The Mycorrhizal Network: Difference between revisions
No edit summary |
|||
| (101 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
<!-- Do not edit this line-->{{Curated}} | |||
<br>By Freya Beinart <br> | |||
<br><br> | |||
==Introduction== | ==Introduction== | ||
<br> <br> | [[File:Blog-fungi-on-small-tree-roots-3.jpg|thumb|300px|left|Fungal colonization of a plant's rhizosphere representing a mycorrhizal symbiosis. [https://www.csuchico.edu/regenerativeagriculture/blog/how-fungus-might-save-us.shtml].]] | ||
A mycorrhiza is the symbiotic relationship between fungi and plants. Mycelium, the vegetative part of a fungus, extends in a branching network of hyphae that secrete enzymes, breaking down organic material into nutrients. The vast mycelial branches of the fungi are made of branching networks of hyphae which, in a mycorrhizal association, colonize the root systems of many plant species, greatly increasing the surface area of a plant’s rhizosphere in which it collects water and essential nutrients <ref name=aa>[Brundrett, M. (1991). <i>Mycorrhizas in natural ecosystems. In Advances in Ecological Research: Vol. 21 </i>(pp. 171–313). Elsevier. doi: 10.1016/S0065-2504(08)60099-9</ref> <ref name=ab>[Johnson, N. C., & Gehring, C. A. (2007). Mycorrhizas: symbiotic mediators of rhizosphere and ecosystem processes. In <i>The Rhizosphere </i>(pp. 73–100). Elsevier. doi: 10.1016/B978-012088775-0/50006-9]]</ref>. In exchange for these nutrients, the plants provide the fungi with carbohydrates that the fungi cannot produce on its own, being heterotrophic. The hyphae of soil fungi colonize the root system of plants, providing the photosynthetic organisms with nutrients and water in exchange for carbohydrates that the heterotrophic fungi cannot produce on its own. This colonization may be intracellular as endomycorrhizal fungi which trade nutrients via a signal transduction pathway, or extracellular as ectomycorrhizal fungi that envelop plant roots <ref name=ac/><ref name=ad>[Smith, S. E., & Read, D. J. (2002). Structure and development of ectomycorrhizal roots. In <i>Mycorrhizal Symbiosis</i> (pp. 163–V). Elsevier. doi: 10.1016/B978-012652840-4/50007-3]</ref>. | |||
<br><br> | |||
About 10% of identified fungal species are known to occur in mycorrhizal symbioses, these fungi are most species of the Glomeromycota, and many species of the Ascomycota and Basidiomycota <ref>Lewis, J. D. (2016). Mycorrhizal fungi, evolution and diversification of. In <i>Encyclopedia of evolutionary biology </i>(pp. 94–99). Elsevier. doi: 10.1016/B978-0-12-800049-6.00251-1</ref>. The fungal symbionts assist host plants to be less susceptible to pathogens and environmental stresses such as lack of nutrient density, drought, and high salinity <ref>Ayub, M. A., Ahmad, H. R., Ali, M., Rizwan, M., Ali, S., Zia ur Rehman, M., & Waris, A. A. (2020). Salinity and its tolerance strategies in plants. In <i>Plant life under changing environment </i>(pp. 47–76). Elsevier. doi: 10.1016/B978-0-12-818204-8.00003-5</ref><ref name=aa/><ref name=ae>[Bunn, R. A., Simpson, D. T., Bullington, L. S., Lekberg, Y., & Janos, D. P. (2019). Revisiting the “direct mineral cycling” hypothesis: arbuscular mycorrhizal fungi colonize leaf litter, but why? <i>The ISME Journal, 13</i>(8), 1891–1898. doi: 10.1038/s41396-019-0403-2]</ref><ref>Evelin, H., Devi, T. S., Gupta, S., & Kapoor, R. (2019). Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: current understanding and new challenges.<i> Frontiers in Plant Science, 10, </i>470. doi: 10.3389/fpls.2019.00470</ref><ref name=bb>[Haskett, T. L., Tkacz, A., & Poole, P. S. (2021). Engineering rhizobacteria for sustainable agriculture. <i>The ISME Journal, 15</i>(4), 949–964. doi: 10.1038/s41396-020-00835-4]</ref>. The vast underground networks of hyphae increase nutrient cycling, erosion resistance, air permeability, and water permeability within the soil itself. Including and maintaining these associations between fungi and plants has proven to be highly beneficial for agricultural health and biogeochemical cycling <ref name=ccc>[Begum, N., Qin, C., Ahanger, M. A., Raza, S., Khan, M. I., Ashraf, M., … Zhang, L. (2019). Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. <i>Frontiers in Plant Science, 10, </i>1068. doi: 10.3389/fpls.2019.01068]</ref><ref name=ae/><ref>Corradi, N., & Bonfante, P. (2012). The arbuscular mycorrhizal symbiosis: origin and evolution of a beneficial plant infection. <i>PLoS Pathogens, 8</i>(4), e1002600. doi: 10.1371/journal.ppat.1002600</ref><ref>Gadgil, R. L., & Gadgil, P. D. (1971). Mycorrhiza and litter decomposition. <i>Nature, 233</i>(5315), 133. doi: 10.1038/233133a0</ref><ref name=bb/><ref name=ag>[Rashid, M. I., Mujawar, L. H., Shahzad, T., Almeelbi, T., Ismail, I. M. I., & Oves, M. (2016). Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. <i>Microbiological Research, 183,</i> 26–41. doi: 10.1016/j.micres.2015.11.007]</ref>. | |||
<br><br> | |||
==Types of Mycorrhizal Symbioses== | |||
[[File:Mycotypes.png|thumb|400px|right|<b>Figure 1.</b> Diagram of different types of mycorrhizal associations on the cellular level. [http://www.davidmoore.org.uk/assets/mostly_mycology/diane_howarth/mycorrhizal%20types.htm].]] | |||
Characterization of mycorrhizal association is dependent upon the location of fungal colonization and is specific to both the plant species and their fungal symbionts. | |||
===Endomycorrhizae=== | |||
Endomycorrhizal symbioses occur when the mycorrhizal fungi colonizes a host plant intracellularly. | |||
<br><br> | |||
<b>Arbuscular Mycorrhizae</b> | |||
<br><br> | |||
Fungi of the phylum Glomeromycota that forms mutualistic symbioses, called arbuscular mycorrhizae, with vascular plants are referred to as arbuscular mycorrhizal fungi, or AMF. These fungi are associated with 90% of vascular plant species. AMF are unable to live without the presence of the host plants, as they depend on the transfer of carbohydrates from the plant tissue, making the fungi obligate biotrophs <ref name=ah>[Smith, S. E., & Reads, D. J. (n.d.). Mycorrhizal Symbiosis. Retrieved April 06, 2021, from https://books.google.com/books?hl=en&lr=&id=qLciOJaG0C4C&oi=fnd&pg=PP1&ots=zrsXoTRxkN&sig=G4cYDH7aK0uYRBaENI2m3JvclNA#v=onepage&q&f=false</ref>. Plant taxa that are observed to exhibit this relationship are bryophyta, pteridophyta, gymnosperms, and angiosperms]<ref>Barman, J., Samanta, A., Saha, B., & Datta, S. (2016). Mycorrhiza. <i>Resonance, 21</i>(12), 1093-1104. doi:10.1007/s12045-016-0421-6</ref>. | |||
<br><br> | |||
Hyphae from a germinating spore infect the host root, passing the epidermis and penetrating cortical cells which form arbuscules - the structure from which the name of these fungi originated <<ref name=ah/>. The arbuscules are networks of extremely fine clustered hyphae within the host cells where nutrient transfer is centralized <ref>Moore, D., Robson, G., & Trinci, A. (2011, July 14). 21St century guidebook TO Fungi: Plant science. Retrieved April 05, 2021, from https://www.cambridge.org/gb/academic/subjects/life-sciences/plant-science/21st-century-guidebook-fungi?format=WW&isbn=9780521186957</ref>.The hyphae may also form vesicles between or within the root cells, acting as an organ for storage with a thickened cell wall which aids in the establishment of new colonies <ref name=aa/> <ref>Müller, A., Ngwene, B., Peiter, E., & George, E. (2017). Quantity and distribution of AMF storage organs within dead roots. Mycorrhiza, 27(3), 201–210. doi: 10.1007/s00572-016-0741-0</ref>. The mycelium which extends from the plant root is called the extraradical mycelium, which connects from plant to plant, forming a continuum of nutrient and water exchange <ref>Bhargava, P., Vats, S., & Gupta, N. (2019). Metagenomics as a tool to explore mycorrhizal fungal communities. Mycorrhizosphere and Pedogenesis, 207-219. doi:10.1007/978-981-13-6480-8_13</ref>. | |||
<br><br> | |||
<b>Ericoid mycorrhizae</b> | |||
