Propionibacterium acnes: A Teenager’s Worst Nightmare Defined: Difference between revisions
| (35 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
<b>By Megan Lydon</b> | <b>By Megan Lydon</b> | ||
<i>Propionibacterium acnes</i> is a bacterium commonly found on human skin, particularly in sebaceous (oil) glands and hair follicles. It is gram-positive and a fairly slow-growing aerotolerant bacterium. A lower density of <i>P. acnes</i> is detected on the skin of adolescents, especially those prepubescent. The bacteria mainly live on fatty acids. The normal habitat of <i>P. acnes</i> is in the sebaceous follicle shared with the yeast <i>Pityrosporum</i> and aerobic staphylococci and micrococci on its surface <ref>Leyden, J. J. (1997). <i>Propionibacterium acnes</i> colonization in acne and non-acne.<i>Journal of Investigative Dermatology</i>, 3(108), 379. From https://www.infona.pl/resource/bwmeta1.element.elsevier-c3dc00b6-a2eb-39e6-b2e6-bdac77b2d48c/</ref>. Despite its name, and its colloquial associations, <i>P. acnes</i> is not solely associated with acne <ref>Bhatia, A., Maisonneuve, J. F., & Persing, D. H. (2004, June). <i>Propionibacterium acnes</i> and chronic diseases. In <i>The Infectious Etiology of Chronic Diseases: Defining the Relationship, Enhancing the Research, and Mitigating the Effects: Workshop Summary., Knobler, SL et al.(eds.)</i>(pp. 74-80). From https://www.ncbi.nlm.nih.gov/books/NBK83685/#/</ref>; it is a normal resident of the skin microbiota in most people. However, it can contribute to the development of acne vulgaris, the most common form of acne, when factors such as excess sebum production, hormonal changes, and inflammation are present. Age-related differences are also noted in which lower levels of <i>P. acnes</i> are found in young children before they hit puberty. Acne is one of the most common skin diseases affecting more than 45 million people in the United States. In addition, in a clinical context, it is estimated that nearly 20% of visits to dermatologists are related to acne and the treatment of the condition <ref>Bhatia, A., Maisonneuve, J. F., & Persing, D. H. (2004, June). <i>Propionibacterium acnes</i> and chronic diseases. In <i>The Infectious Etiology of Chronic Diseases: Defining the Relationship, Enhancing the Research, and Mitigating the Effects: Workshop Summary., Knobler, SL et al.(eds.)</i>(pp. 74-80). From https://www.ncbi.nlm.nih.gov/books/NBK83685/#/</ref>. Varying degrees of acne affects nearly all people between the ages of 15-17 with 15-20% of those cases being moderate to severe <ref>Law, M. P. M., Chuh, A. A. T., Lee, A., & Molinari, N. (2010). Acne prevalence and beyond: acne disability and its predictive factors among Chinese late adolescents in Hong Kong. <i>Clinical and experimental dermatology, 35(1), 16-21.</i> From https://academic.oup.com/ced/article-abstract/35/1/16/6622092/</ref>. | <i>Propionibacterium acnes</i> is a bacterium commonly found on human skin, particularly in sebaceous (oil) glands and hair follicles. It is gram-positive and a fairly slow-growing aerotolerant bacterium. A lower density of <i>P. acnes</i> is detected on the skin of adolescents, especially those prepubescent. The bacteria mainly live on fatty acids. The normal habitat of <i>P. acnes</i> is in the sebaceous follicle shared with the yeast <i>Pityrosporum</i> and aerobic staphylococci and micrococci on its surface <ref name==aa>Leyden, J. J. (1997). <i>Propionibacterium acnes</i> colonization in acne and non-acne.<i>Journal of Investigative Dermatology</i>, 3(108), 379. From https://www.infona.pl/resource/bwmeta1.element.elsevier-c3dc00b6-a2eb-39e6-b2e6-bdac77b2d48c/</ref>. Despite its name, and its colloquial associations, <i>P. acnes</i> is not solely associated with acne <ref name==ab>Bhatia, A., Maisonneuve, J. F., & Persing, D. H. (2004, June). <i>Propionibacterium acnes</i> and chronic diseases. In <i>The Infectious Etiology of Chronic Diseases: Defining the Relationship, Enhancing the Research, and Mitigating the Effects: Workshop Summary., Knobler, SL et al.(eds.)</i>(pp. 74-80). From https://www.ncbi.nlm.nih.gov/books/NBK83685/#/</ref>; it is a normal resident of the skin microbiota in most people. However, it can contribute to the development of acne vulgaris, the most common form of acne, when factors such as excess sebum production, hormonal changes, and inflammation are present. Age-related differences are also noted in which lower levels of <i>P. acnes</i> are found in young children before they hit puberty. Acne is one of the most common skin diseases affecting more than 45 million people in the United States. In addition, in a clinical context, it is estimated that nearly 20% of visits to dermatologists are related to acne and the treatment of the condition <ref name==ab>Bhatia, A., Maisonneuve, J. F., & Persing, D. H. (2004, June). <i>Propionibacterium acnes</i> and chronic diseases. In <i>The Infectious Etiology of Chronic Diseases: Defining the Relationship, Enhancing the Research, and Mitigating the Effects: Workshop Summary., Knobler, SL et al.(eds.)</i>(pp. 74-80). From https://www.ncbi.nlm.nih.gov/books/NBK83685/#/</ref>. Varying degrees of acne affects nearly all people between the ages of 15-17 with 15-20% of those cases being moderate to severe <ref>Law, M. P. M., Chuh, A. A. T., Lee, A., & Molinari, N. (2010). Acne prevalence and beyond: acne disability and its predictive factors among Chinese late adolescents in Hong Kong. <i>Clinical and experimental dermatology, 35(1), 16-21.</i> From https://academic.oup.com/ced/article-abstract/35/1/16/6622092/</ref>. | ||
