Bacillus thuringiensis toxin in C. elegans: Difference between revisions
Adrianowyczs (talk | contribs) No edit summary |
No edit summary |
||
| (114 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
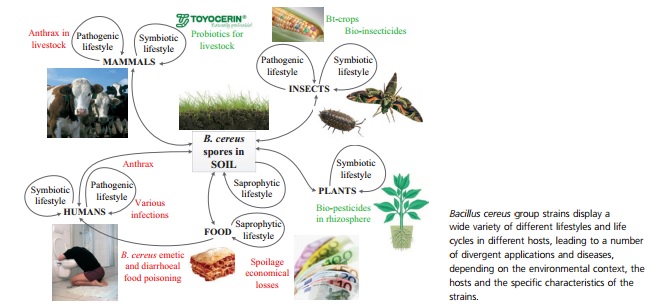
<br>By Sarah Adrianowycz<br><br><br>[[Image:Cueppens2013_1.jpg |thumb|664px|right| Figure 1: Graphical depiction of the dissimilar lifestyles and symbiosis of the <i>Bacillus ceres</i> group, with emphasis on their common soil habitat. <br><http://onlinelibrary.wiley.com/doi/10.1111/1574-6941.12110/pdf/>]] | |||
==<i>Bacillus thuringiensis</i> as an organism== | |||
<i>Bacillus thuringiensis</i> abounds in the soil, and is a Gram–positive, spore-forming member of the <i>Bacillus ceres</i> group. The <i>Bacillus ceres</i> group is a collection of seven genetically similar species, three of which are so alike that they are occasionally considered the same organism, except for their vastly different pathogenic outcomes and lifestyles, some of which are depicted in Figure 1 (Kho et al. 2011). This differing pathogenic ability, or external expression of the organism’s phenotype, is often used as a marker of species distinctness. In the case of <i>B. thuringiensis</i> the organism is typified by the production of specific invertebrate related toxins (Ceuppens et al., 2013). The issue with using phenotypic differences to distinguish among organisms is that the differences frequently stem from virulence factors contained on plasmids, which are extremely variable and transient genetic elements. Plasmids are independent from the primary genome and can be exchanged through horizontal gene transmission, lost in the environment, or selected against during laboratory culturing. Despite the issues with denoting separate species based upon their ability to cause disease, <i>B. thuringiensis</i> is recognized for the ability to form crystal proteins. These proteins are insecticidal in nature and are coded by <i>cry</i> genes (Ceuppens et al.). In the wild, most strains of <i>B. thuringiensis</i> are capable of producing anywhere from 1-4 types of Cry proteins (Kho et al., 2011). The ability to produce multiple <i>cry</i> genes may be an evolutionary advantage for this bacteria. Multiple <i>cry</i> gene expression results in a synergistic effect in taking over a host, as the proteins are more successful when acting together because different hosts have differing levels of susceptibility to individual Cry proteins (E. Schnephf, 1998).<br> | |||
<br>The ability to produce Cry proteins gives <i>B. thuringiensis</i> value as an insecticide and has made it especially interesting to humans who are investigating the proteins' biological applications. These toxins have been utilized both in direct application on crops and as a source of transgenic elements that provide resistance against the orders Lepidoptera, Diptera, and Coleoptera (E. Schnepf, 1998) when incorporated into the genomes of genetically modified organisms. The method of action within this subset of proteins makes them especially effective against insects, but has yet to produce evidence of negative effects against humans or higher trophic level organisms. Since 1996 genetically modified potatoes, cotton, and corn with versions of <i>cry</i> genes have been commercially available to growers and their utilization has spread. Because of these perceived benefits as an insecticide and the lack of obvious toxicity to "beneficial" insects or humans, the utilization of plants that produce Cry proteins has become common place (Schnepf). | |||
==Presence of <i>cry</i> Genes in <i>Bacillus thuringiensis</i>== | |||
of | The genome of <i>B. thuringiensis</i> has between 2.4 – 5.7 million base pairs and is supplemented extensively by plasmids, both linear and circular in nature. The larger varieties of these additional genetic elements are responsible for encoding the crystals released during sporulation (Schnepf). | ||
<br><br>The <i>cry</i> genes are expressed during the stationary phase of <i>B. thuringiensis</i>’s life cycle, and to such an extent that 20-30% of the dry weight of the organism’s spore is made up of the resulting crystals. The regulation of crystal formation and its relationship with the stationary phase are monitored at three levels of regulation: transcriptional, posttranscriptional, and posttranslational. However, not all <i>cry</i> genes are dependent upon the process of spore formation (Schnepf). The geometric structure of the resulting crystals differs based upon the type of Cry protein being expressed, ranging from bipyramidal to spherical, although certain Cry proteins require accessory proteins to assemble into their correct crystal structure (Schnepf). | |||
<br><br>The crystals produced by <i>cry</i> genes are made up of potential toxins that become active only when consumed by a susceptible organism. Susceptible organisms are those that have alkaline conditions in a portion of their digestive system, as is the case for the midgut of insects and the intestines of nematodes. Once the crystal has entered the alkali conditions, the toxin can function after being made soluble, being cleaved by enzymes involved in protein catabolism, and then being inserted into the membrane of the epithelium. Once inserted into the membrane the Cry proteins are able to form pores (Schnepf). The success of various Cry proteins can partially be explained by differences in solubility. In the laboratory, insects have been found to evolve resistance to Cry proteins by preventing the Cry protein from entering solution in their midgut, either through changes in midgut pH or synthesis of mutated enzymes (McGaughey and Whalon, 1992). Once processing is completed, Cry proteins are capable of two main functions, the ability to bind to specific receptors on the target organism and to function as an ion channel. Two methods of pore function have been suggested, one where the pore allows non-specific ion transport and another where the controlled transport of specific ions results in undermining of the membrane potential that is necessary for the cell’s functioning (Schnepf). There is debate over the selectivity of the pore because the wide range of experimental conditions used to test Cry proteins have yielded conflicting results. The pore's perceived inconsistency may be evidence of the pore responding to differing environmental conditions instead of improper scientific experimentation (Schnepf). | |||