<br><br> | |||
Ericoid mycorrhizae are usually found in environments with high acidity and free-draining soils susceptible to droughts. These associations involve a mycorrhizal fungus in the Ascomycota and a host plant of the Ericideae family <ref name=ab/><ref name=ad/><ref>Dighton, J. (2009). Mycorrhizae. In<i> Encyclopedia of Microbiology </i>(pp. 153–162). Elsevier. doi: 10.1016/B978-012373944-5.00327-8</ref>. The hyphal coils are where nutrient transfer occurs within ericoid mycorrhizae. Experimentation of ericoid mycorrhizal fungi growth in axenic has revealed a range of saprotrophic capabilities, allowing the fungi to feed on decaying organic matter <ref>Martino, E., Morin, E., Grelet, G.-A., Kuo, A., Kohler, A., Daghino, S., … Perotto, S. (2018). Comparative genomics and transcriptomics depict ericoid mycorrhizal fungi as versatile saprotrophs and plant mutualists. <i>The New Phytologist, 217</i>(3), 1213–1229. doi: 10.1111/nph.14974</ref>. | |||
<br><br> | |||
===Ectomycorrhizae=== | |||
[[File:Harignet.png|thumb|400px|right|<b>Figure 2.</b> Light micrograph of ectomycorrhizal roots with penetrating fungal hyphae forming the Hartig net structure. [http://www.davidmoore.org.uk/assets/mostly_mycology/diane_howarth/ectomycorrhizas.htm].]] | |||
Fungi that form ectomycorrhizae are majorly Basidiomycota, as well as some Ascomycota and Zygomycetes of the genus <i>Endogone</i>. These associations differ from AM symbioses in the way that the fungi does not penetrate the root cells, instead, it grows intercellularly. Hyphae grow between epidermal and cortical cells of the plant, forming a structure called the Hartig net where nutrient transfer is localized <ref name=ad/>. Hyphae of ectomycorrhizal fungi create sheathes which envelop the exterior of the plant root, called mantle. The hyphae extend outward from the plant root to extend the surface area of the plant root, forming a structure called a rhizomorph. The rhizomorphs’ increased ability to uptake water and nutrients from the soil makes up for the suppression of root hairs by the mantle <ref>Anderson, I. C., & Cairney, J. W. G. (2007). Ectomycorrhizal fungi: exploring the mycelial frontier. <i>FEMS Microbiology Reviews, 31</i>(4), 388–406. doi: 10.1111/j.1574-6976.2007.00073.x</ref>. | |||
<br><br> | |||
Ectomycorrhizae occur in about 10% of plant species, specifically woody plants in certain families of gymnosperms and angiosperms <ref>Wang, B.; Qiu, Y.-L. (July 2006). "Phylogenetic distribution and evolution of mycorrhizas in land plants".<i> Mycorrhiza. 16 </i>(5): 299–363. doi:10.1007/s00572-005-0033-6. PMID 16845554. S2CID 30468942.</ref>. These fungi form fruiting bodies, unlike AMF which only reproduce asexually. Examples of these fruiting bodies are mushrooms of the genus Amanita and genus Tuber. | |||
<br><br> | |||
===Ectendomycorrhizae=== | |||
Ectendomycorrhizal fungi form Hartig net and mantle structures akin to Ectomycorrhizal fungi. Ectendomycorrhizal symbioses differ from ectomycorrhizae as the hyphae penetrate the cortical cells of plant roots, forming an intracellular Hartig net. One type of ectendomycorrhiza is arbutoid mycorrhiza, which is a symbiosis between basidiomycetes and some plant species of the Ericaceae family <ref>Kühdorf, K., Münzenberger, B., Begerow, D., Gómez-Laurito, J., & Hüttl, R. F. (2015). Leotia cf. lubrica forms arbutoid mycorrhiza with Comarostaphylis arbutoides (Ericaceae). <i>Mycorrhiza, 25</i>(2), 109–120. doi: 10.1007/s00572-014-0590-7</ref>. | |||
==Evolution of Mycorrhizae== | |||
[[File:Glomalean.jpg|thumb|400px|left|<b>Figure 3.</b> Fossilized hyphae and spores from the Ordovician period (A, B, C, E, F, G) and spores formed by extant glomalean fungi (D and H). [https://science-sciencemag-org.libproxy.kenyon.edu/content/289/5486/1920].]] | |||
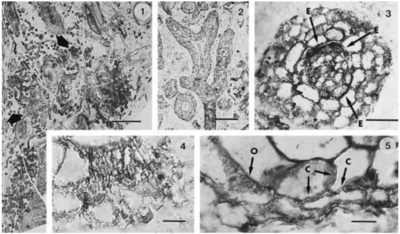
[[File:Hyphaefossil.PNG|thumb|400px|left|<b>Figure 4. </b>Fossil ectomycorrhizae associated with <i>Pinus</i> roots [https://bsapubs-onlinelibrary-wiley-com.libproxy.kenyon.edu/doi/pdfdirect/10.2307/2446014].]] | |||
Fossil records indicate that mycorrhizal fungi predate the evolution of vascular plants, about 460 million years ago in the Ordovician period <ref>Redecker, D., Kodner, R., & Graham, L. E. (2000). Glomalean fungi from the Ordovician. <i>Science, 289</i>(5486), 1920–1921. doi: 10.1126/science.289.5486.1920</ref>. The most ancestral mycorrhizal fungi has been identified as arbuscular, penetrating the root cortical cells in most extant plant taxa. The phylum of fungi that associates with land plants as AMF is that of Glomeromycota <ref>Schüβler, A., Schwarzott, D., & Walker, C. (2001). A new fungal phylum, the Glomeromycota: phylogeny and evolution. <i>Mycological Research, 105</i>(12), 1413–1421. doi: 10.1017/S0953756201005196</ref> The presence of these fungi were involved in the development of soil and the evolution and colonization of vascular land plants, specifically by aiding the non-vascular plants with acquisition of nutrients through fungal hyphae, as the “soil” lacked a significant amount of nutrients for terrestrial plants to survive without their fungal symbionts <ref>Pirozynski, K. A., & Malloch, D. W. (1975). The origin of land plants: a matter of mycotrophism. <i>Bio Systems, 6</i>(3), 153–164. doi: 10.1016/0303-2647(75)90023-4</ref>. As the fungi cycles nutrients such as nitrogen, phosphorus, and sulfur to the plants, the fungus receives and fixes carbon into the ground, assisting in the lowering of atmospheric CO<sup>2</sup> leading to the oxygenation of the atmosphere during the development of terrestrial plants <ref>Mills, B. J. W., Batterman, S. A., & Field, K. J. (2018). Nutrient acquisition by symbiotic fungi governs Palaeozoic climate transition. <i>Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 373</i>(1739). doi: 10.1098/rstb.2016.0503</ref> <ref>Strullu-Derrien, C., Selosse, M.-A., Kenrick, P., & Martin, F. M. (2018). The origin and evolution of mycorrhizal symbioses: from palaeomycology to phylogenomics. <i>The New Phytologist, 220</i>(4), 1012–1030. doi: 10.1111/nph.15076</ref>. | |||
<br><br> | |||
In a study by Krings et al (2007) fossil evidence from the Rhynie chert sediment of the Early Devonian period suggests that fungal endophytes, specifically those which colonize the rhizoids of Nothia aphylla, actively influenced the evolution of these plants due to observed host responses. The responses to fungal infection in N. aphylla - rhizoid bulging, separation of infected cells via thickening of cell walls, and the motile inhibition of hypha - suggests its susceptibility to colonization by fungi at least 400 million years ago, advancing selection between plant species with the increasing complexity of interspecies interactions <ref>Krings, M., Taylor, T. N., Hass, H., Kerp, H., Dotzler, N., & Hermsen, E. J. (2007). Fungal endophytes in a 400-million-yr-old land plant: infection pathways, spatial distribution, and host responses. <i>The New Phytologist, 174</i>(3), 648–657. doi: 10.1111/j.1469-8137.2007.02008.x</ref>. <br><br> | |||
The other form of mycorrhiza associated with the intercellular colonization of fungal symbionts within plants, ectomycorrhiza, evolved much later than its ancestor AMF. Fossil records suggest that ectomycorrhizal fungi may have evolved at least 156 million years ago, as the oldest known extant plant family associated with ectomycorrhizal fungi, Pinaceae, appear to have evolved in that time period <ref>Tedersoo, L., May, T. W., & Smith, M. E. (2010). Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. <i>Mycorrhiza, 20</i>(4), 217–263. doi: 10.1007/s00572-009-0274-x</ref>. Another study shows the morphological identification of ectomycorrhizal fungi based on apparent Hartig net, mantle, and hyphal structures on fossils of <i>Pinus</i> roots dating back 50 million years, clearly representing the established ectomycorrhizal associations (Fig. 4) <ref>Lepage, B., Currah, R., Stockey, R., & Rothwell, G. (1997). Fossil ectomycorrhizae from the Middle Eocene. <i>American Journal of Botany, 84</i>(3), 410. doi: 10.2307/2446014</ref>. | |||
