Mycobacterium bovis: Difference between revisions
No edit summary |
|||
| (57 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | |||
{{Biorealm Genus}} | {{Biorealm Genus}} | ||
==Classification== | ==Classification== | ||
{| | |||
| height="10" bgcolor="#FFDF95" | | |||
'''NCBI: [http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Tree&id=2&lvl=3&lin=f&keep=1&srchmode=1&unlock Taxonomy]''' | |||
|} | |||
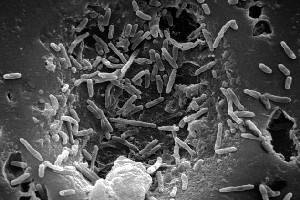
[[Image:M._bovis.jpg]] | |||
Photo Courtesy of CDC | |||
===Higher order taxa=== | ===Higher order taxa=== | ||
Bacteria; Actinobacteria; Actinobacteridae; Actinomycetales; Corynebacterineae; Mycobacteriaceae; Mycobacterium; Mycobacterium tuberculosis complex (1) | ''Bacteria; Actinobacteria; Actinobacteridae; Actinomycetales; Corynebacterineae; Mycobacteriaceae; Mycobacterium; Mycobacterium tuberculosis complex'' (1) | ||
===Genus=== | ===Genus=== | ||
''Mycobacterium bovis'' (1) | |||
Mycobacterium bovis | |||
==Description and significance== | ==Description and significance== | ||
“''Mycobacterium bovis'' is the causative agent of tuberculosis in a range of animal species and man, with world wide annual losses to agriculture of $3 billion.”(2) ''M. bovis'' is the agent responsible for bovine tuberculosis, however it can also cause the disease in humans if there is consumption of infected materials.(1) Pasteurization of milk has been a major preventative factor in stopping transmission of bovine tuberculosis in humans; however in many underdeveloped countries, where pasteurization is not practiced, there is still a concern with infection by ''M. bovis''.(1) ''M. bovis'' AF2122/97 is a fully virulent strain that was isolated from a diseased cow in 1997.(2) | |||
==Genome structure== | ==Genome structure== | ||
M. bovis genome sequence is 4,345,492 base pairs in length, arranged in a single circular | The ''M. bovis'' genome sequence is 4,345,492 base pairs in length, arranged in a single circular chromosome.(2) The genome contains 3,952 genes encoding proteins.(2) The genome is >99.52% identical at the nucleotide level to ''Mycobacterium tuberculosis''.(2) Comparative sequencing with ''M. tuberculosis'' revealed 11 deletions from the genome of ''M. bovis'', ranging in size from 1-12.7 kb, and have been confirmed by the genome sequence data.(2) ''M. bovis'' contains one unique locus termed TbD1, which is absent from the ''M. tuberculosis'' strain; therefore, it appears that deletion has been the mechanism in shaping the ''M. bovis'' genome.(2) | ||
==Cell structure and metabolism== | ==Cell structure and metabolism== | ||
M. bovis is similar in structure and metabolism to M. tuberculosis. M. bovis is a Gram-positive, acid-fast, rod-shaped, aerobic bacteria.(1) Unlike M. tuberculosis, M. bovis lacks pyruvate kinase activity, due to pykA containing a point mutation that affects binding of | ''M. bovis'' is similar in structure and metabolism to ''M. tuberculosis''. ''M. bovis'' is a Gram-positive, acid-fast, rod-shaped, aerobic bacteria.(1) Unlike ''M. tuberculosis'', ''M. bovis'' lacks pyruvate kinase activity, due to pykA containing a point mutation that affects binding of Mg<sup>2+</sup> cofactor.(2) Pyruvate kinase catalyses the final step of glycolysis, the dephosphorylation of phosphorenolpyruvate to pyruvate.(2) Therefore in ''M. bovis'' glycolytic intermediates are unable to enter into oxidative metabolism.(2) Although no specific studies have been performed, it seems that ''M. bovis'' must rely on amino acids or fatty acids as an alternative carbon source for energy metabolism.(2) | ||
==Ecology== | ==Ecology== | ||
''M. bovis'' is an aerobic, host-associated bacteria; it is mesophilic, with its optimal living temperature at 37ºC.(1) It has been able to infect a variety of animals including cows, badgers, buffalo, and lions.(2) An incident at Kruger National Park caused a massive infection affecting several species of animals; this outbreak has severe implications for the biodiversity of this region.(2) | |||
