Neptunomonas: Difference between revisions
m (→Author) |
|||
| (29 intermediate revisions by one other user not shown) | |||
| Line 19: | Line 19: | ||
==Description and Significance== | ==Description and Significance== | ||
Appearance: | Appearance: | ||
The genus includes rod shaped (Bacillus) or slightly curved (vibrio) cells, with approximate sizes of 0.7–0.9 × 2.0–3.0 µm. Spherical (coccoid) body plans appear in older colonies, often associated with a loss of viability. They are capable of producing a capsid and are motile via a single flagellum. [1.] | The genus includes rod shaped (Bacillus) or slightly curved (vibrio) cells, with approximate sizes of 0.7–0.9 × 2.0–3.0 µm. Spherical (coccoid) body plans appear in older colonies, often associated with a loss of viability. They are capable of producing a capsid and are motile via a single flagellum. [1.] | ||
| Line 27: | Line 24: | ||
Habitat: | Habitat: | ||
Often associated with historically polluted marine coastal sediment, such as the Puget sound (Washington, USA), the site of a wood treatment facility and the Mediterranean Sea (Milazzo Harbor, Italy), which has been impacted by municipal wastes. | Often associated with historically polluted marine coastal sediment, such as the Puget sound (Washington, USA), the site of a wood treatment facility and the Mediterranean Sea (Milazzo Harbor, Italy), which has been impacted by municipal wastes. | ||
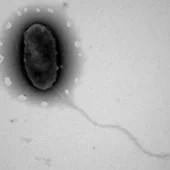
[[Image:osedax_bone.jpg|thumb|300px|right| Worms of the genus Osedax inhabiting the bones of whale fall on the deep ocean floor, with Neptunomonas inhabiting them. Image provided by © 2006 MBARI ]] | |||
In addition to these polluted sites, Neptunomonas is closely related (acting as endosymbionts) to the bone-eating polychaetes in the genus Osedax, which are inhabitants of whale carcasses within the deep sea. These endosymbionts are located within highly branched root tissues which burrow into the bones of these large carcasses, digesting them, releasing the organic carbon which can be used for nutrition. The specifics of this endosymbiosis is not fully known. [2.] | In addition to these polluted sites, Neptunomonas is closely related (acting as endosymbionts) to the bone-eating polychaetes in the genus Osedax, which are inhabitants of whale carcasses within the deep sea. These endosymbionts are located within highly branched root tissues which burrow into the bones of these large carcasses, digesting them, releasing the organic carbon which can be used for nutrition. The specifics of this endosymbiosis is not fully known. [2.] | ||
Importance: | |||
Neptunomonas main focus in scientific research is its capabilities to biodegrade PAH molecules as a result of its relation to the polluted areas it inhabits. These PAH molecules occur naturally in coal, crude oil, and gasoline; they are extremely toxic and hard to break down due to their long chains and long term exposure to them may result in lung cancer (a disease with high mortality rates) as well as other health issues [3.]. Therefore research into the mechanisms of how Neptunomonas is able to break these compounds down may result in the saving of lives in the future. | |||
==Genome Structure== | ==Genome Structure== | ||
===Neptunomonas concharum=== | |||
Neptunomonas concharum was one of the species of choice when examining a genome within this genus as a result of the extensive and complete genome sequencing conducted. The complete genome consisted of 3,561,992 bp, of which G-C base pairs made up 46%. 3273 protein coding sequences were found, with 69 tRNA genes, 15 complete rRNA genes (5 each for 5S, 16S, and 23S), and 4 ncRNAs. The chromosome type is circular. | |||
Bacteria containing prophage are more likely to show antibiotic resistance, greater environmental adaptability and improve adhesion. Concharum contained three incomplete prophage sequences. Additionally CRISPR/Cas systems play an important role in bacterial defence systems, fighting against viruses and other bacterial plasmid invasion, one credible CRISPR sequence containing 22 spacers was acknowledges in the genomic DNA. These sequences recognise some of the uniquely adapted sequences allowing concharum to function in toxic, polluted and otherwise extreme habitats. | |||