As for other opportunistic diseases, P. acnes is known to be involved in endocarditis, osteomyelitis <ref>Jakab, E., Zbinden, R., Gubler, J., Ruef, C., Von Graevenitz, A., & Krause, M. (1996). Severe infections caused by Propionibacterium acnes: an underestimated pathogen in late postoperative infections. <i>The Yale journal of biology and medicine</i>, 69(6), 477. From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2589039//</ref><ref>Söderquist, B., Holmberg, A., & Unemo, M. (2010). <i>Propionibacterium acnes</i> as an etiological agent of arthroplastic and osteosynthetic infections–two cases with specific clinical presentation including formation of draining fistulae. <i>Anaerobe</i>, 16(3), 304-306. From https://www.sciencedirect.com/science/article/pii/S107599640900153X/</ref> arthritis, and postoperative device infection as a result of the insertion of prosthetics and heart valves. | As for other opportunistic diseases, P. acnes is known to be involved in endocarditis, osteomyelitis <ref>Jakab, E., Zbinden, R., Gubler, J., Ruef, C., Von Graevenitz, A., & Krause, M. (1996). Severe infections caused by Propionibacterium acnes: an underestimated pathogen in late postoperative infections. <i>The Yale journal of biology and medicine</i>, 69(6), 477. From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2589039//</ref><ref>Söderquist, B., Holmberg, A., & Unemo, M. (2010). <i>Propionibacterium acnes</i> as an etiological agent of arthroplastic and osteosynthetic infections–two cases with specific clinical presentation including formation of draining fistulae. <i>Anaerobe</i>, 16(3), 304-306. From https://www.sciencedirect.com/science/article/pii/S107599640900153X/</ref> arthritis, and postoperative device infection as a result of the insertion of prosthetics and heart valves. | ||
The genome of <i> P. acnes</i> is 2.5 Mb <ref> Liu, J., Cheng, A., Bangayan, N. J., Barnard, E., Curd, E., Craft, N., & Li, H. (2014). Draft genome sequences of <i>Propionibacterium acnes</i> type strain ATCC6919 and antibiotic-resistant strain HL411PA1. <i>Genome announcements</i>, 2(4), 10-1128. From https://journals.asm.org/doi/full/10.1128/genomea.00740-14/</ref>. <i>P. acnes</i> has genes encoding metabolic enzymes allowing it to survive in microaerophilic conditions. When pathogenic, the evasion of host cells (epithelial cells) is characterized by the meditation of the bacterial surface proteins or adhesions recognizing the extracellular matrix (ECM) components. These components are the ideal target of use by many pathogens for tissue colonization. Other skin-associated bacteria such as those in the genera of <i>Staphylococcus</i> and <i>Streptococcus</i> express skin surface proteins known as MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) that bind to those EMCs. <i>P. acnes</i> is also able to perform this recognition process while adhering to the skin but also is able to promote more reaction by traveling deeper into the skin. | The genome of <i> P. acnes</i> is 2.5 Mb <ref name==ac> Liu, J., Cheng, A., Bangayan, N. J., Barnard, E., Curd, E., Craft, N., & Li, H. (2014). Draft genome sequences of <i>Propionibacterium acnes</i> type strain ATCC6919 and antibiotic-resistant strain HL411PA1. <i>Genome announcements</i>, 2(4), 10-1128. From https://journals.asm.org/doi/full/10.1128/genomea.00740-14/</ref>. <i>P. acnes</i> has genes encoding metabolic enzymes allowing it to survive in microaerophilic conditions. When pathogenic, the evasion of host cells (epithelial cells) is characterized by the meditation of the bacterial surface proteins or adhesions recognizing the extracellular matrix (ECM) components. These components are the ideal target of use by many pathogens for tissue colonization. Other skin-associated bacteria such as those in the genera of <i>Staphylococcus</i> and <i>Streptococcus</i> express skin surface proteins known as MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) that bind to those EMCs. <i>P. acnes</i> is also able to perform this recognition process while adhering to the skin but also is able to promote more reaction by traveling deeper into the skin. | ||
==Skin Microbiome== | ==Skin Microbiome== | ||