==Pathology== | ==Pathology== | ||
The pathology of ''M. bovis'' is similar to ''M. tuberculosis'' in humans, causing chronic debilitation, coughing, and further spread to other organs.(1) In the cow from which ''M. bovis'' AF2122/97 was isolated suffered from necrotic lesions in lung and bronchomediastinal lymph nodes.(2) Infected cows produce mycobacterial mastitis causing the shedding of the bacteria into milk leading to transmission to humans if the milk is ingested without pasteurization.(1) | |||
==Application to Biotechnology== | ==Application to Biotechnology== | ||
''M. bovis'' is the ancestor of the most widely used vaccine against tuberculosis,''M. bovis'' bacillus Calmette-Guérin.(2) BCG is a strain that was created by growing ''M. bovis'' on potato slices soaked in ox-bile and glycerol over a period of 13 years.(2) | |||
==Current Research== | ==Current Research== | ||
'''"First report of ''Mycobacterium bovis'' DNA in human remains from the Iron Age"''' | |||
-Tuberculosis has affected millions of organisms for thousands of years and studies of early human skeletons show tuberculosis lesions dating back to the Neolithic period. Tuberculosis in humans is caused by either ''M. bovis'' or ''M. tuberculosis''. It is possible to extract surviving ancient mycobacterial DNA and amplify it by PCR. Using this technique, a lab performed a study of five Iron Age individuals from Aymyrlyg, South Siberia to determine the type of tuberculosis infection. (3) | |||
'''"Study on the Cell Wall Skeleton Derived from ''Mycobacterium bovis'' BCG Tokyo 172 (SMP-105): Establishment of Preparation and Analytical Methods"''' | |||
-Currently the cell wall of ''M. bovis'' bacillus Calmette-Guérin (BCG) is a drug for tumor immunotherapy; however, the specific biological relationship is not fully understood. In order to properly study the mechanism, large amounts of cell wall skeleton of BCG must be harvested and purified. A lab in Tokyo, Japan has developed a method to secure the cell wall skeleton in a purified form with accuracy and precision. (4) | |||
'''"Differential gene expression between ''Mycobacterium bovis'' and ''Mycobacterium tuberculosis''"''' | |||
-As stated above research has shown that ''M. bovis'' and ''M. tuberculosis'' have a similar genome; even with a similar genome, the physiology of each species is very different as each is a different pathogen. This variation is probably due to variations in gene expression. To examine the different gene expressions, a lab performed a microarray hybridization to compare the total transcriptome of ''M. bovis'' and ''M. tuberculosis'' during the exponential phase of growth. (5) | |||
==References== | ==References== | ||
1) (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=genomeprj&cmd=Retrieve&dopt=Overview&list_uids=89) | |||
2) Thierry Garnier, Karin Eiglmeier, Jean-Christophe Camus, Nadine Medina, Huma Mansoor, Melinda Pryor, Stephanie Duthoy, Sophie Grondin, Celine Lacroix, Christel Monsempe, Sylvie Simon, Barbara Harris, Rebecca Atkin, Jon Doggett, Rebecca Mayes, Lisa Keating, Paul R. Wheeler, Julian Parkhill, Bart G. Barrell, Stewart T. Cole, Stephen V. Gordon, and R. Glyn Hewinson. 2003. “The complete genome sequence of ''Mycobacterium bovis''”. Proceedings of the National Academy of Sciences, vol. 100, no.13, (7877-7882) (http://www.pnas.org/cgi/content/full/100/13/7877) | |||
3) G. Michael Taylor, Eileen Murphy, Richard Hopkins, Paul Rutland, and Yuri Chistov. 2007. “First report of ''Mycobacterium bovis'' DNA in human remains from the Iron Age.” Microbiology, vol. 153, (1243-1249) (http://mic.sgmjournals.org/cgi/content/full/153/4/1243?view=long&pmid=17379733) | |||
