Fusobacterium nucleatum: Difference between revisions
No edit summary |
|||
| (62 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | |||
{{Biorealm Genus}} | {{Biorealm Genus}} | ||
| Line 5: | Line 6: | ||
===Higher order taxa=== | ===Higher order taxa=== | ||
Bacteria; Fusobacteria; Fusobacteria (class); ''Fusobacteriaceae; Fusobacterales; Fusobacterium'' | |||
===Genus=== | ===Genus=== | ||
| Line 20: | Line 21: | ||
==Description and significance== | ==Description and significance== | ||
[[Image:Fnuclea1.JPG|frame|right| Gram-negative stained culture of ''F. nucleatum.'' Image Courtesy of J. Michael Miller, Ph.D.,(D)ABMM of National Center for Zoonotic, Vector-borne, and Enteric Diseases. Picture submitted by him to [http://www.asm.org American Society for Microbiology]]] | |||
''Fusobacterium nucleatum'' is a bacterium that is commonly found in the dental plaque of humans and is frequently associated with gum disease. It is a key component of periodontal plaque due to its abundance and its ability to coaggregate with other species in the oral cavity. The cells of ''F. nucleatum'' are fusiform rods or spindle-shaped of many different lengths. In fact, the name refers to the organism as a small spindle-shaped rod. ''F. nucleatum'' is found in the dental plaque of many primates, thus includes man. This bacteria has been shown to play a central role in dental plaque formation and other diseases like gingivitis. This is due to its ability to adhere to a wide range of both Gram-positive and Gram-negative plaque microorganisms, such as ''Porphyromonas gingivalis''. ''F. nucleatum'' is very much associated with periodontitis, along with invasive human infections of the head and neck, chest, lung, liver and abdomen. Due to its adherence ability, it can be associated with viruses, which adhere to host tissue cells as an invasion and modulate the host's immune response.(1) | |||
''Fusobacterium nucleatum'' | The pathogenic potential of ''Fusobacterium nucleatum'' and its significance in the development of periodontal diseases, as well as in infections in other organs, have gained new interest for several reasons. First, this bacterium has a very high chance to be pathogenic because of its high frequency in periodontal lesions, its production of irritants that affect the tissue, its ability to coaggregate and form mutual synergisms with other bacteria in mixed infections, and its ability to form numerous aggregates with other suspected pathogens in periodontal disease(therefore, it acts as a bridge between early and late colonizers on surfaces of teeth). Second, ''F. nucleatum '' is very common in clinical infections of other body sites. Third, recent new techniques have made it possible to obtain more information about ''F. nucleatum'' on the genetic level, thereby also gaining better knowledge of the structure and functions of the outer membrane proteins(OMPs), which are of great interest with respect to coaggregation, cell nutrition, and antibiotic susceptibility.(2) | ||
==Genome structure== | |||
''Fusobacterium nucleatum'' is a Gram-negative bacterium that does not create spores and is not motile. This bacterium has a G-C content of about 27 to 28 mol%. Its genome size is about 2.4 x 10^6 base pairs (bp). All in all, colony morphology is not a consistent parameter of ''F. nucleatum''. Therefore, it is not sufficient for species identification.(2) | |||
Through phylogenetic grouping through analysis of the 16s rRNA sequences, ''F. nucleatum'' was found to be closely related to ''Bacteroides'' and the flavobacteria. Similarities have been found with ''F. nucleatum'' and the other two species with regards to its DNA and its antigenic composition. In addition, ''F. nucleatum'' is found to exhibit high levels of homology with ''F. alocis, F. periodonticum, and F. simiae''. All these organisms, along with ''F. nucleatum'', colonize in oral cavities.(2) | |||
'' | Native plasmids have been identified in strains of ''F. nucleatum''. Using one of the native plasmid pFN1, a ''F. nucleatum - E. coli'' shuttle vector has been developed.(1) | ||
== | ==Cell structure and metabolism== | ||