Neptunomonas concharum was | Metabolic pathways | ||
Neptunomonas concharum was found to differ from typical forms of glycolysis; the genome sequence contains all the genes encoding for glycolysis and gluconeogenesis, however no genes related to glucose phosphotransferase were found, resulting the lack of utilization of extracellular sugars in metabolism. It has been noted that concharum prefers to utilise | |||
acetate as a carbon source for cellular growth, backed by the identification of genes associated with acetate metabolism (Ack-Pta and Acs pathways, and three acetyl-CoA synthetase genes). [4.] | |||
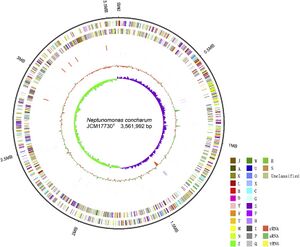
[[Image:Neptunomonas_genome.jpg|thumb|300px|right| Graphical map of the complete genome of N. concharum [4.]]] | |||
===Neptunomonas antarctica=== | |||
Additionally, the marine bacterium Neptunomonas antarctica was also examined and bared similar results. It did however have a longer genome of 4,568,828 bp with a mean G-C content of 45.7%. Despite this, similar functions were found within the genomes, 85 genes are associated with “Virulence, Disease and Defense”, an advantage in the heavily polluted environments these micro-organisms inhabit, aiding in resistance to sediment-associated pollutants including heavy-metal ions, antibiotics and other toxic compounds. | |||
Some additional findings associated with Neptunomonas antarctica were that 62 genes are involved in flagellar motility and 19 genes in bacterial chemotaxis. These would aid in the movement towards nutrient containing sediments and movement away from toxicity and predators, playing an important role in thier sediment adapted lifestyles. | |||
other genome sequences associated with utilizing nutrients in these polluted environments include: 21 nitrate and nitrite ammonification genes, 35 genes related to poisonous aromatic compounds metabolism and 42 genes related to stress response particularly related to the temperature stresses of an arctic environment. [5.] | |||
== | ==Metabolism and Cell Structure== | ||
Metabolism: This species can receive and use resources from a wide range of sources for metabolic engineering such as using some amino acids, carbohydrates, organic acids, or sugar alcohols as sole carbon sources and electron donors. They are aerobically respiring organisms, with selectivity anaerobic capabilities. Oxidising strains are commonly associated with polluted coastal marine sediments. The most notable method within metabolism for Neptunomonas is the catabolism of PAH molecules, a somewhat unique adaptation bought from the polluted coastal marine sediments they inhabit. [2.] | |||
Additionally, this organism is closely related to the endosymbionts of the bone-eating polychaetes in the genus Osedax, occurring in root structures produced by Osedax which penetrate the whale bone. Location and enzyme activity suggest Neptunomonas aid in the digestion of bones for the polychaete, however it is unknown yet if the resulting molecules are used in metabolic processes for the micro-organism. [4.] | |||
Cell structure: Neptunomonas is a gram negative bacterium, having a thin layer of peptidoglycan as compared to some other bacterium, making them harder to kill with treatments such as antibiotics. This Genus is also oxidase and catalase positive, with both of these enzymes being produced and present indicating its aerobic capabilities. [6.] | |||
==Ecology and Pathogenesis== | |||
This organism does not actively cause disease- but it may hold relevance in the treatment of disease as a result of cell structure. They have a recognised resistance to antibiotics meaning despite not causing disease, they may still hold relevance in the field. However research in this field is still required, with its uses in this area still unknown. [6.] | |||
==References== | ==References== | ||