[[File:Microbiome Image.png|thumb|350px|left|Simplified example of a skin microbiome. Photo credit: [https://www.nature.com/articles/s41587-020-0473-8/ Out of your skin]]] | [[File:Microbiome Image.png|thumb|350px|left|Simplified example of a skin microbiome. <ref>Schmidt, C. (2020). Out of your skin. Nat. Biotechnol, 38(4), 392-397. From https://www.nature.com/articles/s41587-020-0473-8/ Out of your skin/</ref>. | ||
Photo credit: [https://www.nature.com/articles/s41587-020-0473-8/ Out of your skin]]] | |||
Microbiomes, in general, serve a greater purpose than living organisms just existing in their habitat. Through a combination of commensal species of microbes and their interactions with their habitat, environments are formed where the host and bacteria can adapt and regulate processes either to their advantage or negative effects of competition. The skin microbiome works identically. There is mass variability in the skin microbiome. As for microbes involved, fungi, bacteria, viruses, and small arthropods contribute to this relationship <ref name==aa>Leyden, J. J. (1997). <i>Propionibacterium acnes</i> colonization in acne and non-acne.<i>Journal of Investigative Dermatology</i>, 3(108), 379. From https://www.infona.pl/resource/bwmeta1.element.elsevier-c3dc00b6-a2eb-39e6-b2e6-bdac77b2d48c/</ref>. | |||
In addition, the microbiome is much more complex than once thought. Past research has tended to focus only on pathogens and opportunistic pathogens rather than the entire spread of microbes in general (even “harmless” to human hosts). In addition to the variability of the microbes present in the skin microbiome, locations of the skin and their own environments are also variable from person to person. However, even in these differences, homeostasis between the microbiome and host is imperative for continued healthy interactions on the epithelium and to avoid the occurrence of disease. The skin ecosystem is continuously variable in humidity, temperature, pH, and composition of antimicrobial peptides and lipids <ref name==aa>Leyden, J. J. (1997). <i>Propionibacterium acnes</i> colonization in acne and non-acne.<i>Journal of Investigative Dermatology</i>, 3(108), 379. From https://www.infona.pl/resource/bwmeta1.element.elsevier-c3dc00b6-a2eb-39e6-b2e6-bdac77b2d48c/</ref>. In addition, the frequency of hair follicles can also determine the production of sebaceous materials and eccrine/apocrine glands. With this variety of environments, it establishes a separate niche for microbes to fill and thrive in. The abundance of certain bacteria is dependent on these niches <ref> Grange, P. A., Raingeaud, J., Morelle, W., Marcelin, A. G., Calvez, V., & Dupin, N. (2017). Characterization of a <i>Propionibacterium acnes</i> surface protein as a fibrinogen-binding protein. <i>Scientific Reports</i>, 7(1), 6428. From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5527093//</ref>. | |||
In addition, the microbiome is much more complex than once thought. Past research has tended to focus only on pathogens and opportunistic pathogens rather than the entire spread of microbes in general (even “harmless” to human hosts). In addition to the variability of the microbes present in the skin microbiome, locations of the skin and their own environments are also variable from person to person. However, even in these differences, homeostasis between the microbiome and host is imperative for continued healthy interactions on the epithelium and to avoid the occurrence of disease. The skin ecosystem is continuously variable in humidity, temperature, pH, and composition of antimicrobial peptides and lipids <ref>Leyden, J. J. (1997). <i>Propionibacterium acnes</i> colonization in acne and non-acne.<i>Journal of Investigative Dermatology</i>, 3(108), 379. From https://www.infona.pl/resource/bwmeta1.element.elsevier-c3dc00b6-a2eb-39e6-b2e6-bdac77b2d48c/</ref>. In addition, the frequency of hair follicles can also determine the production of sebaceous materials and eccrine/apocrine glands. With this variety of environments, it establishes a separate niche for microbes to fill and thrive in. The abundance of certain bacteria is dependent on these niches <ref> Grange, P. A., Raingeaud, J., Morelle, W., Marcelin, A. G., Calvez, V., & Dupin, N. (2017). Characterization of a <i>Propionibacterium acnes</i> surface protein as a fibrinogen-binding protein. <i>Scientific Reports</i>, 7(1), 6428. From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5527093//</ref>. | |||
==Pathogenesis of P. acnes (Acne Vulgeris)== | ==Pathogenesis of P. acnes (Acne Vulgeris)== | ||