<br><br> | |||
==Biogeochemical Cycles== | |||
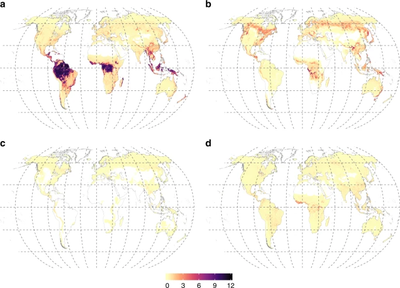
[[File:41467 2019 13019 Fig2 HTML.webp|thumb|400px|right|<b>Figure 5.</b> Amount of carbon stored in plant biomass in vegetation of different mycorrhizal types (Mt C per-grid cell of 15 arcmin). a Arbuscular mycorrhizal plants, b ectomycorrhizal plants, c ericoid mycorrhizal plants, d non-mycorrhizal plants. [https://www.nature.com/articles/s41467-019-13019-2#Fig1].]] | |||
===Carbon Storage=== | |||
Carbon is an extremely important element as it is the primary component of organic compounds and macromolecules. Major amounts of carbon stores are located within the soil, containing more soil than the atmosphere and vegetation combined <ref>Tarnocai et al. 2009. Soil organic carbon pools in the northern circumpolar permafrost region.<i> Global Biogeochemical Cycles, 23</i>(2) doi: 10.1029/2008GB003327</ref>. Saprotrophic fungi play an extremely important role in the carbon cycle as they decompose organic matter, releasing nutrients into the soil. It was believed that most of the carbon found in terrestrial ecosystems was stored in vegetative remains, or leaf litter, however recent studies suggest that most of the stored carbon is derived from the mycelium of mycorrhizal fungi <ref>Clemmensen, K E, Bahr, A., Ovaskainen, O., Dahlberg, A., Ekblad, A., Wallander, H., … Lindahl, B. D. (2013). Roots and associated fungi drive long-term carbon sequestration in boreal forest. <i>Science, 339</i>(6127), 1615–1618. doi: 10.1126/science.1231923</ref><ref>Clemmensen, Karina E, Finlay, R. D., Dahlberg, A., Stenlid, J., Wardle, D. A., & Lindahl, B. D. (2015). Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. <i>The New Phytologist, 205</i>(4), 1525–1536. doi: 10.1111/nph.13208 | |||
</ref>. | |||
<br><br> | |||
High-resolution maps of vegetative biomass associated with mycorrhizal associations elucidate the significant difference in the biogeochemical cycles of ecosystems that are dominated by these symbioses and those that lack mycorrhizae <ref name=bbb>[Soudzilovskaia, N.A., van Bodegom, P.M., Terrer, C. et al. Global mycorrhizal plant distribution linked to terrestrial carbon stocks.<i> Nat Commun</i> 10, 5077 (2019). https://doi.org/10.1038/s41467-019-13019-2]</ref>. This study indicates that non-mycorrhizal vegetation stores 29± 5.5 GT carbon in aboveground biomass, whereas arbuscular, ectomycorrhizal, and ericoid mycorrhizal vegetation store, respectively, 241 ± 15, 100 ± 17, and 7 ± 1.8 GT carbon (Figure 5)<ref name=bbb/>. The same study also quantified the human influence on the biomass of terrestrial ecosystems, reducing ectomycorrhizae in all continents due to the substitution of forests with agricultural lands<ref name=bbb/>. The practice of excess fertilization and destruction of hyphal networks derived from the replacement of natural land with agricultural land is to account for the decrease in global mycorrhizal biomass, which could, in turn, result in loss of total soil carbon<ref name=bbb/>. | |||
<br><br> | |||
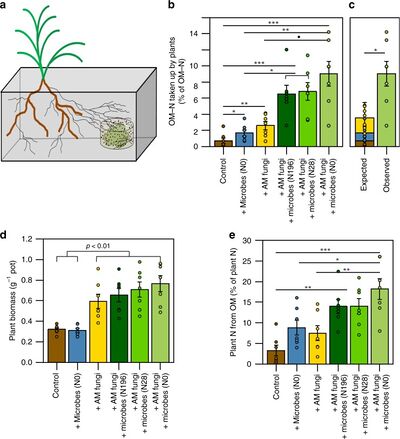
[[File:42003 2019 481 Fig1 HTML.jpg|thumb|400px|right|<b>Figure 6.</b> Effects of fertilization of <i>Brachypodium distachyon</i> with <i>Rhizophagus irregularis</i> and soil microbes. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6588552/figure/Fig1/].]] | |||
===Nitrogen Fixation=== | |||
Nitrogen is an extremely important nutrient for all living organisms as it is essential for amino acid synthesis and therefore the formation of proteins. Despite its importance, nitrogen cannot be used by many organisms until it has been heavily reduced into ammonia <ref>Society, M. (n.d.). Nitrogen cycle: Microbes and the outdoors. Retrieved April 06, 2021, from https://microbiologysociety.org/why-microbiology-matters/what-is-microbiology/microbes-and-the-outdoors/nitrogen-cycle.html#:~:text=Nitrogen%20is%20required%20by%20all,and%20other%20nitrogen%20containing%20compounds.&text=It%20cannot%20be%20used%20in,with%20hydrogen)%2C%20to%20ammonia.</ref>. This anabolic process is very energy-intensive, and therefore many organisms do not possess the ability to fix nitrogen <ref name=ai>[Zahran, H. H. (1999). Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. <i>Microbiology and Molecular Biology Reviews, 63</i>(4), 968–989, table of contents. doi: 10.1128/MMBR.63.4.968-989.1999]</ref> <ref>Kuypers, M. M. M., Marchant, H. K., & Kartal, B. (2018). The microbial nitrogen-cycling network.<i> Nature Reviews. Microbiology, 16</i>(5), 263–276. doi: 10.1038/nrmicro.2018.9</ref>. Organisms that can fix atmospheric nitrogen are known as diazotrophs - bacteria and archaea that do not require fixed nitrogen for their growth or survival <ref>Takai, K. (2019). The nitrogen cycle: A large, fast, and mystifying cycle.<i> Microbes and Environments / JSME, 34</i>(3), 223–225. doi: 10.1264/jsme2.ME3403rh</ref>. | |||
<br><br> | |||
Root nodule symbiosis between plants and nitrogen-fixing bacteria is apparent in plants of the Fabaceae, also known as the legume family<ref name=aj>[Brewin, N. J. (2001). Root Nodules (Legume- <i>Rhizobium </i> Symbiosis). In John Wiley & Sons, Ltd (Ed.), <i>Encyclopedia of life sciences.</i> Chichester, UK: John Wiley & Sons, Ltd. doi: 10.1002/9780470015902.a0003720.pub2]</ref>. Legumes have a symbiotic relationship with rhizobia, single-celled, Gram-negative bacteria <ref name=aj/> <ref name=ak>[Hayman, D. S. (1986). Mycorrhizae of nitrogen-fixing legumes. <i>MIRCEN Journal of Applied Microbiology and Biotechnology, 2</i>(1), 121–145. doi: 10.1007/BF00937189]</ref>. The mutualism between the legumes and rhizobia allows the bacteria to take up atmospheric nitrogen, feeding it to the plant. In return, the plant provides carbohydrates to the bacteria as it is essential for their metabolic processes and growth <ref name=ai/>. Although mycorrhizal symbionts do not possess the metabolism required for nitrogen fixation, studies have shown that mycorrhizae may indirectly affect nitrogen fixation as they increase root nodulation <ref name=aj/> <ref name=ak/><ref name=bc>[Mus, F., Crook, M. B., Garcia, K., Garcia Costas, A., Geddes, B. A., Kouri, E. D., … Peters, J. W. (2016). Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. <i>Applied and Environmental Microbiology, 82</i>(13), 3698–3710. doi: 10.1128/AEM.01055-16]</ref>. | |||
<br><br> | |||
The legume symbiotic signaling (SYM) pathway has been studied in relation to arbuscular mycorrhizal symbiosis <ref name=ac>[Gutjahr, C., Banba, M., Croset, V., An, K., Miyao, A., An, G., … Paszkowski, U. (2008). Arbuscular mycorrhiza-specific signaling in rice transcends the common symbiosis signaling pathway. <i>The Plant Cell, 20</i>(11), 2989–3005. doi: 10.1105/tpc.108.062414]</ref> <ref name=bc/>. The SYM pathway controls both nodulation of legumes and the mycorrhization of land plants, and is suggested to evolve first in legumes and adapted for arbuscular mycorrhizal symbiosis <ref>Role of the SYM pathway in selecting the root microbiota. (n.d.). Retrieved April 6, 2021, from https://gtr.ukri.org/projects?ref=BB%2FR017859%2F1#:~:text=A%20common%20SYM%20pathway%20controls,symbiosis%20(~400%20MYA)%20pathway</ref>. This pathway is characterized by its use of calcium as a second messenger, regulating both arbuscular mycorrhizal symbiosis and rhizobial symbiosis. Due to the SYM pathway evolutionary phylogeny, it is believed that legumes demonstrating root nodule symbiosis adapted the use of AMF to increase their uptake of nitrogen <ref name=bb/>. | |||
<br><br> | |||