<br><br>There have been recent attempts to systematically alter the structure of Cry proteins to increase their insecticidal nature. Although limiting solubility in the midgut is the evolutionary means suggested by which insects decrease their susceptibility to the toxins, human efforts have been focused on increasing the amount of irreversible binding, a process typically associated with insertion of the toxin into the epithelial cells of the organism (Schnepf). | |||
<br> | |||
== | ==The Relationship between <i>B. thuringiensis</i> Cry Proteins and Nematodes== | ||
<i>B. thuringiensis</i> is widespread in the soil and allocates a large amount of energy and resources to the production of Cry proteins. These crystal proteins need to be consumed to be activated, making this allocation of resources counter-intuitive, because most of the research involving Cry proteins and insects has been on insects that spend minimal time feeding directly from the soil (Wei et al., 2003). | |||
< | <br><br> | ||
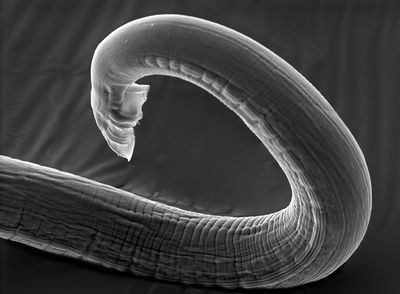
[[Image:C.elegansMale.jpg |thumb|400px|right| Figure 2: Longitudinal view of an adult male <i>C. elegans</i> tail. Image obtained through SEM by Carolyn Marks and David Hall of the Hall Lab. <br><http://www.wormimage.org/image.php?id=320396&page=1/>]] | |||
In contrast, nematodes, specifically <i>Caenorhabditis elegans</i>, pictured at left in Figure 2, inhabit the soil under typical conditions. They are a compelling model organism because they are likely to encounter Cry proteins and other pathogens in their natural habitat and they represent one of over 100,000 species of nematodes that directly consume soil and subsist partially on <i>B. thuringiensis</i> (Wei et al., 2003). This similarity of habitat and the frequent interaction among these organisms means <i>C. elegans</i> may be one of the intended targets for Cry proteins. Nematodes have a number of responses to deal with environmental threats, among them immune responses, although these are costly to initiate. Another less energetically and metabolically expensive option is to supplement immune responses with behavioral changes. By utilizing numerous forms of anti-toxin response, <i>C. elegans</i> an express different degrees of adaption in a rapidly changing environment (Luo et al.,2013). | |||
<br><br> | |||
Although the impact of Cry proteins has been widely studied in insects that harm agricultural products and directly influence human livelihood, less research has been done on the nematode model. It is important to determine the impact of these toxins on <i>C. elegans</i>. Studies are necessary to determine if Cry proteins are toxic to <i>C. elegans</i>, by what method these proteins are toxic, how the two organisms might have coevolved, and the potential for larger environmental impacts if this soil inhabitant is impacted by proteins that have found widespread application in industrial agriculture (Wei et al., 2003). Because of the inclusion of <i>cry</i> genes within staple food crops, nematodes, including <i>C. elegans</i>, have had increasing contact with this pathogen in what were previously stable environments. The typical habitat of <i>C. elegans</i> contained some Cry proteins as a result of <i>B. thuringiensis</i>, but at lower levels than currently. | |||
<br><br> | |||
< | |||
< | |||
== | ==Structure of Cry5B== | ||
To date, 200 types of Cry proteins have been identified, and they range in genetic similarity from less than 20% to more than 90% (Hui et al., 2012). Cry5B is the most researched Cry protein with negative impacts on nematodes, and this toxin functions through binding to specific glycolipid receptors in the worm’s intestines (Hui et al. 2012). Although the sequence similarity between Cry5B and the Cry proteins that are most effective against insects is limited to approximately 20%, the proteins share a similar three component structure. The toxins that are effective in insects that have so far been sequenced have a high degree of structural similarity, but the average difference between Cry5B and this group is even greater than for the most dissimilar insecticidal toxin, Cry2Aa (Hui et al., 2012). | |||
<br><br> | <br><br> | ||
The first domain of Cry5B is believed to be the active mechanism for pore formation, and because of this vital functional role it has the largest degree of similarity to other Cry proteins, which are all believed to function through pore formation (Hui et al., 2012). Although there is only 22% sequence similarity, once the complex is activated by the proteases in the nematode intestine the activated toxin becomes similar to that of Cry4B (Hui et al., 2012). The first domain is made up of five helices grouped together with one focal helix that serves as the initiator for pore formation. The central helix has been identified in all Cry proteins that have so far been structurally investigated (Hui et al., 2012). | |||
<br><br> | <br><br> | ||
Domain two is the most structurally independent of the three components of Cry5B, and has greater structural similarity to a banana lectin than to other Cry proteins because of its homodimer structure. Because of this structural difference from other Cry proteins but the similarity to a manose binding structure, Cry5B is able to accomplish glycan receptor binding that the other Cry proteins can not (Hui et al., 2012). | |||
<br><br> | |||
Domain three is conserved among Cry proteins. Currently, all of the Cry proteins structurally analyzed show marked similarity to molecules capable of binding carbohydrates in this domain, among which some of the mechanisms are well studied (Hui et al., 2012). | |||
== | ==Impact of Cry5B on <i>Caenorhabditis elegans</i>== | ||
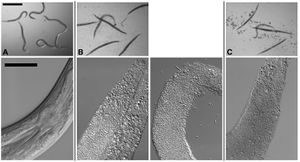
