Sulcia muelleri: Difference between revisions
| (16 intermediate revisions by 2 users not shown) | |||
| Line 20: | Line 20: | ||
==Genome Structure== | ==Genome Structure== | ||
''Sulcia'' has a single circular chromosome around 245 kilobases in length. It is notable for the extreme levels of gene loss that have occurred over the course of its symbiosis with Auchenorrhyncha. It retains only 263 genes, 227 of which are protein-coding. Notably absent are genes for membrane biosynthesis (it depends entirely on host-derived membranes), all but two genes for DNA repair, and even some of the aminoacyl-tRNA | ''Sulcia'' has a single circular chromosome around 245 kilobases in length. It is notable for the extreme levels of gene loss that have occurred over the course of its symbiosis with Auchenorrhyncha. It retains only 263 genes, 227 of which are protein-coding. Notably absent are genes for membrane biosynthesis (it depends entirely on host-derived membranes), all but two genes for DNA repair, and even some of the aminoacyl-tRNA synthetase genes, raising the question of how protein synthesis is accomplished[3]. It is possible that the tRNA synthetases are imported from the host or co-symbiont, or that some of the undetermined proteins in the genome have novel way of carrying out the aminoacylation reaction[6]. | ||
''Sulcia'''s genome is also notable for its paucity of transporters. It seems to express just a single antibiotic resistance transporter, a cation transporter, and a heavy metal ion transporter[6]. No amino acid transporters are evident[6]. It is possible that physical proximity to the co-symbiont and host membranes allows direct transport of necessary compounds. | |||
A disproportionate amount of ''Sulcia'''s genome is dedicated to amino acid synthesis | Its genome displays a very low GC content, around 23.5%[3], which is a common feature of reduced genomes.[4] This is speculated to be due to the universal mutational bias towards AT combined with low selection pressure and a lack of DNA repair machinery. [4][5] | ||
A disproportionate amount of ''Sulcia'''s genome is dedicated to amino acid synthesis (21.3%)[6]. | |||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
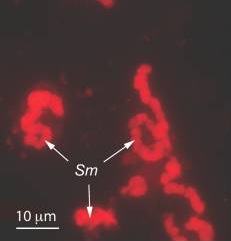
''Sulcia'' has an unusual morphology, with a poorly defined and often grossly elongated shape. This is speculated to be due to the absence of structurally significant proteins such as the MreBCD complex and RodA[4]. | |||
''Sulcia'' is able to generate reducing power in the form of NADH[6]. It transfers this energy through a greatly reduced aerobic electron transport chain consisting of NADH dehydrogenase, Menaquinone reductase, Cytochrome C, with a cbb3-type cytochrome oxidase as the terminal member. This establishes a proton gradient which is used to drive ATP synthase in the usual fashion[6]. Interestingly, the gene for Cytochrome C contains an in-frame stop codon that is well-supported by sequencing data[6]. | |||
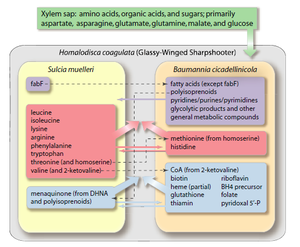
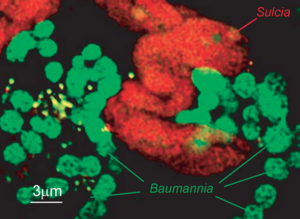
''Sulcia'', befitting its place in a three-way symbiosis, produces compounds necessary for both its host and its co-symbiont. The host feeds exclusively on Xylem sap, which is an extremely nutrient poor and unbalanced food source. It typically contains only a handful of amino acids and some sugars[6]. ''Sulcia'' remedies this situation by synthesizing 8 amino acids ''de novo'', which are used by both the host and co-symbiont. The co-symbiont, for its part, generally synthesizes a variety of vitamins and cofactors. The only cofactor produced by ''Sulcia'' appears to be menaquinone (which is not synthesized in the co-symbiont). Similarly, the cosymbiont appears to be responsible for fatty acid and purine/pyridine synthesis.[6] | |||
[[File:Sulcia_Symbiosis.png|thumb| A diagrammatic representation of the three way symbiosis. From McCutcheon & Moran, PNAS. 2007]] | |||