4) Yuko Uenishi, Takashi Okada, Seiji Okabe, and Makoto Sunagawa. 2007. “Study on the Cell Wall Skeleton Derived from ''Mycobacterium bovis'' BCG Tokyo 172 (SMP-105): Establishment of Preparation and Analytical Methods.” Pharmaceutical Society of Japan: Chemical & Pharmaceutical Bulletin (Tokyo), vol. 55, no. 6, (843-852) (http://cpb.pharm.or.jp/cpb/200706/c06_0843.pdf) | |||
5) Germán Rehren, Shaun Walters, Patricia Fontan, Issar Smith, and Ana M. Zárraga. 2007. “Differential gene expression between ''Mycobacterium bovis'' and ''Mycobacterium tuberculosis''.” Tuberculosis, doi:10.1016/j.tube.2007.02.004 (http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WXK-4NG4C76-1&_user=4429&_coverDate=04%2F11%2F2007&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000059602&_version=1&_urlVersion=0&_userid=4429&md5=f03c4fe1b6f4631d5ec4f02485f8ffd3) | |||
[ | Edited by Steven Lada, student of [mailto:ralarsen@ucsd.edu Rachel Larsen] and [mailto:kpogliano@ucsd.edu Kit Pogliano] | ||
KMG | |||
Latest revision as of 02:25, 9 April 2011
A Microbial Biorealm page on the genus Mycobacterium bovis
Classification
|
NCBI: Taxonomy |
Higher order taxa
Bacteria; Actinobacteria; Actinobacteridae; Actinomycetales; Corynebacterineae; Mycobacteriaceae; Mycobacterium; Mycobacterium tuberculosis complex (1)
Genus
Mycobacterium bovis (1)
Description and significance
“Mycobacterium bovis is the causative agent of tuberculosis in a range of animal species and man, with world wide annual losses to agriculture of $3 billion.”(2) M. bovis is the agent responsible for bovine tuberculosis, however it can also cause the disease in humans if there is consumption of infected materials.(1) Pasteurization of milk has been a major preventative factor in stopping transmission of bovine tuberculosis in humans; however in many underdeveloped countries, where pasteurization is not practiced, there is still a concern with infection by M. bovis.(1) M. bovis AF2122/97 is a fully virulent strain that was isolated from a diseased cow in 1997.(2)
Genome structure
The M. bovis genome sequence is 4,345,492 base pairs in length, arranged in a single circular chromosome.(2) The genome contains 3,952 genes encoding proteins.(2) The genome is >99.52% identical at the nucleotide level to Mycobacterium tuberculosis.(2) Comparative sequencing with M. tuberculosis revealed 11 deletions from the genome of M. bovis, ranging in size from 1-12.7 kb, and have been confirmed by the genome sequence data.(2) M. bovis contains one unique locus termed TbD1, which is absent from the M. tuberculosis strain; therefore, it appears that deletion has been the mechanism in shaping the M. bovis genome.(2)
Cell structure and metabolism
M. bovis is similar in structure and metabolism to M. tuberculosis. M. bovis is a Gram-positive, acid-fast, rod-shaped, aerobic bacteria.(1) Unlike M. tuberculosis, M. bovis lacks pyruvate kinase activity, due to pykA containing a point mutation that affects binding of Mg2+ cofactor.(2) Pyruvate kinase catalyses the final step of glycolysis, the dephosphorylation of phosphorenolpyruvate to pyruvate.(2) Therefore in M. bovis glycolytic intermediates are unable to enter into oxidative metabolism.(2) Although no specific studies have been performed, it seems that M. bovis must rely on amino acids or fatty acids as an alternative carbon source for energy metabolism.(2)
Ecology
M. bovis is an aerobic, host-associated bacteria; it is mesophilic, with its optimal living temperature at 37ºC.(1) It has been able to infect a variety of animals including cows, badgers, buffalo, and lions.(2) An incident at Kruger National Park caused a massive infection affecting several species of animals; this outbreak has severe implications for the biodiversity of this region.(2)
Pathology
The pathology of M. bovis is similar to M. tuberculosis in humans, causing chronic debilitation, coughing, and further spread to other organs.(1) In the cow from which M. bovis AF2122/97 was isolated suffered from necrotic lesions in lung and bronchomediastinal lymph nodes.(2) Infected cows produce mycobacterial mastitis causing the shedding of the bacteria into milk leading to transmission to humans if the milk is ingested without pasteurization.(1)