Cry5B, when produced by <i>E. coli</i> and fed directly to <i>C. elegans</i> results in the death of the nematodes after approximately six days, at a lethal dose of approximately 8µg/ml due to Cry protein intoxication, with no infection by the bacteria. In contrast, when Cry5B is provided for nematodes at a similar concentration but in the presence of <i>B. thuringiensis</i>, death results in 24-48 hours. The bacteria take control, resulting in what the authors refer to as a “Bob” phenotype, or Bag of bacteria, where the internal organs of the nematode are digested and replaced by a combination of bacterial cells and spores surrounded by the cuticle of <i>C. elegans</i> (Kho et al., 2011). Bacterial infection requires both the presence of this pore forming protein (PFP) and the bacterial source, although it does not matter if the Cry protein is produced by the bacteria or only available for uptake in the environment. Additionally, <i>C. elegans</i> cultured in the presence of <i>B. thuringiensis</i> without Cry5B does not result in infection. The “Bob” phenotype can be seen in the accompanying image, Figure 3, as represented in Panel B where the infected nematodes are completely linear, devoid of any internal structures, and lacking their typical color. (Kho et al., 2011). This experiment also showed, in Panel C, that <i>B. anthracis</i>, which does not express Cry proteins, is also able to infect <i>C. elegans</i> in the presence of Cry5B and <i>B. thuringiensis</i>. The researchers utilized <i>B. thuringiensis</i> that produced Cry5B crystals and <i>B. anthracis</i> that expressed green-fluorescent proteins (GFP) in order to evaluate the level of infection in <i>C. elegans</i> by each organism. When <i>B. thuringiensis</i> and <i>B. anthracis</i> spores were combined in a ratio of 16 to 1 and presented to <i>C. elegans</i>, the result was that in some instances the <i>B. anthracis</i> could gain control and infect the nematode, despite the higher concentration and preferential conditions for <i>B. thuringiensis</i> (Kho et al.). | |||
[[Image:Kho2001_1.jpg|thumb|300px|left|Figure 3: Infection of <i>C. elegans</i> by <i>B. thuringiensis</i> and <i>B. anthracis</i>. Top row: Dissecting microscope view of nematodes cultured under various | |||
conditions. Scale bar is 500 mm. Bottom row: Compound microscope view of nematodes cultured under various conditions with anterior of the worm in top right and scale bar is 50 mm. (A) <i>C. elegans</i> cultured in a well with <i>B. thuringiensis</i> without Cry5B. Top row: None of the six nematodes are infected. Bottom row: The internal structures of <i>C. elegans</i> fed <i>B. thuringiensis</i> without Cry5B are all intact. (B) <i>C. elegans</i> cultured in a well with <i>B. thuringiensis</i> and Cry5B. Top row: Five of the six worms are completely infected (rigid, lack of internal structures and normal coloration). Bottom row: Infected animals show complete digestion of internal structures by the bacteria. Vegetative and sporulated bacteria can be seen. (C) Similar images as in (B) except <i>Bacillus anthracis</i> cultured with the nematodes. <http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0029122#pone-0029122-g001/>]] | |||
A subsequent experiment determined that the Cry5B protein is only able to cause death in <i>C. elegans</i> when binding occurs between the toxin and a subset of receptors in the organism’s intestinal track. In mutant nematodes that were devoid of glycolipid receptors, the toxin was incapable of forming a pore, and as a result <i>B. thuringiensis</i> was unable to cause an infection, supporting the hypothesis that the establishment of the pore is a vital virulence factor that permits infection by <i>B. thuringiensis</i> (Kho et al., 2011). | |||
<br><br> | <br><br> | ||
The | Although <i>C. elegans</i> death is almost certain within 48 hours of exposure due to takeover by <i>B. thuringiensis</i> when the Cry protein is also present, in as few as fifteen minutes high level of death can be observed. The authors designed a pulse-chase experiment where juvenile <i>C. elegans</i> were exposed to <i>B. thuringiensis</i> and Cry5B for varying amounts of time. There is no statistical difference in the percentage of <i>C. elegans</i> death for any time points tested between 15 minutes and 8 hours of exposure (15 min, 30 min, 1 hr, 2 hr, 4 hr, or 8 hr), even when organisms were subsequently washed of the excess Cry protein and then immersed in <i>B. megaterium</i> for 48 hours to remove the remaining bacteria and crystal toxins (Kho et al., 2011). | ||
==Means of resistance to Cry5B in <i>Caenorhabditis elegans</i>== | |||
In order to understand the means through which some <i>C. elegans</i> individuals are able to remain resistant to Cry proteins, Huffman et al. selected for mutants that were not killed in the presence of Cry5B and determined that surviving nematodes were defective in <i>bre-1</i> through <i>bre-5</i> functioning (2004). The <i>bre</i> (an abbreviation for Br-toxin resistant) mutants are unable to integrate the pore forming protein Cry5B into the epithelium of the intestine, possibly because the <i>bre</i> genes encode a receptor that permits the uptake of Cry5B, or possibly because the genes influence the level of binding that occurs in the nematode’s intestine. Either way, the result is limited binding of the protein and limited pore formation which results in survival of the organism (Huffman et al., 2004). | |||
==Impact of Cry6A on <i>Caenorhabditis elegans</i>== | |||
Although there has been extensive study of the method of Cry5B’s toxicity in nematodes, until the work of Luo et al., there had been little research into the impact of the structurally distinct Cry protein, Cry6A. Cry6A is a significant departure from other related toxins because it is genetically different, lacking the five conserved sequences that are present in other identified <i>B. thuringiensis</i> Cry proteins. The dramatic genetic difference suggests that Cry6A might act though a mechanism distinct from that previously described for Cry5B, as genetic differences are hypothesized to cause structural differences, although the structure of this protein has not yet been crystallized (Luo et al., 2013). | |||