This organism is possesses an outer membrane, which is very much expected from a Gram-negative bacterium. In addition, the bacterium also has a periplasmic space made of the peptidoglycan layers, in-between the inner and outer cytoplasmic membranes. The inner-membrane is made of a symmetrical phospholipids bilayer with proteins and phospholipids in equal amounts. The outer-membrane, on the other hand, is an asymmetric membrane containing phospholipids, lipopolysaccharides (LPS), lipoproteins, and proteins. It functions as a molecular sieve. The LPS contains 3-deoxy-D-manno-octulosonic acid. Due to the large amount of proteins present in the outer-membrane, it was found that a third of the weight of this bacterium is due to the proteins present in the outer-membrane.(2) | |||
'' | In addition, with its numerous numbers of proteins on the outer cell surface, the bacterium could be found having specific interactions with various complementary structures on the host cell surface. This adherence is mediated by the protein known as adhesin. This adherence is very important in the colonization and establishment of the infection in a susceptible host. In general, adherence is very important to this organisms’ pathogenicity. ''F. nucleatum'' plays an important role in adhesion and coaggregation reactions found in periodontal pockets. LPS extracts from ''F. nucleatum'' helps alongside adhesin to provide adhesion to the saliva-filled environment. All in all, this characteristic is very important to the survival and susceptibility of the ''F. nucleatum'' since fusobacteria adhere very poorly to human cheek epithelial cells. With all these in mind, it is obvious to why research of the bacterium’s adherence proteins: Outer Membrane Proteins (OMP) is currently being conducted.(2) | ||
Due to its non-motile nature, it has been found that ''F. nucleatum'' does not possess pili or flagella. It does, however, possess a mucopolysaccharide capsule surrounding the organism of variable thickness, which may be very important to the organism’s pathogenic capabilities.(2) | |||
This bacterium is an anaerobic creature that grows in an environment with only up to 6% oxygen saturation, and also requires a media containing Trypticase, peptone, or yeast extract. This organism’s ability to produce butyric acid as a major product of fermentation of glucose and peptone is what differentiates Fusobacterium species from other Gram-negative, non-sporing rod-shaped bacterium. ''F. nucleatum'' is one of the few non-sporulating anaerobic species that utilize amino acid catabolism to provide energy. This organism also uses glutamate, histidine, and aspartate. Apparently, ''F. nucleatum'' does not use glucose as its main energy source. Available data indicate that glucose instead is used for the biosynthesis of intracellular molecules and not energy metabolism. This unusual characteristic has been the focus of interest of several studies.(2) | |||
==Ecology== | ==Ecology== | ||
''Fusobacterium nucleatum'' inhabits the mucous environment of an animal oral cavity, serving as a pathogen. It is a predominant member of the human oral flora. Because of its pathogenic and parasitic nature, ''Fusobacterium nucleatum'' does not affect the environment directly. However, it may alter the ecosystem by its effects on the population of infected host animals. This bacterium has a significant impact on the ecology of the oral cavity due to its ability to adhere to many different microbial species and itself. ''F. nucleatum'' is a major component of subgingival plaque.(2) | |||
==Pathology== | ==Pathology== | ||
''Fusobacterium nucleatum'' is an oral bacterium, which means it can only be found in the mouth cavity of mammals, mainly humans. It is generally found in the dental plaque of humans and is frequently associated with various gum diseases. ''Fusobacterium nucleatum'' is not, however, considered a major dental pathogen on its own. ''F. nucleatum'' also has the ability to adhere to and degrade basement membranes in vivo and bind to type 4 collagen. Due to its coaggregation ability (ability to adhere with other plaque organisms, such as ''Porphyromonas gingivalis''), ''F. nucleatum'' could contribute to the development to other diseases such as periodontitis as well as invasive human infections of the head and neck, chest, abdomen, and liver.(2) | |||