[[File:Liu Chart.jpeg|thumb|500px|right|Molecular mechanisms by which <i>P. acnes</i> may contribute to the pathogenesis of acnes vulgaris. Photo credit: [https://www.ingentaconnect.com/content/ben/cdm/2015/00000016/00000004/art00003/ <i>Propionibacterium acnes</i> in the Pathogenesis and Immunotherapy of Acne Vulgaris.] <ref> Liu, J., Cheng, A., Bangayan, N. J., Barnard, E., Curd, E., Craft, N., & Li, H. (2014). Draft genome sequences of <i>Propionibacterium acnes</i> type strain ATCC6919 and antibiotic-resistant strain HL411PA1. <i>Genome announcements</i>, 2(4), 10-1128. From https://journals.asm.org/doi/full/10.1128/genomea.00740-14/</ref>]] | [[File:Liu Chart.jpeg|thumb|500px|right|Molecular mechanisms by which <i>P. acnes</i> may contribute to the pathogenesis of acnes vulgaris. Photo credit: [https://www.ingentaconnect.com/content/ben/cdm/2015/00000016/00000004/art00003/ <i>Propionibacterium acnes</i> in the Pathogenesis and Immunotherapy of Acne Vulgaris.] <ref name==ac> Liu, J., Cheng, A., Bangayan, N. J., Barnard, E., Curd, E., Craft, N., & Li, H. (2014). Draft genome sequences of <i>Propionibacterium acnes</i> type strain ATCC6919 and antibiotic-resistant strain HL411PA1. <i>Genome announcements</i>, 2(4), 10-1128. From https://journals.asm.org/doi/full/10.1128/genomea.00740-14/</ref>]] | ||
<i>P. acnes</i> is present on healthy skin and disrupted skin <ref> Dessinioti, C., & Katsambas, A. D. (2010). The role of <i>Propionibacterium acnes</i> in acne pathogenesis: facts and controversies. <i>Clinics in dermatology</i>, 28(1), 2-7. https://www-sciencedirect-com.libproxy.kenyon.edu/science/article/pii/S0738081X09000583?via%3Dihub/</ref>. Therefore associated conditions cannot be classified as infectious diseases. However, pathogenisis of <i>P. acnes</i> does disrupt normal epithelium and its symptoms look to be treated by many. <i>P. acnes</i> is considered to contribute to the development of acne vulgaris, which can chronically affect 15% of people of all ages and at least 85% of teenagers in the United States <ref> Baldwin, H. E., Friedlander, S. F., Eichenfield, L. F., Mancini, A. J., & Yan, A. C. (2011, September). The effects of culture, skin color, and other nonclinical issues on acne treatment. In Seminars in cutaneous medicine and surgery (Vol. 30, No. 3 Suppl, pp. S12-5). From https://europepmc.org/article/med/21943562/</ref> <ref> Tom, W. L., & Fallon Friedlander, S. (2008). Acne through the ages: case-based observations through childhood and adolescence. <i>Clinical pediatrics</i>, 47(7), 639-651. From https://journals.sagepub.com/doi/abs/10.1177/0009922808315444/</ref> . As for its role in the disruption of the epithelium is the onset of acne vulgeris. This is a condition where painful, red, and inflamed portions of the skin are infected by <i>P. acnes</i>. <i>P. acnes</i> only triggers the disease when it meets a favorable terrain, therefore the dense colonization of bacteria on the skin is necessary but not sufficient for pathogenesis. Research suggests that the density of bacteria has no effect on the frequency of acne vulgeris however there has been some evidence that certain strains of <i>P. acnes</i> can be more pathogenic when met with favorable conditions. However, it has been shown that there is a correlation between high sebum production and <i>P. acnes</i> density <ref> Dessinioti, C., & Katsambas, A. D. (2010). The role of <i>Propionibacterium acnes</i> in acne pathogenesis: facts and controversies. <i>Clinics in dermatology</i>, 28(1), 2-7. https://www-sciencedirect-com.libproxy.kenyon.edu/science/article/pii/S0738081X09000583?via%3Dihub/</ref>. Regular colonization of <i>P. acnes</i> is quite beneficial to the skin microbiome as it can hydrolyze triglycerides and release free fatty acids to maintain acidic pH on the skin surface. This then helps down regulate the density of other pathogenic bacteria such as <i>Streptococcus pyogenes</i> and <i>Staphylococcus aureus</i> <ref> Liu, | <i>P. acnes</i> is present on healthy skin and disrupted skin <ref name==ad> Dessinioti, C., & Katsambas, A. D. (2010). The role of <i>Propionibacterium acnes</i> in acne pathogenesis: facts and controversies. <i>Clinics in dermatology</i>, 28(1), 2-7. https://www-sciencedirect-com.libproxy.kenyon.edu/science/article/pii/S0738081X09000583?via%3Dihub/</ref>. Therefore associated conditions cannot be classified as infectious diseases. However, pathogenisis of <i>P. acnes</i> does disrupt normal epithelium and its symptoms look to be treated by many. <i>P. acnes</i> is considered to contribute to the development of acne vulgaris, which can chronically affect 15% of people of all ages and at least 85% of teenagers in the United States <ref> Baldwin, H. E., Friedlander, S. F., Eichenfield, L. F., Mancini, A. J., & Yan, A. C. (2011, September). The effects of culture, skin color, and other nonclinical issues on acne treatment. In Seminars in cutaneous medicine and surgery (Vol. 30, No. 3 Suppl, pp. S12-5). From https://europepmc.org/article/med/21943562/</ref> <ref> Tom, W. L., & Fallon Friedlander, S. (2008). Acne through the ages: case-based observations through childhood and adolescence. <i>Clinical pediatrics</i>, 47(7), 639-651. From https://journals.sagepub.com/doi/abs/10.1177/0009922808315444/</ref> . As for its role in the disruption of the epithelium is the onset of acne vulgeris. This is a condition where painful, red, and inflamed portions of the skin are infected by <i>P. acnes</i>. <i>P. acnes</i> only triggers the disease when it meets a favorable terrain, therefore the dense colonization of bacteria on the skin is necessary but