1. [https://onlinelibrary.wiley.com/doi/10.1002/9781118960608.gbm01195 Hedlund B.P. "Neptunomonas", ''Neptunomonas''. 2015. p. 1–6.] | |||
[ | 2. [https://link.springer.com/referenceworkentry/10.1007/978-3-540-77587-4_127 Hedlund B.P. & Costa K.C. Neptunomonas. In: Timmis, K.N. (eds) ''Handbook of Hydrocarbon and Lipid Microbiology''. Springer, Berlin, Heidelberg.] | ||
3. [https://pubmed.ncbi.nlm.nih.gov/25911656/#:~:text=Excessive%20exposure%20to%20polycyclic%20aromatic%20hydrocarbons Moorthy B., Chu C., Carlin D.J. Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. "Toxicol Sci". 2015. 145(1), p.5-15.] | |||
4. [https://www.sciencedirect.com/science/article/pii/S1874778720300155 Pu N., Li W., Li J-Z. Complete genome sequence of Neptunomonas concharum JCM17730T: An acetate assimilating bacterium isolated from a dead ark clam, "Marine Genomics", 2020, 53.] | |||
5. [https://www.sciencedirect.com/science/article/pii/S1874778715300489 Rong J-C., Liu M., Li Y., Sun T-Y., Xie B-B., Shi M., Chen X-L., Qin Q-L. Insight into the genome sequence of a sediment-adapted marine bacterium Neptunomonas antarctica S3-22T from Antarctica, "Marine Genomics",2016, 25, p. 29-31.] | |||
6. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5727359/ Diéguez A.L., Pichon P., Balboa S., Magnesen T., Romalde J.L. Complete characterization of new isolates of Neptunomonas phycophila leads to emend its description and opens possibilities of biotechnological applications. "Microbiologyopen". 2017. 6(6).] | |||
==Author== | ==Author== | ||
| Line 66: | Line 81: | ||
Page authored by Nathan Hicks, student of Prof. Bradley Tolar at UNC Wilmington. | Page authored by Nathan Hicks, student of Prof. Bradley Tolar at UNC Wilmington. | ||
Winner: 2023 Weirdest Microbe Award | |||
[[Category:Pages edited by students of Bradley Tolar at UNC Wilmington]] | |||
Latest revision as of 02:39, 13 December 2023
Classification
Higher order taxa
Bacteria; Pseudomonadota; Gammaproteobacteria; Oceanospirillales; Oceanospirillaceae
NCBI: [1]
Genus
Neptunomonas
Description and Significance
Appearance: The genus includes rod shaped (Bacillus) or slightly curved (vibrio) cells, with approximate sizes of 0.7–0.9 × 2.0–3.0 µm. Spherical (coccoid) body plans appear in older colonies, often associated with a loss of viability. They are capable of producing a capsid and are motile via a single flagellum. [1.]
Habitat: Often associated with historically polluted marine coastal sediment, such as the Puget sound (Washington, USA), the site of a wood treatment facility and the Mediterranean Sea (Milazzo Harbor, Italy), which has been impacted by municipal wastes.
In addition to these polluted sites, Neptunomonas is closely related (acting as endosymbionts) to the bone-eating polychaetes in the genus Osedax, which are inhabitants of whale carcasses within the deep sea. These endosymbionts are located within highly branched root tissues which burrow into the bones of these large carcasses, digesting them, releasing the organic carbon which can be used for nutrition. The specifics of this endosymbiosis is not fully known. [2.]
Importance: Neptunomonas main focus in scientific research is its capabilities to biodegrade PAH molecules as a result of its relation to the polluted areas it inhabits. These PAH molecules occur naturally in coal, crude oil, and gasoline; they are extremely toxic and hard to break down due to their long chains and long term exposure to them may result in lung cancer (a disease with high mortality rates) as well as other health issues [3.]. Therefore research into the mechanisms of how Neptunomonas is able to break these compounds down may result in the saving of lives in the future.
Genome Structure
Neptunomonas concharum
Neptunomonas concharum was one of the species of choice when examining a genome within this genus as a result of the extensive and complete genome sequencing conducted. The complete genome consisted of 3,561,992 bp, of which G-C base pairs made up 46%. 3273 protein coding sequences were found, with 69 tRNA genes, 15 complete rRNA genes (5 each for 5S, 16S, and 23S), and 4 ncRNAs. The chromosome type is circular.