<br><br> | <br><br> | ||
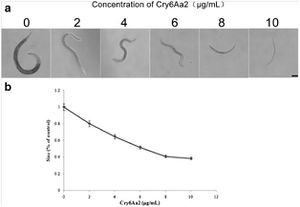
Cry6A severely negatively impacts the typical functioning of <i>C. elegans</i>, impinging growth, stunting brood size, altering movement, and resulting in behavioral changes in an attempt to limit exposure and ingestion of the toxin. Luo et al. found the media lethal dose of Cry6A to be 18.499 μg/mL, a value on par with the previously published value for Cry5B. The similarity in median lethal does speaks to the novel Cry protein’s toxicity in <i>C. elegans</i>. Upon determining this value, the authors sought the sub-lethal impacts of Cry6A and discovered that first stage larvae raised in serially diluted Cry6Aa2 were significantly smaller than those raised in control buffer, with the growth reduced by fifty percent at 6.345 μg/mL, as depicted in Figure 4 (Luo et al.). Size comparisons of the worms were made utilizing photographs of the treated nematodes at x100 magnification, and included analysis of 20 or more individuals per treatment concentration. | |||
[[Image:Luo2013_1.jpg|thumb|300px|right|Figure 4: Growth assay of L1 larvae of <i>C. elegans</i> with Cry6Aa2 toxin. a Microscope views of worms cultured in gradient doses of Cry6Aa2 toxin. Scale bar is 100 μm. b The size percentages of worms cultured in a serial dose of Cry6Aa2 toxin to worms cultured in the absence of Cry6Aa2 toxin. Data represent the average of 20 measurements for each toxin concentration. | |||
Error bars denote standard deviation. <http://link.springer.com/article/10.1007%2Fs00253-013-5249-3/fulltext.html/>]] | |||
The authors also discovered that <i>C. elegans</i> exhibits a number of behavioral adaptations in an attempt to minimize its exposure to Cry6A. In increasing toxin concentration <i>C. elegans</i> decreases ingestion rate, as measured by contraction of the pharynx grinder over the course of 30 seconds. Measured decreases in ingestion were substantial, and over the course of an hour the number of contractions decreased from a baseline value of 41.6 , to 27.2 contractions at a concentration of 21.25 μg/mL, and 9.9 contractions at a Cry protein concentration of 340 μg/mL (Luo et al.). An interesting component of this experiment was that results were measured for both 1 and 6 total hours of exposure, and the overall rate of consumption was higher at every concentration for the longer time interval (Luo et al.). A possible reason for this increase could be that the organism is only capable of evading a toxin using this method alone for a limited amount of time and eventually is forced to return to the baseline level of consumption to sustain itself, even when toxins are present. | |||
<br><br> | <br><br> | ||
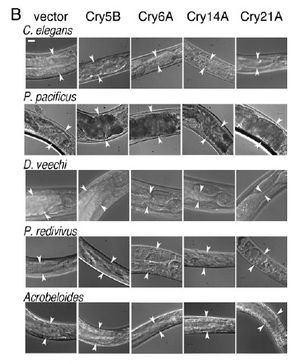
Another study sought to determine the susceptibility of numerous nematode species to a range of Cry proteins, including those from both the Cry5 and Cry6 subfamilies. The Cry5 subfamily is made up of six toxins, all of which have four of the five gene sequences present in other studied <i>B. thuringiensis</i> proteins. The genetic similarity of the proteins indicates their linked ancestry and their relatedness to Cry1A, Cry3A, and Cry4A which are significant insecticides utilized by industrial agriculture (Wie et al.). In contrast, the Cry6 subfamily is made of two proteins which have marked similarity to one another but lack the five conserved sequences the other proteins share (Wie et al.). The work of Wie et al. support the hypothesis that Cry toxins work through the intestines of nematodes, which is similar to the pore formation mechanism in insects. The researchers found correlation between the results of the plate assay, where organisms were fed Cry toxins produced by plated <i>E. coli</i>, and the altered appearance of the nematode’s intestine. When organisms were found dead or intoxicated as a result of the specific Cry protein, they were also discovered to have severely impacted digestive systems. Some of the intestinal changes as a result of the toxin were smaller intestinal cells, pulling in of the intestine away from the rest of the body, and general disturbed appearance as can be seen in Figure 5 (Wie et al.).The gut morphology of <i>C. elegans</i> was negatively impacted by all of the toxins tested, one of which was Cry6A. | |||
[[Image:Wei2003_1.jpeg|thumb|300px|right|Figure 5: Photographs | |||
of the anterior intestine of nematodes fed the four toxic crystal proteins in E. coli. Arrowheads delineate the width of the intestine at position near the | |||
anterior. (Bar 5 20 mm.)<http://www.pnas.org/content/100/5/2760.long/>]] | |||
== | ==Genetic response of <i>Caenorhabditis elegans</i> to <i>Bacillus thuringiensis</i>== | ||
There has been genomic level research utilizing the exceptionally pathogenic strain <i> Bacillus thuringiensis</i> DB27. This strain, which was isolated from a dung beetle, kills <i>C. elegans</i> within 16 hours (Sinha et al.). <i>C. elegans</i> reared on culture of <i>B. thuringiensis</i> DB27 dies within a day, in contrast to nematode survival on three other pathogens, which take at least 2-5 days to cause death(Sinha et al., 2012). <i>C. elegans</i> exhibits pathogen and species specific levels of gene regulation in response to <i>B. thuringiensis</i> DB27. These genetic responses are rapid enough where measurement at the four hour time point allows observation of a substantial transcriptional response (Sinha et al.). At the simplest level, the amount of gene expression is greatest for the bacteria that pose the greatest threat to the host organism. <i>C. elegans</i> was found to express a genetic response in 5688 genes, 97% of which mark an upregulation of gene expression, in contrast to the 249 total genes impacted by exposure to <i>S. aureus</i> (Sinha et al.). Sinha et al. also found that the genomic response of <i>C. elegans</i> to <i>B. thuringiensis</i> DB27 was distinct from the genomic response to another Gram-positive bacterium, <i>S. aureus</i>, with altered expression appearing exclusively for <i>B. thuringiensis</i> in 97 genes. | |||
<br><br> | <br><br> | ||
