Fannyhessea vaginae: Difference between revisions
| (23 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
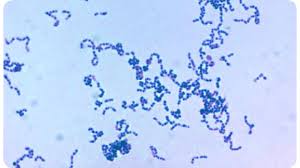
[[Image:Fannyhessea Vaginae.jpg|thumb|300px|right|. Image credit: Google Images]] | [[Image:Fannyhessea Vaginae.jpg|thumb|300px|right|Microscopy of coccus shaped gram negative ''F. vaginae'' . Image credit: Google Images]] | ||
| Line 25: | Line 25: | ||
==Description and Significance== | ==Description and Significance== | ||
Fannyhessea | ''Fannyhessea vaginae'' (formerly ''Atopobium vaginae'') is a gram positive cocci shaped bacterium highly specific to Bacterial Vaginosis (BV) (Sousa et al, 2021). This organism alone does not cause BV, rather in combination with another bacteria with similar characteristics ''Gardnerella'' spp. | ||
As an anaerobe (low to no oxygen) F. Vaginae creates polymicrobial biofilms that layer the vaginal epithelial cells providing the anaerobic conditions for optimal growth ( | As an anaerobe (low to no oxygen) ''F. Vaginae'' creates polymicrobial biofilms that layer the vaginal epithelial cells providing the anaerobic conditions for optimal growth (Sousa et al, 2021). Increase in pH, vaginal discharge and odor are characteristics of BV, and can be partially attributed to the presence of ''F. vaginae''. The Biofilms produced by this microbe serves as a diagnostic marker for BV, which is vital as most of the previous detection methods for BV had a high rate of failure. This is an important connection as bacterial vaginosis affects approximately 29% of reproductive aged women worldwide, and can have adverse effects on childbirth. Discovering this link to ''F. vaginae'' is essential for early detection and treatment. | ||
==Genome Structure== | ==Genome Structure== | ||
The F. | The ''F. vaginae'' genome is a linear chromosome. Of the 24 different strains the primary genome DSM 15829 is sequenced at 1.5 mbp, with the smallest strain only 461 bp. Most of the genes are protein coding, with 1200 genes coding over 18,000 proteins. | ||
DSM 15829 contain a 5s, 16s, 23s rRNA; and a 30s protein s6. Encoded in this genome are sucrose metabolic pathways, glycan biosynthesis, and termination factor rho. In addition, the NusB protein involved in RNA biosynthesis within eubacteria is regulated by modulating transcription and antitermination efficiency. | DSM 15829 contain a 5s, 16s, 23s rRNA; and a 30s protein s6. Encoded in this genome are sucrose metabolic pathways, glycan biosynthesis, and termination factor rho. In addition, the NusB protein involved in RNA biosynthesis within eubacteria is regulated by modulating transcription and antitermination efficiency. | ||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
''F. vaginae'' is Gram-positive, elliptical or rod-shaped cocci, nonmotile and non-spore-forming organism, and given its relatively new discovery from the microflora of a healthy individual, not much is know about the internal structures and processes (Mendling et al., 2019). Research into the life cycle is currently underway as these organisms have been found in the vaginal microflora of healthy women, therefore isolating them has become more available. Mendling et al also mentioned these organisms rarely are found in single species planktonic state, but rather synergistically in the biofilms they create; These biofilms have been attributed to the increased resistance to antibiotics and host immune responses. | |||
This organism utilizes carbohydrates through a glycolysis and pentose phosphorous pathway. The anaerobic glycolytic pathway breaks down glycerin-p to pyruvate to be used in the production of energy. Pentose Phosphorous pathway breaks down fructose-6p that can end up In the input of the glycolytic pathway. In a further complex fashion, after the break down of the glycerin-p rather than continuing the production of pyruvate, this organism can switch its metabolism into an anabolic pathway to build the amino acid methionine from the serine intermediate. | This organism utilizes carbohydrates through a glycolysis and pentose phosphorous pathway. The anaerobic glycolytic pathway breaks down glycerin-p to pyruvate to be used in the production of energy. Pentose Phosphorous pathway breaks down fructose-6p that can end up In the input of the glycolytic pathway. In a further complex fashion, after the break down of the glycerin-p rather than continuing the production of pyruvate, this organism can switch its metabolism into an anabolic pathway to build the amino acid methionine from the serine intermediate. | ||
| Line 41: | Line 42: | ||
==Ecology and Pathogenesis== | ==Ecology and Pathogenesis== | ||
F. vaginae is found in the vaginal microbiota, however, it is rarely found in the microbiota of healthy individuals. This parasitic organism causes many complications for vaginal health by causing a pH in-balance, increase of discharge, foul odor and discomfort. | ''F. vaginae'' is found in the vaginal microbiota, however, it is rarely found in the microbiota of healthy individuals. This parasitic organism causes many complications for vaginal health by causing a pH in-balance, increase of discharge, foul odor and discomfort. Once ''F. vaginae'' creates biofilms and attach to the vaginal epithelial wall, it causes anoxic conditions that exacerbating pH issues from lactic acid production, and increases discharge. | ||
While ''F. vaginae'' does not cause BV alone, it is found coupled with ''Gardnerella'' spp. which is another biofilm producing bacteria associated with BV. ''Gardnerella'' spp. biofilms reduce the ability of lactobacillus adhered to vaginal epithelial cells and produce a lysin that lyses and exfoliates vaginal epithelial cells. From this, ''F. vaginae'' takes hold and the two cause serious issues. As previously mentioned, ''F. vaginae'' is highly specific to BV compared to the other bacteria found in BV positive case studies (Sousa et al, 2021). | |||