==Ecology and Pathogenesis== | ==Ecology and Pathogenesis== | ||
S. muelleri lives in specialized cells | S. muelleri lives in specialized host cells (bacteriocytes) and is involved in an obligate mutualism with its host and certain Gammaproteobacteria. [6] The host primarily feeds on xylem sap which is lacking in amino acids and other important nutrients. In this tripartite symbiosis, a metabolic exchange of metabolites is occurring by each of the members. S. muelleri uses aerobic respiration with the electron donor being carbon found from components of the sap. [6] It is responsible for synthesizing most essential amino acids found in the mutualism and in the host. | ||
Although this bacteria isn't known to be the direct cause of any diseases, its host are considered pests and vectors of some plant diseases such as diseases caused by the pathogen Xylella fastidiosa. | Although this bacteria isn't known to be the direct cause of any diseases, its host are considered pests and vectors of some plant diseases such as diseases caused by the pathogen Xylella fastidiosa. [6] | ||
[[file:Sulcia 2.png|thumb|A depiction of ''Sulcia'' in close proximity to a co-symbiont ]] | |||
[[file:Sulcia 2.png|thumb]] | |||
==References== | ==References== | ||
| Line 49: | Line 54: | ||
3. [https://biocyc.org/CSUL444179/organism-summary?object=CSUL444179 Subhraveti, et al. "Summary of Candidadus ''Sulcia Muelleri''". Biocyc. 2014] | 3. [https://biocyc.org/CSUL444179/organism-summary?object=CSUL444179 Subhraveti, et al. "Summary of Candidadus ''Sulcia Muelleri''". Biocyc. 2014] | ||
4. [http://www.nature.com/nrmicro/journal/v10/n1/full/nrmicro2670.html McCutcheon, J., Moran, N. "Extreme Genome Reduction in Symbiotic Bacteria". ''Nature Reviews Microbiology'. 2012. Volume 10. 13-26.] | |||
5.[http://journals.plos.org/plosgenetics/article?id=10.1371/journal.pgen.1001115 Hershberg, R., Petrov, D. "Evidence that Mutation is Universally Biased towards AT in Bacteria. ''PLoS Genetics'' 2010.] | |||
6.[http://www.pnas.org/content/104/49/19392.full McCutcheon, J., Moran, N. "Parallel Genomic Evolution and Metabolic Interdependence in an Ancient Symbiosis". ''PNAS'' 2007. Volume 104. 19392-19397.] | |||
==Author== | ==Author== | ||
Page authored by | Page authored by Courtney Krause and William Lubetkin, students of Prof. Jay Lennon at Indiana University, Bloomington. | ||
<!-- Do not remove this line-->[[Category:Pages edited by students of Jay Lennon at Indiana University]] | <!-- Do not remove this line-->[[Category:Pages edited by students of Jay Lennon at Indiana University]] | ||
Latest revision as of 12:21, 25 April 2017
Classification
Domain: Bacteria; Phylum; Bacteroidetes; Class: Flavobacteria; Order Flavobacteriales
Species
|
NCBI: Taxonomy |
Sulcia muelleri
Description and Significance
Sulcia muelleri is a bacterial obligate intracellular symbiont primarily found in specialized cells in insects of the suborder Auchenorrhyncha[1] (examples of this suborder include cicadas and treehoppers). It is gram negative, roughly rod-shaped, with a width ranging from 3-5 microns and a widely variable length, up to 80 microns[1]. They are often observed in a coiled or 'balled up' conformation.[1] As Sulcia is found as a symbiont across many different species in Auchenorrhyncha, it is likely that the symbiosis predates the origin of the suborder approximately 260MYA[1]. Today, it is often a partner in tripartite symbiosises with its sap-feeding insect host and other bacteria, such as Baumannia cicadellinicola (Gammaproteobacteria) or Zinderia Insectola (Betaproteobacteria)[2]. It is responsible for amino acid biosynthesis for the insect while the third partner often contributes vitamins and cofactors[2]. These mutualistic interactions have enabled the utilization of otherwise inaccessible niches by Auchenorrhyncha, and thus helped spur their diversification.