not sufficient for pathogenesis. Research suggests that the density of bacteria has no effect on the frequency of acne vulgeris however there has been some evidence that certain strains of <i>P. acnes</i> can be more pathogenic when met with favorable conditions. However, it has been shown that there is a correlation between high sebum production and <i>P. acnes</i> density <ref name==ad> Dessinioti, C., & Katsambas, A. D. (2010). The role of <i>Propionibacterium acnes</i> in acne pathogenesis: facts and controversies. <i>Clinics in dermatology</i>, 28(1), 2-7. https://www-sciencedirect-com.libproxy.kenyon.edu/science/article/pii/S0738081X09000583?via%3Dihub/</ref>. Regular colonization of <i>P. acnes</i> is quite beneficial to the skin microbiome as it can hydrolyze triglycerides and release free fatty acids to maintain acidic pH on the skin surface. This then helps down regulate the density of other pathogenic bacteria such as <i>Streptococcus pyogenes</i> and <i>Staphylococcus aureus</i> <ref name==ac> Liu, J., Cheng, A., Bangayan, N. J., Barnard, E., Curd, E., Craft, N., & Li, H. (2014). Draft genome sequences of <i>Propionibacterium acnes</i> type strain ATCC6919 and antibiotic-resistant strain HL411PA1. <i>Genome announcements</i>, 2(4), 10-1128. From https://journals.asm.org/doi/full/10.1128/genomea.00740-14/</ref>. | ||
===Follicular Epithelium=== | ===Follicular Epithelium=== | ||
| Line 49: | Line 47: | ||
===<b>Antibiotic Resistance</b>=== | ===<b>Antibiotic Resistance</b>=== | ||
The overuse and misuse of antibiotics have led to the widespread development of resistance to antimicrobial therapy by many pathogens including <i>P. acnes</i>. Antibiotic resistance is a very important public health concern, if not maybe, the most important topic. Antimicrobial resistance has resulted from an association between poor therapeutic response and antibiotic resistance. It is estimated that <i>P. acnes</i> antibiotic resistance has grown from an average of 20% being resistant to some form of antibiotic in 1978 to approximately 62% in 1996 <ref>Cooper, A. J. (1998). Systematic review of Propionibacterium acnes resistance to systemic antibiotics. Medical journal of Australia, 169(5), 259-261. From https://onlinelibrary.wiley.com/doi/abs/10.5694/j.1326-5377.1998.tb140250.x/</ref>. The most common resistance was seen of the drugs: erythromycin, clindamycin, tetracycline, doxycycline, and trimethoprim however, resistance to minocycline was rare <ref>Cooper, A. J. (1998). Systematic review of Propionibacterium acnes resistance to systemic antibiotics. Medical journal of Australia, 169(5), 259-261. From https://onlinelibrary.wiley.com/doi/abs/10.5694/j.1326-5377.1998.tb140250.x/</ref>. | The overuse and misuse of antibiotics have led to the widespread development of resistance to antimicrobial therapy by many pathogens including <i>P. acnes</i>. Antibiotic resistance is a very important public health concern, if not maybe, the most important topic. Antimicrobial resistance has resulted from an association between poor therapeutic response and antibiotic resistance. It is estimated that <i>P. acnes</i> antibiotic resistance has grown from an average of 20% being resistant to some form of antibiotic in 1978 to approximately 62% in 1996 <ref name==ae>Cooper, A. J. (1998). Systematic review of Propionibacterium acnes resistance to systemic antibiotics. Medical journal of Australia, 169(5), 259-261. From https://onlinelibrary.wiley.com/doi/abs/10.5694/j.1326-5377.1998.tb140250.x/</ref>. The most common resistance was seen of the drugs: erythromycin, clindamycin, tetracycline, doxycycline, and trimethoprim however, resistance to minocycline was rare <ref name==ae>Cooper, A. J. (1998). Systematic review of Propionibacterium acnes resistance to systemic antibiotics. Medical journal of Australia, 169(5), 259-261. From https://onlinelibrary.wiley.com/doi/abs/10.5694/j.1326-5377.1998.tb140250.x/</ref>. | ||
<i>P. acnes</i> resistance genes have been tied to mutations in 16s and 23s mRNA. This is especially alarming since these genes are closely tied to prokaryotic phylogeny and therefore might be extremely heritable. However, the mechanisms of this antibiotic resistance are a knowledge gap. These strains have been noticed and isolated from Europe, Australia, the United States, and Japan <ref>Ross, J. I., Snelling, A. M., Eady, E. A., Cove, J. H., Cunliffe, W. J., Leyden, J. J., ... & Oshima, S. (2001). Phenotypic and genotypic characterization of antibiotic‐resistant Propionibacterium acnes isolated from acne patients attending dermatology clinics in Europe, the USA, Japan and Australia. British Journal of Dermatology, 144(2), 339-346. From https://academic.oup.com/bjd/article-abstract/144/2/339/6690999/</ref>. | <i>P. acnes</i> resistance genes have been tied to mutations in 16s and 23s mRNA. This is especially alarming since these genes are closely tied to prokaryotic phylogeny and therefore might be extremely heritable. However, the mechanisms of this antibiotic resistance are a knowledge gap. These strains have been noticed and isolated from Europe, Australia, the United States, and Japan <ref>Ross, J. I., Snelling, A. M., Eady, E. A., Cove, J. H., Cunliffe, W. J., Leyden, J. J., ... & Oshima, S. (2001). Phenotypic and genotypic characterization of antibiotic‐resistant Propionibacterium acnes isolated from acne patients attending dermatology clinics in Europe, the USA, Japan and Australia. British Journal of Dermatology, 144(2), 339-346. From https://academic.oup.com/bjd/article-abstract/144/2/339/6690999/</ref>. | ||