Unlike legumes which maintain root nodule symbiosis, AMF have limited accessibility to acquire atmospheric nitrogen. A study has shown that the arbuscular mycorrhizal association between the fungus <i>Rhizophagus irregularis</i> and rhizobia communities increase nitrogen acquisition in the mycorrhizal grass species <i>Brachypodium distachyon</i> <ref name=al>[Averill, C., Turner, B. L., & Finzi, A. C. (2014). Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature, 505(7484), 543–545. doi: 10.1038/nature12901]</ref>. Growth of <i>Brachypodium distachyon</i> fertilized with <i>Rhizophagus irregularis</i> spores acquired two to threefold more nitrogen from organic matter than from the <i>Brachypodium distachyon</i> control without fertilization with AMF or soil microbial communities (Fig. 6)<ref name=al/>. <i>Brachypodium distachyon</i> fertilized with both <i>Rhizophagus irregularis</i> and soil microbial communities acquired ten to twelvefold more nitrogen from organic matter than the control (Fig. 6)<ref name=al/>. The increased uptake of organic nitrogen in host plants with AM symbiosis represents the extreme degree of importance that AM symbiosis has on nutrient cycles within global ecosystems. | |||
<br><br> | |||
== | ==Applications== | ||
< | There has been plenty of research pertaining to the efficacy of introducing fungal spores into crop fertilizer, specifically to utilize and maintain mycorrhizal symbiosis between plants and fungi <ref name=ag/>. The branching networks of mycelium that the fungi form increase the absorption of water within the soil as well as aerate the soil to aid in plant growth and colonization. Introducing hyphae into environments has the ability to promote plant growth and diversity upon other forms of agricultural intervention. | ||
<br><br> | |||
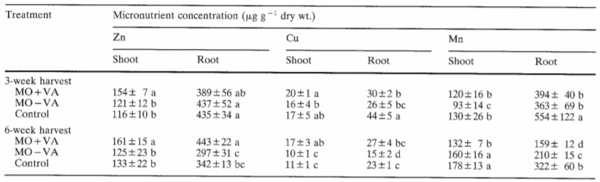
== | [[File:Nutrientcyclemyco.PNG|thumb|600px|left|<b>Table 1.</b>Micronutrient concentrations in shoots and roots of maize plants grown in different soil treatments for 3 and 6 weeks. Values within a single column followed by different letters are significantly different (Duncan test, P_<0.05). [https://link-springer-com.libproxy.kenyon.edu/content/pdf/10.1007/BF00207404.pdf].]] | ||
< | ===Nutrient Cycling=== | ||
Studies have analyzed the effect of the addition of AMF into the soil of agricultural plants and found increased nutrient acquisition and plant growth with the utilization of this symbiosis<ref name = aaa>[Kothari, S. K., Marschner, H., & Römheld, V. (1991). Contribution of the VA mycorrhizal hyphae in the acquisition of phosphorus and zinc by maize grown in a calcareous soil. <i>Plant and Soil, 131</i>(2), 177–185. doi: 10.1007/BF00009447]</ref><ref name = aac>[Azaizeh, H. A., Marschner, H., Römheld, V., & Wittenmayer, L. (1995). Effects of a vesicular-arbuscular mycorrhizal fungus and other soil microorganisms on growth, mineral nutrient acquisition, and root exudation of soil-grown maize plants.<i> Mycorrhiza, 5</i>(5), 321–327. doi: 10.1007/BF00207404]</ref><ref name = acc>[Arines, J., Vilarino, A., & Sainz, M. (1989). Effect of Different Inocula of Vesicular-Arbuscular Mycorrhizal Fungi on Manganese Content and Concentration in Red Clover (Trifolium pratense L.) Plants. <i>The New Phytologist, 112</i>(2), 215-219. Retrieved April 17, 2021, from http://www.jstor.org/stable/2556903]</ref>. One study demonstrated the effectiveness of arbuscular mycorrhizae with the growth of soil-grown maize plants, showing a significant increase in shoot dry weight and the shoot: root ratio of the maize plants after only 6 weeks of infection with AMF compared to the control plants<ref name=aac/>. After six weeks of infection, it was also shown that there were significant increases in nutrient concentrations of zinc, copper, nitrogen, and phosphorus in both the shoots and roots of the maize plants (Table 1)<ref name=aac/>. Another study found similar results, studying the acquisition of P and Zn by maize plants inoculated with AMF, demonstrating a 1.3 to 3-fold increase of P concentration in the maize roots and shoots, as well as a 1.7 to 3.8-fold increase of Zn concentration in shoots after AMF inoculation<ref name = aaa/>. The study by Arines et al (1989) also demonstrated a significant decrease in manganese concentrations within the plants, an essential nutrient for plant growth, but can be toxic in high concentrations<ref name=aac/><ref name = aad>[S. V., . K. V. H., & . S. J. (2007). Characterization of manganese toxicity and its influence on nutrient uptake, antioxidant enzymes and biochemical parameters in tea. <i>Research Journal of Phytochemistry, 1</i>(2), 52–60. doi: 10.3923/rjphyto.2007.52.60]</ref><ref name = aae>[Millaleo, R., Reyes- Diaz, M., Ivanov, A. G., Mora, M. L., & Alberdi, M. (2010). Manganese as essential and toxic element for plants: transport, accumulation and resistance mechanisms.<i> Journal of Soil Science and Plant Nutrition, 10</i>(4), 470–481. doi: 10.4067/S0718-95162010000200008]</ref>. It is deduced that the decrease in Mn concentration may be related to the AMF decreasing the amount of Mn-reducing bacteria, or the increase of Mn-oxidizing bacteria<ref name=aac/><ref name = aaf>[Posta, K., Marschner, H., & Römheld, V. (1994). Manganese reduction in the rhizosphere of mycorrhizal and nonmycorrhizal maize. <i>Mycorrhiza, 5</i>(2), 119–124. doi: 10.1007/BF00202343]</ref>. | |||
== | <br><br> | ||
< | ===Disease Resistance=== | ||
Mycorrhizal associations have been important symbioses with research regarding natural biofertilizers, not only for their help in nutrient aggregation in soil, but for their implications in plant disease control<ref name=ddd>[Berdeni, D., Cotton, T. E. A., Daniell, T. J., Bidartondo, M. I., Cameron, D. D., & Evans, K. L. (2018). The Effects of Arbuscular Mycorrhizal Fungal Colonisation on Nutrient Status, Growth, Productivity, and Canker Resistance of Apple (Malus pumila). Frontiers in Microbiology, 9, 1461. doi: 10.3389/fmicb.2018.01461]</ref><ref name=eee>[Mukerji, K. G., & Ciancio, A. (2007). Mycorrhizae in the integrated pest and disease management. In A. Ciancio & K. G. Mukerji (Eds.), General concepts in integrated pest and disease management (pp. 245–266). Dordrecht: Springer Netherlands. doi: 10.1007/978-1-4020-6061-8_10]</ref></ref><ref name=ccc/></ref><ref name=fff>[Berruti, A., Lumini, E., Balestrini, R., & Bianciotto, V. (2015). Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Successes. Frontiers in Microbiology, 6, 1559. doi: 10.3389/fmicb.2015.01559]</ref>. A study found that reduced fertilizer applications will allow for benefits in phosphorus and nitrogen uptake in plants due to its effect on AMF<ref name=ddd/>. By the third year of suggested fertilizer application, P and N concentrations in<i> Malus pumila</i> leaves were significantly reduced due to both the reduced biomass of the plant roots and of the hyphal networks from AMF<ref name=ddd/>. The study had also studied the mycorrhizal effects on disease resistance, finding that the inoculation of AMF improved the resistance of <i> Malus pumila</i> to the fungal pathogen <i>N. ditissima</i> by decreasing the amount of infected material by 18%<ref name=ddd/>. The pathway for the increased disease resistance is not fully understood and requires further study, however, it is clear from a multitude of studies that AMF colonization increases nutrient uptake and disease resistance in crops<ref name=zzz>[ Wang, Y.-Y., Yin, Q.-S., Qu, Y., Li, G.-Z., & Hao, L. (2018). Arbuscular mycorrhiza-mediated resistance in tomato against Cladosporium fulvum -induced mould disease. Journal of Phytopathology, 166(1), 67–74. doi: 10.1111/jph.12662 ]</ref><ref name=qqq>[Huang, J., Luo, S., & Zeng, R. (2003). [Mechanisms of plant disease resistance induced by arbuscular mycorrhizal fungi]. Ying Yong Sheng Tai Xue Bao = the Journal of Applied Ecology, 14(5), 819–822.]</ref>. | |||
<br><br> | |||
==Conclusion== | ==Conclusion== | ||