Genome Structure
Sulcia has a single circular chromosome around 245 kilobases in length. It is notable for the extreme levels of gene loss that have occurred over the course of its symbiosis with Auchenorrhyncha. It retains only 263 genes, 227 of which are protein-coding. Notably absent are genes for membrane biosynthesis (it depends entirely on host-derived membranes), all but two genes for DNA repair, and even some of the aminoacyl-tRNA synthetase genes, raising the question of how protein synthesis is accomplished[3]. It is possible that the tRNA synthetases are imported from the host or co-symbiont, or that some of the undetermined proteins in the genome have novel way of carrying out the aminoacylation reaction[6].
Sulcia's genome is also notable for its paucity of transporters. It seems to express just a single antibiotic resistance transporter, a cation transporter, and a heavy metal ion transporter[6]. No amino acid transporters are evident[6]. It is possible that physical proximity to the co-symbiont and host membranes allows direct transport of necessary compounds.
Its genome displays a very low GC content, around 23.5%[3], which is a common feature of reduced genomes.[4] This is speculated to be due to the universal mutational bias towards AT combined with low selection pressure and a lack of DNA repair machinery. [4][5]
A disproportionate amount of Sulcia's genome is dedicated to amino acid synthesis (21.3%)[6].
Cell Structure, Metabolism and Life Cycle
Sulcia has an unusual morphology, with a poorly defined and often grossly elongated shape. This is speculated to be due to the absence of structurally significant proteins such as the MreBCD complex and RodA[4].
Sulcia is able to generate reducing power in the form of NADH[6]. It transfers this energy through a greatly reduced aerobic electron transport chain consisting of NADH dehydrogenase, Menaquinone reductase, Cytochrome C, with a cbb3-type cytochrome oxidase as the terminal member. This establishes a proton gradient which is used to drive ATP synthase in the usual fashion[6]. Interestingly, the gene for Cytochrome C contains an in-frame stop codon that is well-supported by sequencing data[6].
Sulcia, befitting its place in a three-way symbiosis, produces compounds necessary for both its host and its co-symbiont. The host feeds exclusively on Xylem sap, which is an extremely nutrient poor and unbalanced food source. It typically contains only a handful of amino acids and some sugars[6]. Sulcia remedies this situation by synthesizing 8 amino acids de novo, which are used by both the host and co-symbiont. The co-symbiont, for its part, generally synthesizes a variety of vitamins and cofactors. The only cofactor produced by Sulcia appears to be menaquinone (which is not synthesized in the co-symbiont). Similarly, the cosymbiont appears to be responsible for fatty acid and purine/pyridine synthesis.[6]
Ecology and Pathogenesis
S. muelleri lives in specialized host cells (bacteriocytes) and is involved in an obligate mutualism with its host and certain Gammaproteobacteria. [6] The host primarily feeds on xylem sap which is lacking in amino acids and other important nutrients. In this tripartite symbiosis, a metabolic exchange of metabolites is occurring by each of the members. S. muelleri uses aerobic respiration with the electron donor being carbon found from components of the sap. [6] It is responsible for synthesizing most essential amino acids found in the mutualism and in the host.
Although this bacteria isn't known to be the direct cause of any diseases, its host are considered pests and vectors of some plant diseases such as diseases caused by the pathogen Xylella fastidiosa. [6]
References
3. Subhraveti, et al. "Summary of Candidadus Sulcia Muelleri". Biocyc. 2014
Author
Page authored by Courtney Krause and William Lubetkin, students of Prof. Jay Lennon at Indiana University, Bloomington.