vivo" J Virol. 2011 May; 85(10):5197-201.
20. Rios M., Zhang M.J., Grinev A., Srinivasan K., Daniel S., Wood O., Hewlett I.K., Dayton A.I. "Monocytes-macrophages are a potential target in human infection with West Nile virus through blood transfusion." Transfusion. 2006;46:659–667.
21. Centers for Disease Control and Prevention. 2011. "West Nile Virus." retrieved april 28 2013.