< | Although many people commonly associate fungi with disease or death, filamentous fungi demonstrate remarkable benefits for terrestrial organisms and the global environment. Mycorrhizal fungi were involved in the colonization of land plants, and continue to form symbiotic relationships, increasing plant diversity and helping to eliminate selection pressures from the environment. It is essential that mycorrhizal fungi and symbioses continue to be studied, as results increase our understanding of how AMF continues to be beneficial to the global environment. Integrating the various disciplines of mycorrhizal understanding is extremely relevant as our understanding of these associations have merit in reducing the negative effects of climate change and can increase yield of agricultural production on a large scale without the use of fertilizers and pesticides that damage the environment<ref name=www>[Torres, N., Antolín, M. C., & Goicoechea, N. (2018). Arbuscular mycorrhizal symbiosis as a promising resource for improving berry quality in grapevines under changing environments. <i>Frontiers in Plant Science, 9</i>, 897. doi: 10.3389/fpls.2018.00897]</ref><ref name=wwe>[Bettoni, M.M., Mogor, Á.F., Pauletti, V. et al. The interaction between mycorrhizal inoculation, humic acids supply and elevated atmospheric CO2 increases energetic and antioxidant properties and sweetness of yellow onion. <i>Hortic. Environ. Biotechnol. </i>58, 432–440 (2017). https://doi.org/10.1007/s13580-017-0122-4]</ref><ref name=wwr>[ Baslam, M, Antolín, M. C., Gogorcena, Y., Muñoz, F., & Goicoechea, N. (2014). Changes in alfalfa forage quality and stem carbohydrates induced by arbuscular mycorrhizal fungi and elevated atmospheric CO2. <i>The Annals of Applied Biology, 164</i>(2), 190–199. doi: 10.1111/aab.12092 ]</ref><ref name=wwt>[Baslam, Marouane, Garmendia, I., & Goicoechea, N. (2011). Arbuscular mycorrhizal fungi (AMF) improved growth and nutritional quality of greenhouse-grown lettuce. <i>Journal of Agricultural and Food Chemistry, 59</i>(10), 5504–5515. doi: 10.1021/jf200501c]</ref><ref name=wwy>[ Baslam, Marouane, Garmendia, I., & Goicoechea, N. (2013). The arbuscular mycorrhizal symbiosis can overcome reductions in yield and nutritional quality in greenhouse-lettuces cultivated at inappropriate growing seasons. <i>Scientia Horticulturae, 164</i>, 145–154. doi: 10.1016/j.scienta.2013.09.021]</ref>. | ||
<br><br> | |||
==References== | ==References== | ||
<references/> | |||
<br><br>Authored for BIOL 238 Microbiology, taught by [mailto:slonczewski@kenyon.edu Joan Slonczewski], 2021, [http://www.kenyon.edu/index.xml Kenyon College]. | |||
Latest revision as of 14:11, 17 April 2021
By Freya Beinart
Introduction

A mycorrhiza is the symbiotic relationship between fungi and plants. Mycelium, the vegetative part of a fungus, extends in a branching network of hyphae that secrete enzymes, breaking down organic material into nutrients. The vast mycelial branches of the fungi are made of branching networks of hyphae which, in a mycorrhizal association, colonize the root systems of many plant species, greatly increasing the surface area of a plant’s rhizosphere in which it collects water and essential nutrients [1] [2]. In exchange for these nutrients, the plants provide the fungi with carbohydrates that the fungi cannot produce on its own, being heterotrophic. The hyphae of soil fungi colonize the root system of plants, providing the photosynthetic organisms with nutrients and water in exchange for carbohydrates that the heterotrophic fungi cannot produce on its own. This colonization may be intracellular as endomycorrhizal fungi which trade nutrients via a signal transduction pathway, or extracellular as ectomycorrhizal fungi that envelop plant roots [3][4].
About 10% of identified fungal species are known to occur in mycorrhizal symbioses, these fungi are most species of the Glomeromycota, and many species of the Ascomycota and Basidiomycota [5]. The fungal symbionts assist host plants to be less susceptible to pathogens and environmental stresses such as lack of nutrient density, drought, and high salinity [6][1][7][8][9]. The vast underground networks of hyphae increase nutrient cycling, erosion resistance, air permeability, and water permeability within the soil itself. Including and maintaining these associations between fungi and plants has proven to be highly beneficial for agricultural health and biogeochemical cycling [10][7][11][12][9][13].
Types of Mycorrhizal Symbioses
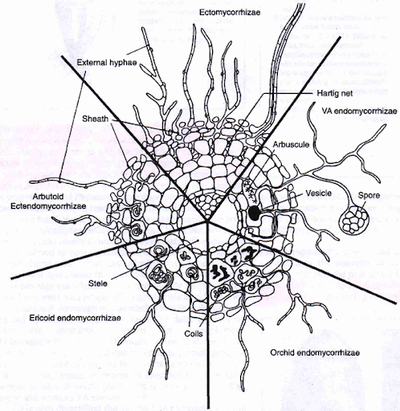
Characterization of mycorrhizal association is dependent upon the location of fungal colonization and is specific to both the plant species and their fungal symbionts.
Endomycorrhizae
Endomycorrhizal symbioses occur when the mycorrhizal fungi colonizes a host plant intracellularly.
Arbuscular Mycorrhizae
Fungi of the phylum Glomeromycota that forms mutualistic symbioses, called arbuscular mycorrhizae, with vascular plants are referred to as arbuscular mycorrhizal fungi, or AMF. These fungi are associated with 90% of vascular plant species. AMF are unable to live without the presence of the host plants, as they depend on the transfer of carbohydrates from the plant tissue, making the fungi obligate biotrophs [14]. Plant taxa that are observed to exhibit this relationship are bryophyta, pteridophyta, gymnosperms, and angiosperms][15].
Hyphae from a germinating spore infect the host root, passing the epidermis and penetrating cortical cells which form arbuscules - the structure from which the name of these fungi originated <[14]. The arbuscules are networks of extremely fine clustered hyphae within the host cells where nutrient transfer is centralized [16].The hyphae may also form vesicles between or within the root cells, acting as an organ for storage with a thickened cell wall which aids in the establishment of new colonies [1] [17]. The mycelium which extends from the plant root is called the extraradical mycelium, which connects from plant to plant, forming a continuum of nutrient and water exchange [18].
Ericoid mycorrhizae
Ericoid mycorrhizae are usually found in environments with high acidity and free-draining soils susceptible to droughts. These associations involve a mycorrhizal fungus in the Ascomycota and a host plant of the Ericideae family [2][4][19]. The hyphal coils are where nutrient transfer occurs within ericoid mycorrhizae. Experimentation of ericoid mycorrhizal fungi growth in axenic has revealed a range of saprotrophic capabilities, allowing the fungi to feed on decaying organic matter [20].
Ectomycorrhizae
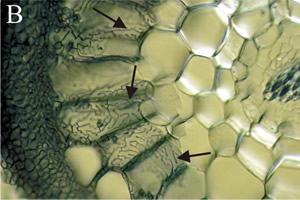
Fungi that form ectomycorrhizae are majorly Basidiomycota, as well as some Ascomycota and Zygomycetes of the genus Endogone. These associations differ from AM symbioses in the way that the fungi does not penetrate the root cells, instead, it grows intercellularly. Hyphae grow between epidermal and cortical cells of the plant, forming a structure called the Hartig net where nutrient transfer is localized [4]. Hyphae of ectomycorrhizal fungi create sheathes which envelop the exterior of the plant root, called mantle. The hyphae extend outward from the plant root to extend the surface area of the plant root, forming a structure called a rhizomorph. The rhizomorphs’ increased ability to uptake water and nutrients from the soil makes up for the suppression of root hairs by the mantle [21].
Ectomycorrhizae occur in about 10% of plant species, specifically woody plants in certain families of gymnosperms and angiosperms [22]. These fungi form fruiting bodies, unlike AMF which only reproduce asexually. Examples of these fruiting bodies are mushrooms of the genus Amanita and genus Tuber.
Ectendomycorrhizae
Ectendomycorrhizal fungi form Hartig net and mantle structures akin to Ectomycorrhizal fungi. Ectendomycorrhizal symbioses differ from ectomycorrhizae as the hyphae penetrate the cortical cells of plant roots, forming an intracellular Hartig net. One type of ectendomycorrhiza is arbutoid mycorrhiza, which is a symbiosis between basidiomycetes and some plant species of the Ericaceae family [23].