Out of all periodontal species that are statistically related with periodontal disease, it is the most common in clinical infections found in other body sites. Some bodily infections that this oral bacterium affects include tropical skin ulcers, peritonsillar abscesses, pyomyositis and septic arthritis, bacteremia and liver abscesses, intrauterine infections, bacterial vaginosis, urinary tract infections, pericarditis and endocarditis, and lung and pleuropulmonary infections. Coincidentally, it has been more frequently found in a child’s body.(1) | |||
''F. nucleatum'' is known to have the potential to be a periodontal pathogen by using the production of toxic metabolites. These toxic components have the ability to kill or arrest the proliferation of the normal nearby cells of the periodontium (the fibroblasts). The formation of sulfides by ''F. nucleatum'' may provide a way for the bacteria to avoid the host immune system. Butyrate (in the form of the tissue irritant butyric acid), propionate, and ammonium ions, which are produced by ''F. nucleatum'', inhibit proliferation of human gingival fibroblasts. In addition, it may have the ability to penetrate the gingival epithelium, and are present in elevated levels in plaque associated with periodontitis. Therefore, they may have a very important role in producing oral diseases, such as gingivitis. The effects of the toxins is not fatal to the cells, but the inhibition of fibroblast proliferation is severe because the potential for rapid wound healing is compromised. ''F. nucleatum'' also possesses major OMPs that may be important for virulence.(2) | |||
==Application to Biotechnology== | ==Application to Biotechnology== | ||
Aminopeptidase is nutritionally very important for ''Fusobacterium nucleatum''. This peptidase is found to be cell-associated by isolating the culture from disrupted chemostat-grown cells. The enzyme was inactivated by chelators, bestatin, ''p''hydroxymercuribenzoate and some heavy metals. Aminopeptidase, therefore, appears to be a cobalt-activated metallo-peptidase. Together with other peptidases, Aminopeptidase would be vital to the growth and survival, in the subgingival environment of the mouth, of ''F. nucleatum''.(5) | |||
A ''Fusobacterium nucleatum'' shuttle plasmid, pHS17, capable of transforming ''E. coli'' and ''F. nucleatum'' ATCC10953, was constructed with pFN1. Shuttle plasmid pHS17 was stably maintained in the ''F. nucleatum'' transformants. The differences in the transformation efficiencies suggested the presence of a restriction-modification system in ''Fusobacterium nucleatum''.(4) | |||
''Fusobacterium nucleatum ATCC23726'' is one type of ''Fusobacterium nucleatum'' that is still having its full assembly in progress.(1) | |||
==Current Research== | ==Current Research== | ||
Here are some of the more current research regarding ''F. nucleatum'': | |||
"Identification and analysis of fipA, a ''Fusobacterium nucleatum'' immunosuppressive factor gene" – Extracts of ''Fusobacterium nucleatum'' FDC 364 were capable of inhibiting human T-cell responses to mitogens and antigens. The purified ''F. nucleatum'' immunosuppressive protein (FIP) inhibits T-cell activation by stopping or arresting cells in the middle of the G1 phase of the cell cycle. FIP impairs the expression of the cell nuclear antigen during proliferation. One of the FIP component, 44 kDa, has a FipA polypeptide, from the fipA gene, that is sufficient in mediating the immunosuppressive activities of the host protein complex.(6) | |||
"Effect of ''Fusobacterium nucleatum'' on the T and B cell responses to ''Porphyromonas gingivalis'' in a mouse model" – Mice were used in this experiment. The host mice injected with ''P. gingivalis'' followed by ''F. nucleatum'' produced equal levels of both anti-''P. gingivalis'' and anti-''F. nucleatum'' antibodies. It was observed that ''F. nucleatum'' immunization does not affect the splenic T cell cytokine response to ''P. gingivalis''. However, ''F. nucleatum'' immunization prior to that of ''P. gingivalis'' inhibited the production of anti-''P. gingivalis'' antibodies. ''P. gingivalis'' injection before ''F. nucleatum'' demonstrated a partial inhibitory effect by ''P. gingivalis'' on antibody production to ''F. nucleatum''. These results suggest that ''P. gingivalis '' and ''F. nucleatum'' do not allow the production of cross-reactive antibodies to other similar oral microorganisms. Ultimately, it shows that human periodontal diseases are very hard to determine its main cause.(7) | |||