Some of the gene varieties with increased expression after exposure to <i>B. thuringiensis</I> are those that influence the number of proteins with lipid processing abilities and those with similar structures. Lipases are frequently a major component of <i>C. elegans</I>’s response to pathogens (Sinha et al., 2012). There is also increased gene expression that is non-specific to <i>B. thuringiensis</i>, and that is conserved among bacterial threats, including upregulation of regions involved with both protein processing and ATPase functioning (Sinha et al.) | |||
< | |||
== | ==Conclusion== | ||
It was first hypothesized that because Cry proteins function through a number of complex mechanisms that insects would be unable to adapt to these novel environmental challenges. However, because Cry proteins have so many mechanisms of action, insects can mutate in a number of ways to increase survival (McGaughey and Whalon 1992). The result is an arms race between industrial agriculture and the insects that prey on staple crops that drives synthesis of Cry proteins with increasing toxicity. This enhanced toxicity may temporarily result in the death of pests, but their presence in the environment can also harm other organisms that happen to encounter the Cry proteins. The importance of studying the impact of <i> Bacillus thuringiensis </i> and its toxins on native soil communities should not be underestimated. <i> C. elegans </i> is one of the many organisms necessary for aeration and proper functioning of the soil, and changes in toxin concentration, intentional or unintentional, deserve proper research as they could seriously hamper the survival of this nematode and have unforeseen environmental impacts. | |||
== | ==References== | ||
== | [http://onlinelibrary.wiley.com/doi/10.1111/1574-6941.12110/pdf Ceuppens, Siele, Nico Boon, and Mieke Uyttendaele. "Diversity of Bacillus cereus group strains is reflected in their broad range of pathogenicity and diverse ecological lifestyles." FEMS microbiology ecology 84.3 (2013): 433-450.] | ||
<br><br>[http://ac.els-cdn.com/S1438422104702003/1-s2.0-S1438422104702003-main.pdf?_tid=3000d772-cb0c-11e3-ac92-00000aab0f27&acdnat=1398274075_ef625d4780bb8631243f41b67f0cd5ec Huffman, Danielle L., et al. "Pore worms: Using< i> Caenorhabditis elegans</i> to study how bacterial toxins interact with their target host." International Journal of Medical Microbiology 293.7 (2004): 599-607.] | |||
<br><br> | |||
[http://pubs.acs.org/doi/pdf/10.1021/bi301386q Hui, Fan, et al. "Structure and glycolipid binding properties of the nematicidal protein Cry5B." Biochemistry 51.49 (2012): 9911-9921.] | |||
<br><br> | |||
[http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0029122#pone-0029122-g005 Kho, Melanie F., et al. "The pore-forming protein Cry5B elicits the pathogenicity of Bacillus sp. against Caenorhabditis elegans." PloS one 6.12 (2011): e29122.] | |||
<br><br> | |||
[http://link.springer.com/article/10.1007/s00253-013-5249-3/fulltext.html Luo, Hui, et al. "The effects of Bacillus thuringiensis Cry6A on the survival, growth, reproduction, locomotion, and behavioral response of Caenorhabditis elegans." Applied microbiology and biotechnology 97.23 (2013): 10135-10142.] | |||
<br><br>[http://www.sciencemag.org/content/258/5087/1451.full.pdf McGaughey, William H., and Mark E. Whalon. "Managing insect resistance to Bacillus thuringiensis toxins." Science 258.5087 (1992): 1451-1455.] | |||
<br><br> | |||
[http://mmbr.asm.org/content/62/3/775.full Schnepf, E., et al. "Bacillus thuringiensis and its pesticidal crystal proteins." Microbiology and molecular biology reviews 62.3 (1998): 775-806.] | |||
<br><br> | |||
[http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0044255#pone-0044255-g005 Sinha, Amit, et al. "System wide analysis of the evolution of innate immunity in the nematode model species Caenorhabditis elegans and Pristionchus pacificus." PloS one 7.9 (2012): e44255.] | |||
<br><br> | |||
[http://www.pnas.org/content/100/5/2760.full.pdf+html Wei, Jun-Zhi, et al. "Bacillus thuringiensis crystal proteins that target nematodes." Proceedings of the National Academy of Sciences 100.5 (2003): 2760-2765.] | |||
Latest revision as of 17:59, 29 September 2015
By Sarah Adrianowycz
<http://onlinelibrary.wiley.com/doi/10.1111/1574-6941.12110/pdf/>
Bacillus thuringiensis as an organism
Bacillus thuringiensis abounds in the soil, and is a Gram–positive, spore-forming member of the Bacillus ceres group. The Bacillus ceres group is a collection of seven genetically similar species, three of which are so alike that they are occasionally considered the same organism, except for their vastly different pathogenic outcomes and lifestyles, some of which are depicted in Figure 1 (Kho et al. 2011). This differing pathogenic ability, or external expression of the organism’s phenotype, is often used as a marker of species distinctness. In the case of B. thuringiensis the organism is typified by the production of specific invertebrate related toxins (Ceuppens et al., 2013). The issue with using phenotypic differences to distinguish among organisms is that the differences frequently stem from virulence factors contained on plasmids, which are extremely variable and transient genetic elements. Plasmids are independent from the primary genome and can be exchanged through horizontal gene transmission, lost in the environment, or selected against during laboratory culturing. Despite the issues with denoting separate species based upon their ability to cause disease, B. thuringiensis is recognized for the ability to form crystal proteins. These proteins are insecticidal in nature and are coded by cry genes (Ceuppens et al.). In the wild, most strains of B. thuringiensis are capable of producing anywhere from 1-4 types of Cry proteins (Kho et al., 2011). The ability to produce multiple cry genes may be an evolutionary advantage for this bacteria. Multiple cry gene expression results in a synergistic effect in taking over a host, as the proteins are more successful when acting together because different hosts have differing levels of susceptibility to individual Cry proteins (E. Schnephf, 1998).