Evolution of Mycorrhizae
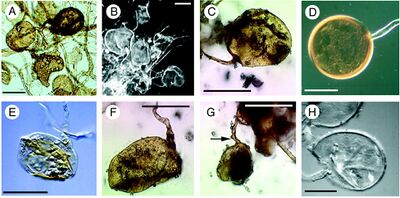
Fossil records indicate that mycorrhizal fungi predate the evolution of vascular plants, about 460 million years ago in the Ordovician period [24]. The most ancestral mycorrhizal fungi has been identified as arbuscular, penetrating the root cortical cells in most extant plant taxa. The phylum of fungi that associates with land plants as AMF is that of Glomeromycota [25] The presence of these fungi were involved in the development of soil and the evolution and colonization of vascular land plants, specifically by aiding the non-vascular plants with acquisition of nutrients through fungal hyphae, as the “soil” lacked a significant amount of nutrients for terrestrial plants to survive without their fungal symbionts [26]. As the fungi cycles nutrients such as nitrogen, phosphorus, and sulfur to the plants, the fungus receives and fixes carbon into the ground, assisting in the lowering of atmospheric CO2 leading to the oxygenation of the atmosphere during the development of terrestrial plants [27] [28].
In a study by Krings et al (2007) fossil evidence from the Rhynie chert sediment of the Early Devonian period suggests that fungal endophytes, specifically those which colonize the rhizoids of Nothia aphylla, actively influenced the evolution of these plants due to observed host responses. The responses to fungal infection in N. aphylla - rhizoid bulging, separation of infected cells via thickening of cell walls, and the motile inhibition of hypha - suggests its susceptibility to colonization by fungi at least 400 million years ago, advancing selection between plant species with the increasing complexity of interspecies interactions [29].
The other form of mycorrhiza associated with the intercellular colonization of fungal symbionts within plants, ectomycorrhiza, evolved much later than its ancestor AMF. Fossil records suggest that ectomycorrhizal fungi may have evolved at least 156 million years ago, as the oldest known extant plant family associated with ectomycorrhizal fungi, Pinaceae, appear to have evolved in that time period [30]. Another study shows the morphological identification of ectomycorrhizal fungi based on apparent Hartig net, mantle, and hyphal structures on fossils of Pinus roots dating back 50 million years, clearly representing the established ectomycorrhizal associations (Fig. 4) [31].
Biogeochemical Cycles
Carbon Storage
Carbon is an extremely important element as it is the primary component of organic compounds and macromolecules. Major amounts of carbon stores are located within the soil, containing more soil than the atmosphere and vegetation combined [32]. Saprotrophic fungi play an extremely important role in the carbon cycle as they decompose organic matter, releasing nutrients into the soil. It was believed that most of the carbon found in terrestrial ecosystems was stored in vegetative remains, or leaf litter, however recent studies suggest that most of the stored carbon is derived from the mycelium of mycorrhizal fungi [33][34].
High-resolution maps of vegetative biomass associated with mycorrhizal associations elucidate the significant difference in the biogeochemical cycles of ecosystems that are dominated by these symbioses and those that lack mycorrhizae [35]. This study indicates that non-mycorrhizal vegetation stores 29± 5.5 GT carbon in aboveground biomass, whereas arbuscular, ectomycorrhizal, and ericoid mycorrhizal vegetation store, respectively, 241 ± 15, 100 ± 17, and 7 ± 1.8 GT carbon (Figure 5)[35]. The same study also quantified the human influence on the biomass of terrestrial ecosystems, reducing ectomycorrhizae in all continents due to the substitution of forests with agricultural lands[35]. The practice of excess fertilization and destruction of hyphal networks derived from the replacement of natural land with agricultural land is to account for the decrease in global mycorrhizal biomass, which could, in turn, result in loss of total soil carbon[35].
Nitrogen Fixation
Nitrogen is an extremely important nutrient for all living organisms as it is essential for amino acid synthesis and therefore the formation of proteins. Despite its importance, nitrogen cannot be used by many organisms until it has been heavily reduced into ammonia [36]. This anabolic process is very energy-intensive, and therefore many organisms do not possess the ability to fix nitrogen [37] [38]. Organisms that can fix atmospheric nitrogen are known as diazotrophs - bacteria and archaea that do not require fixed nitrogen for their growth or survival [39].
Root nodule symbiosis between plants and nitrogen-fixing bacteria is apparent in plants of the Fabaceae, also known as the legume family[40]. Legumes have a symbiotic relationship with rhizobia, single-celled, Gram-negative bacteria [40] [41]. The mutualism between the legumes and rhizobia allows the bacteria to take up atmospheric nitrogen, feeding it to the plant. In return, the plant provides carbohydrates to the bacteria as it is essential for their metabolic processes and growth [37]. Although mycorrhizal symbionts do not possess the metabolism required for nitrogen fixation, studies have shown that mycorrhizae may indirectly affect nitrogen fixation as they increase root nodulation [40] [41][42].
The legume symbiotic signaling (SYM) pathway has been studied in relation to arbuscular mycorrhizal symbiosis [3] [42]. The SYM pathway controls both nodulation of legumes and the mycorrhization of land plants, and is suggested to evolve first in legumes and adapted for arbuscular mycorrhizal symbiosis [43]. This pathway is characterized by its use of calcium as a second messenger, regulating both arbuscular mycorrhizal symbiosis and rhizobial symbiosis. Due to the SYM pathway evolutionary phylogeny, it is believed that legumes demonstrating root nodule symbiosis adapted the use of AMF to increase their uptake of nitrogen [9].
Unlike legumes which maintain root nodule symbiosis, AMF have limited accessibility to acquire atmospheric nitrogen. A study has shown that the arbuscular mycorrhizal association between the fungus Rhizophagus irregularis and rhizobia communities increase nitrogen acquisition in the mycorrhizal grass species Brachypodium distachyon [44]. Growth of Brachypodium distachyon fertilized with Rhizophagus irregularis spores acquired two to threefold more nitrogen from organic matter than from the Brachypodium distachyon control without fertilization with AMF or soil microbial communities (Fig. 6)[44]. Brachypodium distachyon fertilized with both Rhizophagus irregularis and soil microbial communities acquired ten to twelvefold more nitrogen from organic matter than the control (Fig. 6)[44]. The increased uptake of organic nitrogen in host plants with AM symbiosis represents the extreme degree of importance that AM symbiosis has on nutrient cycles within global ecosystems.
Applications
There has been plenty of research pertaining to the efficacy of introducing fungal spores into crop fertilizer, specifically to utilize and maintain mycorrhizal symbiosis between plants and fungi [13]. The branching networks of mycelium that the fungi form increase the absorption of water within the soil as well as aerate the soil to aid in plant growth and colonization. Introducing hyphae into environments has the ability to promote plant growth and diversity upon other forms of agricultural intervention.
Nutrient Cycling
Studies have analyzed the effect of the addition of AMF into the soil of agricultural plants and found increased nutrient acquisition and plant growth with the utilization of this symbiosis[45][46][47]. One study demonstrated the effectiveness of arbuscular mycorrhizae with the growth of soil-grown maize plants, showing a significant increase in shoot dry weight and the shoot: root ratio of the maize plants after only 6 weeks of infection with AMF compared to the control plants[46]. After six weeks of infection, it was also shown that there were significant increases in nutrient concentrations of zinc, copper, nitrogen, and phosphorus in both the shoots and roots of the maize plants (Table 1)[46]. Another study found similar results, studying the acquisition of P and Zn by maize plants inoculated with AMF, demonstrating a 1.3 to 3-fold increase of P concentration in the maize roots and shoots, as well as a 1.7 to 3.8-fold increase of Zn concentration in shoots after AMF inoculation[45]. The study by Arines et al (1989) also demonstrated a significant decrease in manganese concentrations within the plants, an essential nutrient for plant growth, but can be toxic in high concentrations[46][48][49]. It is deduced that the decrease in Mn concentration may be related to the AMF decreasing the amount of Mn-reducing bacteria, or the increase of Mn-oxidizing bacteria[46][50].
Disease Resistance
Mycorrhizal associations have been important symbioses with research regarding natural biofertilizers, not only for their help in nutrient aggregation in soil, but for their implications in plant disease control[51][52]</ref>[10]</ref>[53]. A study found that reduced fertilizer applications will allow for benefits in phosphorus and nitrogen uptake in plants due to its effect on AMF[51]. By the third year of suggested fertilizer application, P and N concentrations in Malus pumila leaves were significantly reduced due to both the reduced biomass of the plant roots and of the hyphal networks from AMF[51]. The study had also studied the mycorrhizal effects on disease resistance, finding that the inoculation of AMF improved the resistance of Malus pumila to the fungal pathogen N. ditissima by decreasing the amount of infected material by 18%[51]. The pathway for the increased disease resistance is not fully understood and requires further study, however, it is clear from a multitude of studies that AMF colonization increases nutrient uptake and disease resistance in crops[54][55].