"Enhanced pathogenicity of ''Fusobacterium nucleatum'' adapted to oxidative stress" – In this research, characterization of ''F. nucleatum''’s response to oxidative stress is observed by studying its cellular morphology and pathogenicity. This would allow the understanding of how this anaerobic bacterium survives during an invasive process of oxygenated tissues in the host oral cavities. Once again, mice were used to conduct this experiment. A wild-strain of ''F. nucleatum'' and an aero-strain were injected into the mice. Mice with aero-strain showed drastic changes in cellular morphology compared to the wild-strain mice. Also, these aero-strain mice showed hyperemia, an increased number of inflammatory cells, and steatosis in the liver. The results showed that the adaptation to oxidative stress might influence the pathogenicity of ''F. nucleatum''. This is a big deal since most hosts that would be exposed to ''F. nucleatum'' contain cells with oxidative characteristics.(8) | |||
"''Fusobacterium nucleatum'' pericarditis" – ''F. nucleatum'' pericarditis was found in a the chest of a 49-year old man, who was suspected to be affected by mycobacteria. Antituberculosis drugs were used on him thinking that mycobacteria were the cause. However, it was the work of ''F. nucleatum''. This finding gives the realization that entry of ''F. nucleatum'' through oropharyngeal portal is the cause of diseases such as this. This is one of the first earlier findings that ''F. nucleatum'' could also affect other bodily cavities, besides the mouth.(9) | |||
==References== | ==References== | ||
[ | 1. [http://www.hgsc.bcm.tmc.edu/projects/microbial/microbial-index.xsp HGSC at Baylor College of Medicine] | ||
2. [http://cmr.asm.org/cgi/reprint/9/1/55 Bolstad, A.I., Jensen, H.B., Bakken, V. “Taxonomy, Biology, and Periodontal Aspects of ''Fusobacterium nucleatum''.” Clinical of Microbiology Reviews. Jan. 1996. pp. 55-71.] | |||
3. [http://www.pubmedcentral.nih.gov/ PubMed Central] | |||
4. [http://jb.asm.org/cgi/reprint/182/4/1176.pdf Haake, S.A., Yoder, S.C., Attarian, G., Podkaminer, K. “Native Plasmids of ''Fusobacterium nucleatum'': Characterization and Use in Development of Genetic Systems.” Journal of Bacteriology. Feb. 2000. pp. 1176-1180.] | |||
5. [http://mic.sgmjournals.org/cgi/reprint/144/7/1807.pdf Rogers, A.H., Gunadi, A., Gully, N.J., Zilm, P.S. “An aminopeptidase nutritionally important to ''Fusobacterium nucleatum''.” Microbiology. 1998. pp. 1807-1813.] | |||
6. [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=173923 Demuth, D.R., Savary, R., Golub, E., Shenker, B.J. “Identification and analysis of fipA, a ''Fusobacterium nucleatum'' immunosuppressive factor gene.” Infect Immun. April 1996. 1335-1341.] | |||
7. [http://www.ingentaconnect.com/content/bsc/cei/2002/00000128/00000002/art00006 Gemmell, E., Bird, P.S., Carter, C.L., Drysdale, K.E., Seymour, G.J. “Effect of ''Fusobacterium nucleatum'' on the T and B cell responses to ''Porphyromonas gingivalis'' in a mouse model.” Clinical & Experimental Immunology, Vol. 128, Number 2. May 2002, pp. 238-244] | |||
8. [http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=16125361&dopt=Abstract Silva, V.L., Diniz C.G., Cara D.C., Santos, S.G., Nicoli J.R., Carvalho, M.A., Farias, L.M. “Enhanced pathogenicity of ''Fusobacterium nucleatum'' adapted to oxidative stress.” Microbial Pathogenisis. Oct. 2005. pp. 131-138.] | |||
9. [http://jcm.asm.org/cgi/reprint/17/2/349.pdf Truant A.L., Menge, S., Milliorn, K., Lairscey, R., Kelly, M.T. “''Fusobacterium nucleatum'' pericarditis.” Journal of Clinical Microbiology. Feb. 1983. pp. 349-351.] | |||
Edited by Jason Homan, student of [mailto:ralarsen@ucsd.edu Rachel Larsen] and Kit Pogliano | Edited by Jason Homan, student of [mailto:ralarsen@ucsd.edu Rachel Larsen] and Kit Pogliano | ||
Latest revision as of 15:36, 2 June 2011
A Microbial Biorealm page on the genus Fusobacterium nucleatum
Classification
Higher order taxa
Bacteria; Fusobacteria; Fusobacteria (class); Fusobacteriaceae; Fusobacterales; Fusobacterium
Genus
Fusobacterium
|
NCBI: Taxonomy |
Description and significance
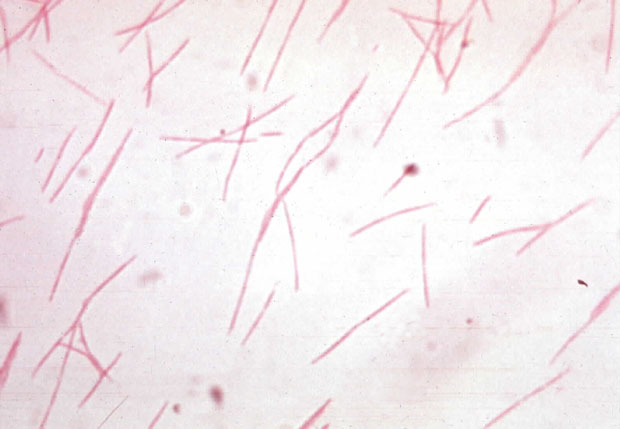