The ability to produce Cry proteins gives B. thuringiensis value as an insecticide and has made it especially interesting to humans who are investigating the proteins' biological applications. These toxins have been utilized both in direct application on crops and as a source of transgenic elements that provide resistance against the orders Lepidoptera, Diptera, and Coleoptera (E. Schnepf, 1998) when incorporated into the genomes of genetically modified organisms. The method of action within this subset of proteins makes them especially effective against insects, but has yet to produce evidence of negative effects against humans or higher trophic level organisms. Since 1996 genetically modified potatoes, cotton, and corn with versions of cry genes have been commercially available to growers and their utilization has spread. Because of these perceived benefits as an insecticide and the lack of obvious toxicity to "beneficial" insects or humans, the utilization of plants that produce Cry proteins has become common place (Schnepf).
Presence of cry Genes in Bacillus thuringiensis
The genome of B. thuringiensis has between 2.4 – 5.7 million base pairs and is supplemented extensively by plasmids, both linear and circular in nature. The larger varieties of these additional genetic elements are responsible for encoding the crystals released during sporulation (Schnepf).
The cry genes are expressed during the stationary phase of B. thuringiensis’s life cycle, and to such an extent that 20-30% of the dry weight of the organism’s spore is made up of the resulting crystals. The regulation of crystal formation and its relationship with the stationary phase are monitored at three levels of regulation: transcriptional, posttranscriptional, and posttranslational. However, not all cry genes are dependent upon the process of spore formation (Schnepf). The geometric structure of the resulting crystals differs based upon the type of Cry protein being expressed, ranging from bipyramidal to spherical, although certain Cry proteins require accessory proteins to assemble into their correct crystal structure (Schnepf).
The crystals produced by cry genes are made up of potential toxins that become active only when consumed by a susceptible organism. Susceptible organisms are those that have alkaline conditions in a portion of their digestive system, as is the case for the midgut of insects and the intestines of nematodes. Once the crystal has entered the alkali conditions, the toxin can function after being made soluble, being cleaved by enzymes involved in protein catabolism, and then being inserted into the membrane of the epithelium. Once inserted into the membrane the Cry proteins are able to form pores (Schnepf). The success of various Cry proteins can partially be explained by differences in solubility. In the laboratory, insects have been found to evolve resistance to Cry proteins by preventing the Cry protein from entering solution in their midgut, either through changes in midgut pH or synthesis of mutated enzymes (McGaughey and Whalon, 1992). Once processing is completed, Cry proteins are capable of two main functions, the ability to bind to specific receptors on the target organism and to function as an ion channel. Two methods of pore function have been suggested, one where the pore allows non-specific ion transport and another where the controlled transport of specific ions results in undermining of the membrane potential that is necessary for the cell’s functioning (Schnepf). There is debate over the selectivity of the pore because the wide range of experimental conditions used to test Cry proteins have yielded conflicting results. The pore's perceived inconsistency may be evidence of the pore responding to differing environmental conditions instead of improper scientific experimentation (Schnepf).
There have been recent attempts to systematically alter the structure of Cry proteins to increase their insecticidal nature. Although limiting solubility in the midgut is the evolutionary means suggested by which insects decrease their susceptibility to the toxins, human efforts have been focused on increasing the amount of irreversible binding, a process typically associated with insertion of the toxin into the epithelial cells of the organism (Schnepf).
The Relationship between B. thuringiensis Cry Proteins and Nematodes
B. thuringiensis is widespread in the soil and allocates a large amount of energy and resources to the production of Cry proteins. These crystal proteins need to be consumed to be activated, making this allocation of resources counter-intuitive, because most of the research involving Cry proteins and insects has been on insects that spend minimal time feeding directly from the soil (Wei et al., 2003).
<http://www.wormimage.org/image.php?id=320396&page=1/>
In contrast, nematodes, specifically Caenorhabditis elegans, pictured at left in Figure 2, inhabit the soil under typical conditions. They are a compelling model organism because they are likely to encounter Cry proteins and other pathogens in their natural habitat and they represent one of over 100,000 species of nematodes that directly consume soil and subsist partially on B. thuringiensis (Wei et al., 2003). This similarity of habitat and the frequent interaction among these organisms means C. elegans may be one of the intended targets for Cry proteins. Nematodes have a number of responses to deal with environmental threats, among them immune responses, although these are costly to initiate. Another less energetically and metabolically expensive option is to supplement immune responses with behavioral changes. By utilizing numerous forms of anti-toxin response, C. elegans an express different degrees of adaption in a rapidly changing environment (Luo et al.,2013).
Although the impact of Cry proteins has been widely studied in insects that harm agricultural products and directly influence human livelihood, less research has been done on the nematode model. It is important to determine the impact of these toxins on C. elegans. Studies are necessary to determine if Cry proteins are toxic to C. elegans, by what method these proteins are toxic, how the two organisms might have coevolved, and the potential for larger environmental impacts if this soil inhabitant is impacted by proteins that have found widespread application in industrial agriculture (Wei et al., 2003). Because of the inclusion of cry genes within staple food crops, nematodes, including C. elegans, have had increasing contact with this pathogen in what were previously stable environments. The typical habitat of C. elegans contained some Cry proteins as a result of B. thuringiensis, but at lower levels than currently.
Structure of Cry5B
To date, 200 types of Cry proteins have been identified, and they range in genetic similarity from less than 20% to more than 90% (Hui et al., 2012). Cry5B is the most researched Cry protein with negative impacts on nematodes, and this toxin functions through binding to specific glycolipid receptors in the worm’s intestines (Hui et al. 2012). Although the sequence similarity between Cry5B and the Cry proteins that are most effective against insects is limited to approximately 20%, the proteins share a similar three component structure. The toxins that are effective in insects that have so far been sequenced have a high degree of structural similarity, but the average difference between Cry5B and this group is even greater than for the most dissimilar insecticidal toxin, Cry2Aa (Hui et al., 2012).
The first domain of Cry5B is believed to be the active mechanism for pore formation, and because of this vital functional role it has the largest degree of similarity to other Cry proteins, which are all believed to function through pore formation (Hui et al., 2012). Although there is only 22% sequence similarity, once the complex is activated by the proteases in the nematode intestine the activated toxin becomes similar to that of Cry4B (Hui et al., 2012). The first domain is made up of five helices grouped together with one focal helix that serves as the initiator for pore formation. The central helix has been identified in all Cry proteins that have so far been structurally investigated (Hui et al., 2012).