While F. | |||
==References== | ==References== | ||
Sousa, L. G., Castro, J., França, A., Almeida, C., Muzny, C. A., & Cerca, N. (2021). A new PNA-fish probe targeting Fannyhessea Vaginae. Frontiers in Cellular and Infection Microbiology, 11. https://doi.org/10.3389/fcimb.2021.779376 | |||
Mendling, Werner, et al. “An update on the role of Atopobium Vaginae in bacterial vaginosis: What to consider when choosing a treatment? A mini review.” Archives of Gynecology and Obstetrics, vol. 300, no. 1, 2019, pp. 1–6, https://doi.org/10.1007/s00404-019-05142-8. | |||
==Author== | ==Author== | ||
| Line 56: | Line 56: | ||
Page authored by John T., student of Prof. Bradley Tolar at UNC Wilmington. | Page authored by John T., student of Prof. Bradley Tolar at UNC Wilmington. | ||
Winner: 2023 Coolest Microbe Award | |||
<!-- Do not remove this line-->[[Category:Pages edited by students of Bradley Tolar at UNC Wilmington]] | <!-- Do not remove this line-->[[Category:Pages edited by students of Bradley Tolar at UNC Wilmington]] | ||
Latest revision as of 18:34, 13 December 2023
Classification
Bacteria; Actinimycetota; Actinomycetes ; Actinomycetales; Actinomycetaceae; Fannyhessea
Species
Fannyhessea vaginae
|
NCBI: [1] |
Description and Significance
Fannyhessea vaginae (formerly Atopobium vaginae) is a gram positive cocci shaped bacterium highly specific to Bacterial Vaginosis (BV) (Sousa et al, 2021). This organism alone does not cause BV, rather in combination with another bacteria with similar characteristics Gardnerella spp.
As an anaerobe (low to no oxygen) F. Vaginae creates polymicrobial biofilms that layer the vaginal epithelial cells providing the anaerobic conditions for optimal growth (Sousa et al, 2021). Increase in pH, vaginal discharge and odor are characteristics of BV, and can be partially attributed to the presence of F. vaginae. The Biofilms produced by this microbe serves as a diagnostic marker for BV, which is vital as most of the previous detection methods for BV had a high rate of failure. This is an important connection as bacterial vaginosis affects approximately 29% of reproductive aged women worldwide, and can have adverse effects on childbirth. Discovering this link to F. vaginae is essential for early detection and treatment.
Genome Structure
The F. vaginae genome is a linear chromosome. Of the 24 different strains the primary genome DSM 15829 is sequenced at 1.5 mbp, with the smallest strain only 461 bp. Most of the genes are protein coding, with 1200 genes coding over 18,000 proteins.
DSM 15829 contain a 5s, 16s, 23s rRNA; and a 30s protein s6. Encoded in this genome are sucrose metabolic pathways, glycan biosynthesis, and termination factor rho. In addition, the NusB protein involved in RNA biosynthesis within eubacteria is regulated by modulating transcription and antitermination efficiency.
Cell Structure, Metabolism and Life Cycle
F. vaginae is Gram-positive, elliptical or rod-shaped cocci, nonmotile and non-spore-forming organism, and given its relatively new discovery from the microflora of a healthy individual, not much is know about the internal structures and processes (Mendling et al., 2019). Research into the life cycle is currently underway as these organisms have been found in the vaginal microflora of healthy women, therefore isolating them has become more available. Mendling et al also mentioned these organisms rarely are found in single species planktonic state, but rather synergistically in the biofilms they create; These biofilms have been attributed to the increased resistance to antibiotics and host immune responses.
This organism utilizes carbohydrates through a glycolysis and pentose phosphorous pathway. The anaerobic glycolytic pathway breaks down glycerin-p to pyruvate to be used in the production of energy. Pentose Phosphorous pathway breaks down fructose-6p that can end up In the input of the glycolytic pathway. In a further complex fashion, after the break down of the glycerin-p rather than continuing the production of pyruvate, this organism can switch its metabolism into an anabolic pathway to build the amino acid methionine from the serine intermediate.
Ecology and Pathogenesis
F. vaginae is found in the vaginal microbiota, however, it is rarely found in the microbiota of healthy individuals. This parasitic organism causes many complications for vaginal health by causing a pH in-balance, increase of discharge, foul odor and discomfort. Once F. vaginae creates biofilms and attach to the vaginal epithelial wall, it causes anoxic conditions that exacerbating pH issues from lactic acid production, and increases discharge.
While F. vaginae does not cause BV alone, it is found coupled with Gardnerella spp. which is another biofilm producing bacteria associated with BV. Gardnerella spp. biofilms reduce the ability of lactobacillus adhered to vaginal epithelial cells and produce a lysin that lyses and exfoliates vaginal epithelial cells. From this, F. vaginae takes hold and the two cause serious issues. As previously mentioned, F. vaginae is highly specific to BV compared to the other bacteria found in BV positive case studies (Sousa et al, 2021).
References
Sousa, L. G., Castro, J., França, A., Almeida, C., Muzny, C. A., & Cerca, N. (2021). A new PNA-fish probe targeting Fannyhessea Vaginae. Frontiers in Cellular and Infection Microbiology, 11. https://doi.org/10.3389/fcimb.2021.779376
Mendling, Werner, et al. “An update on the role of Atopobium Vaginae in bacterial vaginosis: What to consider when choosing a treatment? A mini review.” Archives of Gynecology and Obstetrics, vol. 300, no. 1, 2019, pp. 1–6, https://doi.org/10.1007/s00404-019-05142-8.
Author
Page authored by John T., student of Prof. Bradley Tolar at UNC Wilmington.
Winner: 2023 Coolest Microbe Award