Fusobacterium nucleatum is a bacterium that is commonly found in the dental plaque of humans and is frequently associated with gum disease. It is a key component of periodontal plaque due to its abundance and its ability to coaggregate with other species in the oral cavity. The cells of F. nucleatum are fusiform rods or spindle-shaped of many different lengths. In fact, the name refers to the organism as a small spindle-shaped rod. F. nucleatum is found in the dental plaque of many primates, thus includes man. This bacteria has been shown to play a central role in dental plaque formation and other diseases like gingivitis. This is due to its ability to adhere to a wide range of both Gram-positive and Gram-negative plaque microorganisms, such as Porphyromonas gingivalis. F. nucleatum is very much associated with periodontitis, along with invasive human infections of the head and neck, chest, lung, liver and abdomen. Due to its adherence ability, it can be associated with viruses, which adhere to host tissue cells as an invasion and modulate the host's immune response.(1)
The pathogenic potential of Fusobacterium nucleatum and its significance in the development of periodontal diseases, as well as in infections in other organs, have gained new interest for several reasons. First, this bacterium has a very high chance to be pathogenic because of its high frequency in periodontal lesions, its production of irritants that affect the tissue, its ability to coaggregate and form mutual synergisms with other bacteria in mixed infections, and its ability to form numerous aggregates with other suspected pathogens in periodontal disease(therefore, it acts as a bridge between early and late colonizers on surfaces of teeth). Second, F. nucleatum is very common in clinical infections of other body sites. Third, recent new techniques have made it possible to obtain more information about F. nucleatum on the genetic level, thereby also gaining better knowledge of the structure and functions of the outer membrane proteins(OMPs), which are of great interest with respect to coaggregation, cell nutrition, and antibiotic susceptibility.(2)
Genome structure
Fusobacterium nucleatum is a Gram-negative bacterium that does not create spores and is not motile. This bacterium has a G-C content of about 27 to 28 mol%. Its genome size is about 2.4 x 10^6 base pairs (bp). All in all, colony morphology is not a consistent parameter of F. nucleatum. Therefore, it is not sufficient for species identification.(2)
Through phylogenetic grouping through analysis of the 16s rRNA sequences, F. nucleatum was found to be closely related to Bacteroides and the flavobacteria. Similarities have been found with F. nucleatum and the other two species with regards to its DNA and its antigenic composition. In addition, F. nucleatum is found to exhibit high levels of homology with F. alocis, F. periodonticum, and F. simiae. All these organisms, along with F. nucleatum, colonize in oral cavities.(2)
Native plasmids have been identified in strains of F. nucleatum. Using one of the native plasmid pFN1, a F. nucleatum - E. coli shuttle vector has been developed.(1)
Cell structure and metabolism
This organism is possesses an outer membrane, which is very much expected from a Gram-negative bacterium. In addition, the bacterium also has a periplasmic space made of the peptidoglycan layers, in-between the inner and outer cytoplasmic membranes. The inner-membrane is made of a symmetrical phospholipids bilayer with proteins and phospholipids in equal amounts. The outer-membrane, on the other hand, is an asymmetric membrane containing phospholipids, lipopolysaccharides (LPS), lipoproteins, and proteins. It functions as a molecular sieve. The LPS contains 3-deoxy-D-manno-octulosonic acid. Due to the large amount of proteins present in the outer-membrane, it was found that a third of the weight of this bacterium is due to the proteins present in the outer-membrane.(2)
In addition, with its numerous numbers of proteins on the outer cell surface, the bacterium could be found having specific interactions with various complementary structures on the host cell surface. This adherence is mediated by the protein known as adhesin. This adherence is very important in the colonization and establishment of the infection in a susceptible host. In general, adherence is very important to this organisms’ pathogenicity. F. nucleatum plays an important role in adhesion and coaggregation reactions found in periodontal pockets. LPS extracts from F. nucleatum helps alongside adhesin to provide adhesion to the saliva-filled environment. All in all, this characteristic is very important to the survival and susceptibility of the F. nucleatum since fusobacteria adhere very poorly to human cheek epithelial cells. With all these in mind, it is obvious to why research of the bacterium’s adherence proteins: Outer Membrane Proteins (OMP) is currently being conducted.(2)