Domain two is the most structurally independent of the three components of Cry5B, and has greater structural similarity to a banana lectin than to other Cry proteins because of its homodimer structure. Because of this structural difference from other Cry proteins but the similarity to a manose binding structure, Cry5B is able to accomplish glycan receptor binding that the other Cry proteins can not (Hui et al., 2012).
Domain three is conserved among Cry proteins. Currently, all of the Cry proteins structurally analyzed show marked similarity to molecules capable of binding carbohydrates in this domain, among which some of the mechanisms are well studied (Hui et al., 2012).
Impact of Cry5B on Caenorhabditis elegans
Cry5B, when produced by E. coli and fed directly to C. elegans results in the death of the nematodes after approximately six days, at a lethal dose of approximately 8µg/ml due to Cry protein intoxication, with no infection by the bacteria. In contrast, when Cry5B is provided for nematodes at a similar concentration but in the presence of B. thuringiensis, death results in 24-48 hours. The bacteria take control, resulting in what the authors refer to as a “Bob” phenotype, or Bag of bacteria, where the internal organs of the nematode are digested and replaced by a combination of bacterial cells and spores surrounded by the cuticle of C. elegans (Kho et al., 2011). Bacterial infection requires both the presence of this pore forming protein (PFP) and the bacterial source, although it does not matter if the Cry protein is produced by the bacteria or only available for uptake in the environment. Additionally, C. elegans cultured in the presence of B. thuringiensis without Cry5B does not result in infection. The “Bob” phenotype can be seen in the accompanying image, Figure 3, as represented in Panel B where the infected nematodes are completely linear, devoid of any internal structures, and lacking their typical color. (Kho et al., 2011). This experiment also showed, in Panel C, that B. anthracis, which does not express Cry proteins, is also able to infect C. elegans in the presence of Cry5B and B. thuringiensis. The researchers utilized B. thuringiensis that produced Cry5B crystals and B. anthracis that expressed green-fluorescent proteins (GFP) in order to evaluate the level of infection in C. elegans by each organism. When B. thuringiensis and B. anthracis spores were combined in a ratio of 16 to 1 and presented to C. elegans, the result was that in some instances the B. anthracis could gain control and infect the nematode, despite the higher concentration and preferential conditions for B. thuringiensis (Kho et al.).
A subsequent experiment determined that the Cry5B protein is only able to cause death in C. elegans when binding occurs between the toxin and a subset of receptors in the organism’s intestinal track. In mutant nematodes that were devoid of glycolipid receptors, the toxin was incapable of forming a pore, and as a result B. thuringiensis was unable to cause an infection, supporting the hypothesis that the establishment of the pore is a vital virulence factor that permits infection by B. thuringiensis (Kho et al., 2011).
Although C. elegans death is almost certain within 48 hours of exposure due to takeover by B. thuringiensis when the Cry protein is also present, in as few as fifteen minutes high level of death can be observed. The authors designed a pulse-chase experiment where juvenile C. elegans were exposed to B. thuringiensis and Cry5B for varying amounts of time. There is no statistical difference in the percentage of C. elegans death for any time points tested between 15 minutes and 8 hours of exposure (15 min, 30 min, 1 hr, 2 hr, 4 hr, or 8 hr), even when organisms were subsequently washed of the excess Cry protein and then immersed in B. megaterium for 48 hours to remove the remaining bacteria and crystal toxins (Kho et al., 2011).
Means of resistance to Cry5B in Caenorhabditis elegans
In order to understand the means through which some C. elegans individuals are able to remain resistant to Cry proteins, Huffman et al. selected for mutants that were not killed in the presence of Cry5B and determined that surviving nematodes were defective in bre-1 through bre-5 functioning (2004). The bre (an abbreviation for Br-toxin resistant) mutants are unable to integrate the pore forming protein Cry5B into the epithelium of the intestine, possibly because the bre genes encode a receptor that permits the uptake of Cry5B, or possibly because the genes influence the level of binding that occurs in the nematode’s intestine. Either way, the result is limited binding of the protein and limited pore formation which results in survival of the organism (Huffman et al., 2004).
Impact of Cry6A on Caenorhabditis elegans
Although there has been extensive study of the method of Cry5B’s toxicity in nematodes, until the work of Luo et al., there had been little research into the impact of the structurally distinct Cry protein, Cry6A. Cry6A is a significant departure from other related toxins because it is genetically different, lacking the five conserved sequences that are present in other identified B. thuringiensis Cry proteins. The dramatic genetic difference suggests that Cry6A might act though a mechanism distinct from that previously described for Cry5B, as genetic differences are hypothesized to cause structural differences, although the structure of this protein has not yet been crystallized (Luo et al., 2013).
Cry6A severely negatively impacts the typical functioning of C. elegans, impinging growth, stunting brood size, altering movement, and resulting in behavioral changes in an attempt to limit exposure and ingestion of the toxin. Luo et al. found the media lethal dose of Cry6A to be 18.499 μg/mL, a value on par with the previously published value for Cry5B. The similarity in median lethal does speaks to the novel Cry protein’s toxicity in C. elegans. Upon determining this value, the authors sought the sub-lethal impacts of Cry6A and discovered that first stage larvae raised in serially diluted Cry6Aa2 were significantly smaller than those raised in control buffer, with the growth reduced by fifty percent at 6.345 μg/mL, as depicted in Figure 4 (Luo et al.). Size comparisons of the worms were made utilizing photographs of the treated nematodes at x100 magnification, and included analysis of 20 or more individuals per treatment concentration.