| Line 55: | Line 53: | ||
===<b>Epidemiology</b>=== | ===<b>Epidemiology</b>=== | ||
Not much is known about the epidemiology of acne vulgaris. Clinical and histological features are well defined however much less appears to be written about its epidemiology. This is quite strange because acne is a condition that is defined colloquially as a disease and is almost always present in the teenage years <ref>Bhate, K., & Williams, H. C. (2013). Epidemiology of acne vulgaris. British Journal of Dermatology, 168(3), 474-485. From https://onlinelibrary.wiley.com/doi/epdf/10.1111/bjd.12149/</ref>. | Not much is known about the epidemiology of acne vulgaris. Clinical and histological features are well defined however much less appears to be written about its epidemiology. This is quite strange because acne is a condition that is defined colloquially as a disease and is almost always present in the teenage years <ref name==af>Bhate, K., & Williams, H. C. (2013). Epidemiology of acne vulgaris. British Journal of Dermatology, 168(3), 474-485. From https://onlinelibrary.wiley.com/doi/epdf/10.1111/bjd.12149/</ref>. | ||
====Diet==== | ====Diet==== | ||
[[File:Acne Image.jpg|thumb| | [[File:Acne Image.jpg|thumb|250px|right| Moderate inflammatory acne accompanied by scarring. Photo credit: | ||
[https://onlinelibrary.wiley.com/doi/epdf/10.1111/bjd.12149./ Epidemiology of acne vulgaris]<ref>Bhate, K., & Williams, H. C. (2013). Epidemiology of acne vulgaris. British Journal of Dermatology, 168(3), 474-485. From https://onlinelibrary.wiley.com/doi/epdf/10.1111/bjd.12149/</ref>]] | [https://onlinelibrary.wiley.com/doi/epdf/10.1111/bjd.12149./ Epidemiology of acne vulgaris]<ref name==af>Bhate, K., & Williams, H. C. (2013). Epidemiology of acne vulgaris. British Journal of Dermatology, 168(3), 474-485. From https://onlinelibrary.wiley.com/doi/epdf/10.1111/bjd.12149/</ref>]] | ||
Historically, dietary advice was very common when discussing acenes treatments. Early studies hinted that patients with acne had skewed glucose tolerance and altered carbohydrate metabolism. Therefore, patients were advised to avoid excessive carbohydrates and sugary goods <ref>CAMPBELL, G. G. (1931). The relation of sugar intolerance to certain diseases of the skin. British Journal of Dermatology, 43(6), 297-304. From https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2133.1931.tb09505.x/</ref>. On the other hand, later studies have found that students who consumed a high carbohydrate diet had no flare-ups over time. Granted, this specific study had a small sample size and no control group <ref>Taylor, S. C., Cook-Bolden, F., Rahman, Z., & Strachan, D. (2002). Acne vulgaris in skin of color. Journal of the American Academy of Dermatology, 46(2), S98-S106. From https://www.sciencedirect.com/science/article/pii/S0190962202107407/</ref>. However, an additional comprehensive review of seven studies found no clear positive evidence that any dietary components increase acne risk <ref>Magin, P., Pond, D., Smith, W., & Watson, A. (2005). A systematic review of the evidence for ‘myths and misconceptions’ in acne management: diet, face-washing and sunlight. Family practice, 22(1), 62-70. From https://academic.oup.com/fampra/article/22/1/62/440463/</ref>.What needs to be defined however is if acne levels and dietary makeup are being demonstrated based on acne occurrence as opposed to food that could influence the severity of disease flares of those already predisposed to higher levels of acne. | Historically, dietary advice was very common when discussing acenes treatments. Early studies hinted that patients with acne had skewed glucose tolerance and altered carbohydrate metabolism. Therefore, patients were advised to avoid excessive carbohydrates and sugary goods <ref>CAMPBELL, G. G. (1931). The relation of sugar intolerance to certain diseases of the skin. British Journal of Dermatology, 43(6), 297-304. From https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2133.1931.tb09505.x/</ref>. On the other hand, later studies have found that students who consumed a high carbohydrate diet had no flare-ups over time. Granted, this specific study had a small sample size and no control group <ref>Taylor, S. C., Cook-Bolden, F., Rahman, Z., & Strachan, D. (2002). Acne vulgaris in skin of color. Journal of the American Academy of Dermatology, 46(2), S98-S106. From https://www.sciencedirect.com/science/article/pii/S0190962202107407/</ref>. However, an additional comprehensive review of seven studies found no clear positive evidence that any dietary components increase acne risk <ref>Magin, P., Pond, D., Smith, W., & Watson, A. (2005). A systematic review of the evidence for ‘myths and misconceptions’ in acne management: diet, face-washing and sunlight. Family practice, 22(1), 62-70. From https://academic.oup.com/fampra/article/22/1/62/440463/</ref>.What needs to be defined however is if acne levels and dietary makeup are being demonstrated based on acne occurrence as opposed to food that could influence the severity of disease flares of those already predisposed to higher levels of acne. | ||