Conclusion
Although many people commonly associate fungi with disease or death, filamentous fungi demonstrate remarkable benefits for terrestrial organisms and the global environment. Mycorrhizal fungi were involved in the colonization of land plants, and continue to form symbiotic relationships, increasing plant diversity and helping to eliminate selection pressures from the environment. It is essential that mycorrhizal fungi and symbioses continue to be studied, as results increase our understanding of how AMF continues to be beneficial to the global environment. Integrating the various disciplines of mycorrhizal understanding is extremely relevant as our understanding of these associations have merit in reducing the negative effects of climate change and can increase yield of agricultural production on a large scale without the use of fertilizers and pesticides that damage the environment[56][57][58][59][60].
References
- ↑ 1.0 1.1 1.2 [Brundrett, M. (1991). Mycorrhizas in natural ecosystems. In Advances in Ecological Research: Vol. 21 (pp. 171–313). Elsevier. doi: 10.1016/S0065-2504(08)60099-9
- ↑ 2.0 2.1 [Johnson, N. C., & Gehring, C. A. (2007). Mycorrhizas: symbiotic mediators of rhizosphere and ecosystem processes. In The Rhizosphere (pp. 73–100). Elsevier. doi: 10.1016/B978-012088775-0/50006-9]]
- ↑ 3.0 3.1 [Gutjahr, C., Banba, M., Croset, V., An, K., Miyao, A., An, G., … Paszkowski, U. (2008). Arbuscular mycorrhiza-specific signaling in rice transcends the common symbiosis signaling pathway. The Plant Cell, 20(11), 2989–3005. doi: 10.1105/tpc.108.062414]
- ↑ 4.0 4.1 4.2 [Smith, S. E., & Read, D. J. (2002). Structure and development of ectomycorrhizal roots. In Mycorrhizal Symbiosis (pp. 163–V). Elsevier. doi: 10.1016/B978-012652840-4/50007-3]
- ↑ Lewis, J. D. (2016). Mycorrhizal fungi, evolution and diversification of. In Encyclopedia of evolutionary biology (pp. 94–99). Elsevier. doi: 10.1016/B978-0-12-800049-6.00251-1
- ↑ Ayub, M. A., Ahmad, H. R., Ali, M., Rizwan, M., Ali, S., Zia ur Rehman, M., & Waris, A. A. (2020). Salinity and its tolerance strategies in plants. In Plant life under changing environment (pp. 47–76). Elsevier. doi: 10.1016/B978-0-12-818204-8.00003-5
- ↑ 7.0 7.1 [Bunn, R. A., Simpson, D. T., Bullington, L. S., Lekberg, Y., & Janos, D. P. (2019). Revisiting the “direct mineral cycling” hypothesis: arbuscular mycorrhizal fungi colonize leaf litter, but why? The ISME Journal, 13(8), 1891–1898. doi: 10.1038/s41396-019-0403-2]
- ↑ Evelin, H., Devi, T. S., Gupta, S., & Kapoor, R. (2019). Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: current understanding and new challenges. Frontiers in Plant Science, 10, 470. doi: 10.3389/fpls.2019.00470
- ↑ 9.0 9.1 9.2 [Haskett, T. L., Tkacz, A., & Poole, P. S. (2021). Engineering rhizobacteria for sustainable agriculture. The ISME Journal, 15(4), 949–964. doi: 10.1038/s41396-020-00835-4]
- ↑ 10.0 10.1 [Begum, N., Qin, C., Ahanger, M. A., Raza, S., Khan, M. I., Ashraf, M., … Zhang, L. (2019). Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Frontiers in Plant Science, 10, 1068. doi: 10.3389/fpls.2019.01068]
- ↑ Corradi, N., & Bonfante, P. (2012). The arbuscular mycorrhizal symbiosis: origin and evolution of a beneficial plant infection. PLoS Pathogens, 8(4), e1002600. doi: 10.1371/journal.ppat.1002600
- ↑ Gadgil, R. L., & Gadgil, P. D. (1971). Mycorrhiza and litter decomposition. Nature, 233(5315), 133. doi: 10.1038/233133a0
- ↑ 13.0 13.1 [Rashid, M. I., Mujawar, L. H., Shahzad, T., Almeelbi, T., Ismail, I. M. I., & Oves, M. (2016). Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiological Research, 183, 26–41. doi: 10.1016/j.micres.2015.11.007]
- ↑ 14.0 14.1 [Smith, S. E., & Reads, D. J. (n.d.). Mycorrhizal Symbiosis. Retrieved April 06, 2021, from https://books.google.com/books?hl=en&lr=&id=qLciOJaG0C4C&oi=fnd&pg=PP1&ots=zrsXoTRxkN&sig=G4cYDH7aK0uYRBaENI2m3JvclNA#v=onepage&q&f=false
- ↑ Barman, J., Samanta, A., Saha, B., & Datta, S. (2016). Mycorrhiza. Resonance, 21(12), 1093-1104. doi:10.1007/s12045-016-0421-6
- ↑ Moore, D., Robson, G., & Trinci, A. (2011, July 14). 21St century guidebook TO Fungi: Plant science. Retrieved April 05, 2021, from https://www.cambridge.org/gb/academic/subjects/life-sciences/plant-science/21st-century-guidebook-fungi?format=WW&isbn=9780521186957
- ↑ Müller, A., Ngwene, B., Peiter, E., & George, E. (2017). Quantity and distribution of AMF storage organs within dead roots. Mycorrhiza, 27(3), 201–210. doi: 10.1007/s00572-016-0741-0
- ↑ Bhargava, P., Vats, S., & Gupta, N. (2019). Metagenomics as a tool to explore mycorrhizal fungal communities. Mycorrhizosphere and Pedogenesis, 207-219. doi:10.1007/978-981-13-6480-8_13
- ↑ Dighton, J. (2009). Mycorrhizae. In Encyclopedia of Microbiology (pp. 153–162). Elsevier. doi: 10.1016/B978-012373944-5.00327-8
- ↑ Martino, E., Morin, E., Grelet, G.-A., Kuo, A., Kohler, A., Daghino, S., … Perotto, S. (2018). Comparative genomics and transcriptomics depict ericoid mycorrhizal fungi as versatile saprotrophs and plant mutualists. The New Phytologist, 217(3), 1213–1229. doi: 10.1111/nph.14974
- ↑ Anderson, I. C., & Cairney, J. W. G. (2007). Ectomycorrhizal fungi: exploring the mycelial frontier. FEMS Microbiology Reviews, 31(4), 388–406. doi: 10.1111/j.1574-6976.2007.00073.x
- ↑ Wang, B.; Qiu, Y.-L. (July 2006). "Phylogenetic distribution and evolution of mycorrhizas in land plants". Mycorrhiza. 16 (5): 299–363. doi:10.1007/s00572-005-0033-6. PMID 16845554. S2CID 30468942.
- ↑ Kühdorf, K., Münzenberger, B., Begerow, D., Gómez-Laurito, J., & Hüttl, R. F. (2015). Leotia cf. lubrica forms arbutoid mycorrhiza with Comarostaphylis arbutoides (Ericaceae). Mycorrhiza, 25(2), 109–120. doi: 10.1007/s00572-014-0590-7
- ↑ Redecker, D., Kodner, R., & Graham, L. E. (2000). Glomalean fungi from the Ordovician. Science, 289(5486), 1920–1921. doi: 10.1126/science.289.5486.1920
- ↑ Schüβler, A., Schwarzott, D., & Walker, C. (2001). A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycological Research, 105(12), 1413–1421. doi: 10.1017/S0953756201005196
- ↑ Pirozynski, K. A., & Malloch, D. W. (1975). The origin of land plants: a matter of mycotrophism. Bio Systems, 6(3), 153–164. doi: 10.1016/0303-2647(75)90023-4
- ↑ Mills, B. J. W., Batterman, S. A., & Field, K. J. (2018). Nutrient acquisition by symbiotic fungi governs Palaeozoic climate transition. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 373(1739). doi: 10.1098/rstb.2016.0503
- ↑ Strullu-Derrien, C., Selosse, M.-A., Kenrick, P., & Martin, F. M. (2018). The origin and evolution of mycorrhizal symbioses: from palaeomycology to phylogenomics. The New Phytologist, 220(4), 1012–1030. doi: 10.1111/nph.15076
- ↑ Krings, M., Taylor, T. N., Hass, H., Kerp, H., Dotzler, N., & Hermsen, E. J. (2007). Fungal endophytes in a 400-million-yr-old land plant: infection pathways, spatial distribution, and host responses. The New Phytologist, 174(3), 648–657. doi: 10.1111/j.1469-8137.2007.02008.x
- ↑ Tedersoo, L., May, T. W., & Smith, M. E. (2010). Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza, 20(4), 217–263. doi: 10.1007/s00572-009-0274-x
- ↑ Lepage, B., Currah, R., Stockey, R., & Rothwell, G. (1997). Fossil ectomycorrhizae from the Middle Eocene. American Journal of Botany, 84(3), 410. doi: 10.2307/2446014
- ↑ Tarnocai et al. 2009. Soil organic carbon pools in the northern circumpolar permafrost region. Global Biogeochemical Cycles, 23(2) doi: 10.1029/2008GB003327
- ↑ Clemmensen, K E, Bahr, A., Ovaskainen, O., Dahlberg, A., Ekblad, A., Wallander, H., … Lindahl, B. D. (2013). Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science, 339(6127), 1615–1618. doi: 10.1126/science.1231923
- ↑ Clemmensen, Karina E, Finlay, R. D., Dahlberg, A., Stenlid, J., Wardle, D. A., & Lindahl, B. D. (2015). Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. The New Phytologist, 205(4), 1525–1536. doi: 10.1111/nph.13208
- ↑ 35.0 35.1 35.2 35.3 [Soudzilovskaia, N.A., van Bodegom, P.M., Terrer, C. et al. Global mycorrhizal plant distribution linked to terrestrial carbon stocks. Nat Commun 10, 5077 (2019). https://doi.org/10.1038/s41467-019-13019-2]
- ↑ Society, M. (n.d.). Nitrogen cycle: Microbes and the outdoors. Retrieved April 06, 2021, from https://microbiologysociety.org/why-microbiology-matters/what-is-microbiology/microbes-and-the-outdoors/nitrogen-cycle.html#:~:text=Nitrogen%20is%20required%20by%20all,and%20other%20nitrogen%20containing%20compounds.&text=It%20cannot%20be%20used%20in,with%20hydrogen)%2C%20to%20ammonia.