The authors also discovered that C. elegans exhibits a number of behavioral adaptations in an attempt to minimize its exposure to Cry6A. In increasing toxin concentration C. elegans decreases ingestion rate, as measured by contraction of the pharynx grinder over the course of 30 seconds. Measured decreases in ingestion were substantial, and over the course of an hour the number of contractions decreased from a baseline value of 41.6 , to 27.2 contractions at a concentration of 21.25 μg/mL, and 9.9 contractions at a Cry protein concentration of 340 μg/mL (Luo et al.). An interesting component of this experiment was that results were measured for both 1 and 6 total hours of exposure, and the overall rate of consumption was higher at every concentration for the longer time interval (Luo et al.). A possible reason for this increase could be that the organism is only capable of evading a toxin using this method alone for a limited amount of time and eventually is forced to return to the baseline level of consumption to sustain itself, even when toxins are present.
Another study sought to determine the susceptibility of numerous nematode species to a range of Cry proteins, including those from both the Cry5 and Cry6 subfamilies. The Cry5 subfamily is made up of six toxins, all of which have four of the five gene sequences present in other studied B. thuringiensis proteins. The genetic similarity of the proteins indicates their linked ancestry and their relatedness to Cry1A, Cry3A, and Cry4A which are significant insecticides utilized by industrial agriculture (Wie et al.). In contrast, the Cry6 subfamily is made of two proteins which have marked similarity to one another but lack the five conserved sequences the other proteins share (Wie et al.). The work of Wie et al. support the hypothesis that Cry toxins work through the intestines of nematodes, which is similar to the pore formation mechanism in insects. The researchers found correlation between the results of the plate assay, where organisms were fed Cry toxins produced by plated E. coli, and the altered appearance of the nematode’s intestine. When organisms were found dead or intoxicated as a result of the specific Cry protein, they were also discovered to have severely impacted digestive systems. Some of the intestinal changes as a result of the toxin were smaller intestinal cells, pulling in of the intestine away from the rest of the body, and general disturbed appearance as can be seen in Figure 5 (Wie et al.).The gut morphology of C. elegans was negatively impacted by all of the toxins tested, one of which was Cry6A.
Genetic response of Caenorhabditis elegans to Bacillus thuringiensis
There has been genomic level research utilizing the exceptionally pathogenic strain Bacillus thuringiensis DB27. This strain, which was isolated from a dung beetle, kills C. elegans within 16 hours (Sinha et al.). C. elegans reared on culture of B. thuringiensis DB27 dies within a day, in contrast to nematode survival on three other pathogens, which take at least 2-5 days to cause death(Sinha et al., 2012). C. elegans exhibits pathogen and species specific levels of gene regulation in response to B. thuringiensis DB27. These genetic responses are rapid enough where measurement at the four hour time point allows observation of a substantial transcriptional response (Sinha et al.). At the simplest level, the amount of gene expression is greatest for the bacteria that pose the greatest threat to the host organism. C. elegans was found to express a genetic response in 5688 genes, 97% of which mark an upregulation of gene expression, in contrast to the 249 total genes impacted by exposure to S. aureus (Sinha et al.). Sinha et al. also found that the genomic response of C. elegans to B. thuringiensis DB27 was distinct from the genomic response to another Gram-positive bacterium, S. aureus, with altered expression appearing exclusively for B. thuringiensis in 97 genes.
Some of the gene varieties with increased expression after exposure to B. thuringiensis are those that influence the number of proteins with lipid processing abilities and those with similar structures. Lipases are frequently a major component of C. elegans’s response to pathogens (Sinha et al., 2012). There is also increased gene expression that is non-specific to B. thuringiensis, and that is conserved among bacterial threats, including upregulation of regions involved with both protein processing and ATPase functioning (Sinha et al.)
Conclusion
It was first hypothesized that because Cry proteins function through a number of complex mechanisms that insects would be unable to adapt to these novel environmental challenges. However, because Cry proteins have so many mechanisms of action, insects can mutate in a number of ways to increase survival (McGaughey and Whalon 1992). The result is an arms race between industrial agriculture and the insects that prey on staple crops that drives synthesis of Cry proteins with increasing toxicity. This enhanced toxicity may temporarily result in the death of pests, but their presence in the environment can also harm other organisms that happen to encounter the Cry proteins. The importance of studying the impact of Bacillus thuringiensis and its toxins on native soil communities should not be underestimated. C. elegans is one of the many organisms necessary for aeration and proper functioning of the soil, and changes in toxin concentration, intentional or unintentional, deserve proper research as they could seriously hamper the survival of this nematode and have unforeseen environmental impacts.
References
Ceuppens, Siele, Nico Boon, and Mieke Uyttendaele. "Diversity of Bacillus cereus group strains is reflected in their broad range of pathogenicity and diverse ecological lifestyles." FEMS microbiology ecology 84.3 (2013): 433-450.
Huffman, Danielle L., et al. "Pore worms: Using< i> Caenorhabditis elegans to study how bacterial toxins interact with their target host." International Journal of Medical Microbiology 293.7 (2004): 599-607.
Hui, Fan, et al. "Structure and glycolipid binding properties of the nematicidal protein Cry5B." Biochemistry 51.49 (2012): 9911-9921.
Kho, Melanie F., et al. "The pore-forming protein Cry5B elicits the pathogenicity of Bacillus sp. against Caenorhabditis elegans." PloS one 6.12 (2011): e29122.
Luo, Hui, et al. "The effects of Bacillus thuringiensis Cry6A on the survival, growth, reproduction, locomotion, and behavioral response of Caenorhabditis elegans." Applied microbiology and biotechnology 97.23 (2013): 10135-10142.
McGaughey, William H., and Mark E. Whalon. "Managing insect resistance to Bacillus thuringiensis toxins." Science 258.5087 (1992): 1451-1455.
Schnepf, E., et al. "Bacillus thuringiensis and its pesticidal crystal proteins." Microbiology and molecular biology reviews 62.3 (1998): 775-806.
Sinha, Amit, et al. "System wide analysis of the evolution of innate immunity in the nematode model species Caenorhabditis elegans and Pristionchus pacificus." PloS one 7.9 (2012): e44255.
Wei, Jun-Zhi, et al. "Bacillus thuringiensis crystal proteins that target nematodes." Proceedings of the National Academy of Sciences 100.5 (2003): 2760-2765.