Due to its non-motile nature, it has been found that F. nucleatum does not possess pili or flagella. It does, however, possess a mucopolysaccharide capsule surrounding the organism of variable thickness, which may be very important to the organism’s pathogenic capabilities.(2)
This bacterium is an anaerobic creature that grows in an environment with only up to 6% oxygen saturation, and also requires a media containing Trypticase, peptone, or yeast extract. This organism’s ability to produce butyric acid as a major product of fermentation of glucose and peptone is what differentiates Fusobacterium species from other Gram-negative, non-sporing rod-shaped bacterium. F. nucleatum is one of the few non-sporulating anaerobic species that utilize amino acid catabolism to provide energy. This organism also uses glutamate, histidine, and aspartate. Apparently, F. nucleatum does not use glucose as its main energy source. Available data indicate that glucose instead is used for the biosynthesis of intracellular molecules and not energy metabolism. This unusual characteristic has been the focus of interest of several studies.(2)
Ecology
Fusobacterium nucleatum inhabits the mucous environment of an animal oral cavity, serving as a pathogen. It is a predominant member of the human oral flora. Because of its pathogenic and parasitic nature, Fusobacterium nucleatum does not affect the environment directly. However, it may alter the ecosystem by its effects on the population of infected host animals. This bacterium has a significant impact on the ecology of the oral cavity due to its ability to adhere to many different microbial species and itself. F. nucleatum is a major component of subgingival plaque.(2)
Pathology
Fusobacterium nucleatum is an oral bacterium, which means it can only be found in the mouth cavity of mammals, mainly humans. It is generally found in the dental plaque of humans and is frequently associated with various gum diseases. Fusobacterium nucleatum is not, however, considered a major dental pathogen on its own. F. nucleatum also has the ability to adhere to and degrade basement membranes in vivo and bind to type 4 collagen. Due to its coaggregation ability (ability to adhere with other plaque organisms, such as Porphyromonas gingivalis), F. nucleatum could contribute to the development to other diseases such as periodontitis as well as invasive human infections of the head and neck, chest, abdomen, and liver.(2)
Out of all periodontal species that are statistically related with periodontal disease, it is the most common in clinical infections found in other body sites. Some bodily infections that this oral bacterium affects include tropical skin ulcers, peritonsillar abscesses, pyomyositis and septic arthritis, bacteremia and liver abscesses, intrauterine infections, bacterial vaginosis, urinary tract infections, pericarditis and endocarditis, and lung and pleuropulmonary infections. Coincidentally, it has been more frequently found in a child’s body.(1)
F. nucleatum is known to have the potential to be a periodontal pathogen by using the production of toxic metabolites. These toxic components have the ability to kill or arrest the proliferation of the normal nearby cells of the periodontium (the fibroblasts). The formation of sulfides by F. nucleatum may provide a way for the bacteria to avoid the host immune system. Butyrate (in the form of the tissue irritant butyric acid), propionate, and ammonium ions, which are produced by F. nucleatum, inhibit proliferation of human gingival fibroblasts. In addition, it may have the ability to penetrate the gingival epithelium, and are present in elevated levels in plaque associated with periodontitis. Therefore, they may have a very important role in producing oral diseases, such as gingivitis. The effects of the toxins is not fatal to the cells, but the inhibition of fibroblast proliferation is severe because the potential for rapid wound healing is compromised. F. nucleatum also possesses major OMPs that may be important for virulence.(2)