| Line 68: | Line 66: | ||
==Acne Vulgeris Treatments== | ==Acne Vulgeris Treatments== | ||
Topical or combined agents are used with the majority of patients with mild to moderate acne. Combination treatment is beneficial to target multiple causes and sites where acne vulgaris pathogenizes. Systemic therapy can be used for severe cases with nodules, cysts, or even scarring. Our current understanding of the pathophysiology of the inflammatory scene supports some sort of combination therapy aimed at eradicating pathogenic <i>P. acnes</i> and normalizing abnormal makeup of the follicular glands (such as an increase in the production of sebaceous oils). This will then eliminate an environment where <i>P. acnes</i> will proliferate extensively and then cause infection. In turn, this will downregulate the creation of the microcomedone which leads to acne lesions and mature comedones <ref> Leyden, J. J. (1998). Topical treatment of acne vulgaris: retinoids and cutaneous irritation. Journal of the American Academy of Dermatology, 38(4), S1-S4. From https://www.jaad.org/article/S0190-9622(98)70138-0/fulltext/</ref>. | Contributing factors of the pathogenesis of acne vulgaris by <i>P. acnes</i> are hyperkeratinization and obstruction of the follicular epithelium. In addition, stimulated sebum production and inflammation are targets and reasons for complications of effective new treatments <ref name==af>Bhate, K., & Williams, H. C. (2013). Epidemiology of acne vulgaris. British Journal of Dermatology, 168(3), 474-485. From https://onlinelibrary.wiley.com/doi/epdf/10.1111/bjd.12149/</ref>. | ||
Topical or combined agents are used with the majority of patients with mild to moderate acne. Combination treatment is beneficial to target multiple causes and sites where acne vulgaris pathogenizes. Systemic therapy can be used for severe cases with nodules, cysts, or even scarring. Our current understanding of the pathophysiology of the inflammatory scene supports some sort of combination therapy aimed at eradicating pathogenic <i>P. acnes</i> and normalizing abnormal makeup of the follicular glands (such as an increase in the production of sebaceous oils). This will then eliminate an environment where <i>P. acnes</i> will proliferate extensively and then cause infection. In turn, this will downregulate the creation of the microcomedone which leads to acne lesions and mature comedones <ref name==ag> Leyden, J. J. (1998). Topical treatment of acne vulgaris: retinoids and cutaneous irritation. Journal of the American Academy of Dermatology, 38(4), S1-S4. From https://www.jaad.org/article/S0190-9622(98)70138-0/fulltext/</ref>. | |||
===Topical/Systemic Antibiotics=== | ===Topical/Systemic Antibiotics=== | ||
| Line 83: | Line 82: | ||
===Benzoyl Peroxide=== | ===Benzoyl Peroxide=== | ||
[[File:Topical.webp|thumb|300px|right|Topical Benzoyl peroxide gel. Photo credit: [https://www.webmd.com/drugs/2/drug-1344/benzoyl-peroxide-topical/details/ WebMD.]]] | |||
Benzoyl peroxide is an alternative to antibiotics in the treatment of acne vulgaris. It is widely used due to its efficacy and favorable tolerability profile (very low amounts of adverse effects). Benzoyl peroxide contains mediums that have antibacterial, anti-inflammatory keratolytic, and wound healing properties <ref>Merker, P. C. (2002). Benzoyl peroxide: a history of early research and researchers. International journal of dermatology, 41(3). From https://eds.p.ebscohost.com/eds/detail/detail?vid=0&sid=9ff8152d-d475-442b-94d5-1587efe839a0%40redis&bdata=JnNpdGU9ZWRzLWxpdmU%3d#AN=6697926&db=aph/</ref>. Combination therapies including benzoyl peroxide are also very beneficial at targeting <i>P. acnes</i> in a pathogenic state. It is a powerful oxidizing agent derived from chlorohydroxyquinoline, a by-product of coal tar <ref>Pace, W. E. (1965). A benzoyl peroxide-sulfur cream for acne vulgaris. Canadian Medical Association Journal, 93(6), 252. From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1928665//</ref>. The compound is highly lipophilic and easily able to penetrate and enter the pilosebaceous follicle <ref>Ibbotson, S. H., Lambert, C. R., Moran, M. N., Lynch, M. C., & Kochevar, I. E. (1998). Benzoyl peroxide increases UVA-induced plasma membrane damage and lipid oxidation in murine leukemia L1210 cells. Journal of