Bacteria containing prophage are more likely to show antibiotic resistance, greater environmental adaptability and improve adhesion. Concharum contained three incomplete prophage sequences. Additionally CRISPR/Cas systems play an important role in bacterial defence systems, fighting against viruses and other bacterial plasmid invasion, one credible CRISPR sequence containing 22 spacers was acknowledges in the genomic DNA. These sequences recognise some of the uniquely adapted sequences allowing concharum to function in toxic, polluted and otherwise extreme habitats.
Metabolic pathways Neptunomonas concharum was found to differ from typical forms of glycolysis; the genome sequence contains all the genes encoding for glycolysis and gluconeogenesis, however no genes related to glucose phosphotransferase were found, resulting the lack of utilization of extracellular sugars in metabolism. It has been noted that concharum prefers to utilise acetate as a carbon source for cellular growth, backed by the identification of genes associated with acetate metabolism (Ack-Pta and Acs pathways, and three acetyl-CoA synthetase genes). [4.]
Neptunomonas antarctica
Additionally, the marine bacterium Neptunomonas antarctica was also examined and bared similar results. It did however have a longer genome of 4,568,828 bp with a mean G-C content of 45.7%. Despite this, similar functions were found within the genomes, 85 genes are associated with “Virulence, Disease and Defense”, an advantage in the heavily polluted environments these micro-organisms inhabit, aiding in resistance to sediment-associated pollutants including heavy-metal ions, antibiotics and other toxic compounds.
Some additional findings associated with Neptunomonas antarctica were that 62 genes are involved in flagellar motility and 19 genes in bacterial chemotaxis. These would aid in the movement towards nutrient containing sediments and movement away from toxicity and predators, playing an important role in thier sediment adapted lifestyles.
other genome sequences associated with utilizing nutrients in these polluted environments include: 21 nitrate and nitrite ammonification genes, 35 genes related to poisonous aromatic compounds metabolism and 42 genes related to stress response particularly related to the temperature stresses of an arctic environment. [5.]
Metabolism and Cell Structure
Metabolism: This species can receive and use resources from a wide range of sources for metabolic engineering such as using some amino acids, carbohydrates, organic acids, or sugar alcohols as sole carbon sources and electron donors. They are aerobically respiring organisms, with selectivity anaerobic capabilities. Oxidising strains are commonly associated with polluted coastal marine sediments. The most notable method within metabolism for Neptunomonas is the catabolism of PAH molecules, a somewhat unique adaptation bought from the polluted coastal marine sediments they inhabit. [2.]
Additionally, this organism is closely related to the endosymbionts of the bone-eating polychaetes in the genus Osedax, occurring in root structures produced by Osedax which penetrate the whale bone. Location and enzyme activity suggest Neptunomonas aid in the digestion of bones for the polychaete, however it is unknown yet if the resulting molecules are used in metabolic processes for the micro-organism. [4.]
Cell structure: Neptunomonas is a gram negative bacterium, having a thin layer of peptidoglycan as compared to some other bacterium, making them harder to kill with treatments such as antibiotics. This Genus is also oxidase and catalase positive, with both of these enzymes being produced and present indicating its aerobic capabilities. [6.]
Ecology and Pathogenesis
This organism does not actively cause disease- but it may hold relevance in the treatment of disease as a result of cell structure. They have a recognised resistance to antibiotics meaning despite not causing disease, they may still hold relevance in the field. However research in this field is still required, with its uses in this area still unknown. [6.]
References
1. Hedlund B.P. "Neptunomonas", Neptunomonas. 2015. p. 1–6.
Author
Page authored by Nathan Hicks, student of Prof. Bradley Tolar at UNC Wilmington.
Winner: 2023 Weirdest Microbe Award