Application to Biotechnology
Aminopeptidase is nutritionally very important for Fusobacterium nucleatum. This peptidase is found to be cell-associated by isolating the culture from disrupted chemostat-grown cells. The enzyme was inactivated by chelators, bestatin, phydroxymercuribenzoate and some heavy metals. Aminopeptidase, therefore, appears to be a cobalt-activated metallo-peptidase. Together with other peptidases, Aminopeptidase would be vital to the growth and survival, in the subgingival environment of the mouth, of F. nucleatum.(5)
A Fusobacterium nucleatum shuttle plasmid, pHS17, capable of transforming E. coli and F. nucleatum ATCC10953, was constructed with pFN1. Shuttle plasmid pHS17 was stably maintained in the F. nucleatum transformants. The differences in the transformation efficiencies suggested the presence of a restriction-modification system in Fusobacterium nucleatum.(4)
Fusobacterium nucleatum ATCC23726 is one type of Fusobacterium nucleatum that is still having its full assembly in progress.(1)
Current Research
Here are some of the more current research regarding F. nucleatum:
"Identification and analysis of fipA, a Fusobacterium nucleatum immunosuppressive factor gene" – Extracts of Fusobacterium nucleatum FDC 364 were capable of inhibiting human T-cell responses to mitogens and antigens. The purified F. nucleatum immunosuppressive protein (FIP) inhibits T-cell activation by stopping or arresting cells in the middle of the G1 phase of the cell cycle. FIP impairs the expression of the cell nuclear antigen during proliferation. One of the FIP component, 44 kDa, has a FipA polypeptide, from the fipA gene, that is sufficient in mediating the immunosuppressive activities of the host protein complex.(6)
"Effect of Fusobacterium nucleatum on the T and B cell responses to Porphyromonas gingivalis in a mouse model" – Mice were used in this experiment. The host mice injected with P. gingivalis followed by F. nucleatum produced equal levels of both anti-P. gingivalis and anti-F. nucleatum antibodies. It was observed that F. nucleatum immunization does not affect the splenic T cell cytokine response to P. gingivalis. However, F. nucleatum immunization prior to that of P. gingivalis inhibited the production of anti-P. gingivalis antibodies. P. gingivalis injection before F. nucleatum demonstrated a partial inhibitory effect by P. gingivalis on antibody production to F. nucleatum. These results suggest that P. gingivalis and F. nucleatum do not allow the production of cross-reactive antibodies to other similar oral microorganisms. Ultimately, it shows that human periodontal diseases are very hard to determine its main cause.(7)
"Enhanced pathogenicity of Fusobacterium nucleatum adapted to oxidative stress" – In this research, characterization of F. nucleatum’s response to oxidative stress is observed by studying its cellular morphology and pathogenicity. This would allow the understanding of how this anaerobic bacterium survives during an invasive process of oxygenated tissues in the host oral cavities. Once again, mice were used to conduct this experiment. A wild-strain of F. nucleatum and an aero-strain were injected into the mice. Mice with aero-strain showed drastic changes in cellular morphology compared to the wild-strain mice. Also, these aero-strain mice showed hyperemia, an increased number of inflammatory cells, and steatosis in the liver. The results showed that the adaptation to oxidative stress might influence the pathogenicity of F. nucleatum. This is a big deal since most hosts that would be exposed to F. nucleatum contain cells with oxidative characteristics.(8)
"Fusobacterium nucleatum pericarditis" – F. nucleatum pericarditis was found in a the chest of a 49-year old man, who was suspected to be affected by mycobacteria. Antituberculosis drugs were used on him thinking that mycobacteria were the cause. However, it was the work of F. nucleatum. This finding gives the realization that entry of F. nucleatum through oropharyngeal portal is the cause of diseases such as this. This is one of the first earlier findings that F. nucleatum could also affect other bodily cavities, besides the mouth.(9)
References
1. HGSC at Baylor College of Medicine
Edited by Jason Homan, student of Rachel Larsen and Kit Pogliano