- ↑ 37.0 37.1 [Zahran, H. H. (1999). Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiology and Molecular Biology Reviews, 63(4), 968–989, table of contents. doi: 10.1128/MMBR.63.4.968-989.1999]
- ↑ Kuypers, M. M. M., Marchant, H. K., & Kartal, B. (2018). The microbial nitrogen-cycling network. Nature Reviews. Microbiology, 16(5), 263–276. doi: 10.1038/nrmicro.2018.9
- ↑ Takai, K. (2019). The nitrogen cycle: A large, fast, and mystifying cycle. Microbes and Environments / JSME, 34(3), 223–225. doi: 10.1264/jsme2.ME3403rh
- ↑ 40.0 40.1 40.2 [Brewin, N. J. (2001). Root Nodules (Legume- Rhizobium Symbiosis). In John Wiley & Sons, Ltd (Ed.), Encyclopedia of life sciences. Chichester, UK: John Wiley & Sons, Ltd. doi: 10.1002/9780470015902.a0003720.pub2]
- ↑ 41.0 41.1 [Hayman, D. S. (1986). Mycorrhizae of nitrogen-fixing legumes. MIRCEN Journal of Applied Microbiology and Biotechnology, 2(1), 121–145. doi: 10.1007/BF00937189]
- ↑ 42.0 42.1 [Mus, F., Crook, M. B., Garcia, K., Garcia Costas, A., Geddes, B. A., Kouri, E. D., … Peters, J. W. (2016). Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Applied and Environmental Microbiology, 82(13), 3698–3710. doi: 10.1128/AEM.01055-16]
- ↑ Role of the SYM pathway in selecting the root microbiota. (n.d.). Retrieved April 6, 2021, from https://gtr.ukri.org/projects?ref=BB%2FR017859%2F1#:~:text=A%20common%20SYM%20pathway%20controls,symbiosis%20(~400%20MYA)%20pathway
- ↑ 44.0 44.1 44.2 [Averill, C., Turner, B. L., & Finzi, A. C. (2014). Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature, 505(7484), 543–545. doi: 10.1038/nature12901]
- ↑ 45.0 45.1 [Kothari, S. K., Marschner, H., & Römheld, V. (1991). Contribution of the VA mycorrhizal hyphae in the acquisition of phosphorus and zinc by maize grown in a calcareous soil. Plant and Soil, 131(2), 177–185. doi: 10.1007/BF00009447]
- ↑ 46.0 46.1 46.2 46.3 46.4 [Azaizeh, H. A., Marschner, H., Römheld, V., & Wittenmayer, L. (1995). Effects of a vesicular-arbuscular mycorrhizal fungus and other soil microorganisms on growth, mineral nutrient acquisition, and root exudation of soil-grown maize plants. Mycorrhiza, 5(5), 321–327. doi: 10.1007/BF00207404]
- ↑ [Arines, J., Vilarino, A., & Sainz, M. (1989). Effect of Different Inocula of Vesicular-Arbuscular Mycorrhizal Fungi on Manganese Content and Concentration in Red Clover (Trifolium pratense L.) Plants. The New Phytologist, 112(2), 215-219. Retrieved April 17, 2021, from http://www.jstor.org/stable/2556903]
- ↑ [S. V., . K. V. H., & . S. J. (2007). Characterization of manganese toxicity and its influence on nutrient uptake, antioxidant enzymes and biochemical parameters in tea. Research Journal of Phytochemistry, 1(2), 52–60. doi: 10.3923/rjphyto.2007.52.60]
- ↑ [Millaleo, R., Reyes- Diaz, M., Ivanov, A. G., Mora, M. L., & Alberdi, M. (2010). Manganese as essential and toxic element for plants: transport, accumulation and resistance mechanisms. Journal of Soil Science and Plant Nutrition, 10(4), 470–481. doi: 10.4067/S0718-95162010000200008]
- ↑ [Posta, K., Marschner, H., & Römheld, V. (1994). Manganese reduction in the rhizosphere of mycorrhizal and nonmycorrhizal maize. Mycorrhiza, 5(2), 119–124. doi: 10.1007/BF00202343]
- ↑ 51.0 51.1 51.2 51.3 [Berdeni, D., Cotton, T. E. A., Daniell, T. J., Bidartondo, M. I., Cameron, D. D., & Evans, K. L. (2018). The Effects of Arbuscular Mycorrhizal Fungal Colonisation on Nutrient Status, Growth, Productivity, and Canker Resistance of Apple (Malus pumila). Frontiers in Microbiology, 9, 1461. doi: 10.3389/fmicb.2018.01461]
- ↑ [Mukerji, K. G., & Ciancio, A. (2007). Mycorrhizae in the integrated pest and disease management. In A. Ciancio & K. G. Mukerji (Eds.), General concepts in integrated pest and disease management (pp. 245–266). Dordrecht: Springer Netherlands. doi: 10.1007/978-1-4020-6061-8_10]
- ↑ [Berruti, A., Lumini, E., Balestrini, R., & Bianciotto, V. (2015). Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Successes. Frontiers in Microbiology, 6, 1559. doi: 10.3389/fmicb.2015.01559]
- ↑ [ Wang, Y.-Y., Yin, Q.-S., Qu, Y., Li, G.-Z., & Hao, L. (2018). Arbuscular mycorrhiza-mediated resistance in tomato against Cladosporium fulvum -induced mould disease. Journal of Phytopathology, 166(1), 67–74. doi: 10.1111/jph.12662 ]
- ↑ [Huang, J., Luo, S., & Zeng, R. (2003). [Mechanisms of plant disease resistance induced by arbuscular mycorrhizal fungi]. Ying Yong Sheng Tai Xue Bao = the Journal of Applied Ecology, 14(5), 819–822.]
- ↑ [Torres, N., Antolín, M. C., & Goicoechea, N. (2018). Arbuscular mycorrhizal symbiosis as a promising resource for improving berry quality in grapevines under changing environments. Frontiers in Plant Science, 9, 897. doi: 10.3389/fpls.2018.00897]
- ↑ [Bettoni, M.M., Mogor, Á.F., Pauletti, V. et al. The interaction between mycorrhizal inoculation, humic acids supply and elevated atmospheric CO2 increases energetic and antioxidant properties and sweetness of yellow onion. Hortic. Environ. Biotechnol. 58, 432–440 (2017). https://doi.org/10.1007/s13580-017-0122-4]
- ↑ [ Baslam, M, Antolín, M. C., Gogorcena, Y., Muñoz, F., & Goicoechea, N. (2014). Changes in alfalfa forage quality and stem carbohydrates induced by arbuscular mycorrhizal fungi and elevated atmospheric CO2. The Annals of Applied Biology, 164(2), 190–199. doi: 10.1111/aab.12092 ]
- ↑ [Baslam, Marouane, Garmendia, I., & Goicoechea, N. (2011). Arbuscular mycorrhizal fungi (AMF) improved growth and nutritional quality of greenhouse-grown lettuce. Journal of Agricultural and Food Chemistry, 59(10), 5504–5515. doi: 10.1021/jf200501c]
- ↑ [ Baslam, Marouane, Garmendia, I., & Goicoechea, N. (2013). The arbuscular mycorrhizal symbiosis can overcome reductions in yield and nutritional quality in greenhouse-lettuces cultivated at inappropriate growing seasons. Scientia Horticulturae, 164, 145–154. doi: 10.1016/j.scienta.2013.09.021]
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2021, Kenyon College.