Application to Biotechnology
M. bovis is the ancestor of the most widely used vaccine against tuberculosis,M. bovis bacillus Calmette-Guérin.(2) BCG is a strain that was created by growing M. bovis on potato slices soaked in ox-bile and glycerol over a period of 13 years.(2)
Current Research
"First report of Mycobacterium bovis DNA in human remains from the Iron Age"
-Tuberculosis has affected millions of organisms for thousands of years and studies of early human skeletons show tuberculosis lesions dating back to the Neolithic period. Tuberculosis in humans is caused by either M. bovis or M. tuberculosis. It is possible to extract surviving ancient mycobacterial DNA and amplify it by PCR. Using this technique, a lab performed a study of five Iron Age individuals from Aymyrlyg, South Siberia to determine the type of tuberculosis infection. (3)
"Study on the Cell Wall Skeleton Derived from Mycobacterium bovis BCG Tokyo 172 (SMP-105): Establishment of Preparation and Analytical Methods"
-Currently the cell wall of M. bovis bacillus Calmette-Guérin (BCG) is a drug for tumor immunotherapy; however, the specific biological relationship is not fully understood. In order to properly study the mechanism, large amounts of cell wall skeleton of BCG must be harvested and purified. A lab in Tokyo, Japan has developed a method to secure the cell wall skeleton in a purified form with accuracy and precision. (4)
"Differential gene expression between Mycobacterium bovis and Mycobacterium tuberculosis"
-As stated above research has shown that M. bovis and M. tuberculosis have a similar genome; even with a similar genome, the physiology of each species is very different as each is a different pathogen. This variation is probably due to variations in gene expression. To examine the different gene expressions, a lab performed a microarray hybridization to compare the total transcriptome of M. bovis and M. tuberculosis during the exponential phase of growth. (5)
References
1) (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=genomeprj&cmd=Retrieve&dopt=Overview&list_uids=89)
2) Thierry Garnier, Karin Eiglmeier, Jean-Christophe Camus, Nadine Medina, Huma Mansoor, Melinda Pryor, Stephanie Duthoy, Sophie Grondin, Celine Lacroix, Christel Monsempe, Sylvie Simon, Barbara Harris, Rebecca Atkin, Jon Doggett, Rebecca Mayes, Lisa Keating, Paul R. Wheeler, Julian Parkhill, Bart G. Barrell, Stewart T. Cole, Stephen V. Gordon, and R. Glyn Hewinson. 2003. “The complete genome sequence of Mycobacterium bovis”. Proceedings of the National Academy of Sciences, vol. 100, no.13, (7877-7882) (http://www.pnas.org/cgi/content/full/100/13/7877)
3) G. Michael Taylor, Eileen Murphy, Richard Hopkins, Paul Rutland, and Yuri Chistov. 2007. “First report of Mycobacterium bovis DNA in human remains from the Iron Age.” Microbiology, vol. 153, (1243-1249) (http://mic.sgmjournals.org/cgi/content/full/153/4/1243?view=long&pmid=17379733)
4) Yuko Uenishi, Takashi Okada, Seiji Okabe, and Makoto Sunagawa. 2007. “Study on the Cell Wall Skeleton Derived from Mycobacterium bovis BCG Tokyo 172 (SMP-105): Establishment of Preparation and Analytical Methods.” Pharmaceutical Society of Japan: Chemical & Pharmaceutical Bulletin (Tokyo), vol. 55, no. 6, (843-852) (http://cpb.pharm.or.jp/cpb/200706/c06_0843.pdf)
5) Germán Rehren, Shaun Walters, Patricia Fontan, Issar Smith, and Ana M. Zárraga. 2007. “Differential gene expression between Mycobacterium bovis and Mycobacterium tuberculosis.” Tuberculosis, doi:10.1016/j.tube.2007.02.004 (http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WXK-4NG4C76-1&_user=4429&_coverDate=04%2F11%2F2007&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000059602&_version=1&_urlVersion=0&_userid=4429&md5=f03c4fe1b6f4631d5ec4f02485f8ffd3)
Edited by Steven Lada, student of Rachel Larsen and Kit Pogliano
KMG