investigative dermatology, 110(1), 79-83. From https://www.sciencedirect.com/science/article/pii/S0022202X15373711/</ref>. With antibiotic-resistant bacteria more prevalent, benzoyl peroxide is an effective alternative to antibiotic monotherapy in the treatment of mild to moderate acne vulgaris. | Benzoyl peroxide is an alternative to antibiotics in the treatment of acne vulgaris. It is widely used due to its efficacy and favorable tolerability profile (very low amounts of adverse effects). Benzoyl peroxide contains mediums that have antibacterial, anti-inflammatory keratolytic, and wound healing properties <ref>Merker, P. C. (2002). Benzoyl peroxide: a history of early research and researchers. International journal of dermatology, 41(3). From https://eds.p.ebscohost.com/eds/detail/detail?vid=0&sid=9ff8152d-d475-442b-94d5-1587efe839a0%40redis&bdata=JnNpdGU9ZWRzLWxpdmU%3d#AN=6697926&db=aph/</ref>. Combination therapies including benzoyl peroxide are also very beneficial at targeting <i>P. acnes</i> in a pathogenic state. It is a powerful oxidizing agent derived from chlorohydroxyquinoline, a by-product of coal tar <ref>Pace, W. E. (1965). A benzoyl peroxide-sulfur cream for acne vulgaris. Canadian Medical Association Journal, 93(6), 252. From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1928665//</ref>. The compound is highly lipophilic and easily able to penetrate and enter the pilosebaceous follicle <ref>Ibbotson, S. H., Lambert, C. R., Moran, M. N., Lynch, M. C., & Kochevar, I. E. (1998). Benzoyl peroxide increases UVA-induced plasma membrane damage and lipid oxidation in murine leukemia L1210 cells. Journal of investigative dermatology, 110(1), 79-83. From https://www.sciencedirect.com/science/article/pii/S0022202X15373711/</ref>. With antibiotic-resistant bacteria more prevalent, benzoyl peroxide is an effective alternative to antibiotic monotherapy in the treatment of mild to moderate acne vulgaris. | ||
| Line 96: | Line 97: | ||
===Acne Vaccine Treatments=== | ===Acne Vaccine Treatments=== | ||
The majority of acne treatments are isolated to topical/systemic antibiotics, oral/topical isotretinoin, chemicals like benzoyl peroxide, oral contraceptives, and corticosteroids. A possible <i>P. acnes</i> vaccine may be in development using a multifaceted approach of traditional immunological and biochemical antigen discovery strategies as well as genomic. Vaccines against <i>P. acnes</i> could be quite clinically valuable. It has been proved that G-coated bacteria are present in comedones of acne patients showing that antibodies produced from immunization should be able to reach <i>P. acnes</i> | The majority of acne treatments are isolated to topical/systemic antibiotics, oral/topical isotretinoin, chemicals like benzoyl peroxide, oral contraceptives, and corticosteroids. A possible <i>P. acnes</i> vaccine may be in development using a multifaceted approach of traditional immunological and biochemical antigen discovery strategies as well as genomic. Vaccines against <i>P. acnes</i> could be quite clinically valuable. It has been proved that G-coated bacteria are present in comedones of acne patients showing that antibodies produced from immunization should be able to reach <i>P. acnes</i> <ref name==ab>Bhatia, A., Maisonneuve, J. F., & Persing, D. H. (2004, June). <i>Propionibacterium acnes</i> and chronic diseases. In <i>The Infectious Etiology of Chronic Diseases: Defining the Relationship, Enhancing the Research, and Mitigating the Effects: Workshop Summary., Knobler, SL et al.(eds.)</i>(pp. 74-80). From https://www.ncbi.nlm.nih.gov/books/NBK83685/#/</ref>. These antibodies would be able to target secretory virulence factor-induced inflammation rather than specific bacteria themselves. This would be beneficial to keep the healthy microbiome in order and only respond to bacteria releasing toxins in it virulent state, keeping high levels of inflammation at bay. | ||
==Conclusion== | |||
Conditions caused by the pathogenesis of <i>P. acnes</i> are rarely lethal. However, I find the behavior surrounding the treatment of conditions such as acne vulgeris quite interesting, especially in the United States. It is easily understood that the cosmetic associations with or with not having acne would be treated. However, to the extent of such therapies as isotretinoin derived therapeutics are incredibly interesting and worth studying. This question begins to creep into a psychological discipline that is worth researching. | |||
=<b>References</b>= | =<b>References</b>= | ||
Latest revision as of 21:47, 14 April 2024
General Background
By Megan Lydon
Propionibacterium acnes is a bacterium commonly found on human skin, particularly in sebaceous (oil) glands and hair follicles. It is gram-positive and a fairly slow-growing aerotolerant bacterium. A lower density of P. acnes is detected on the skin of adolescents, especially those prepubescent. The bacteria mainly live on fatty acids. The normal habitat of P. acnes is in the sebaceous follicle shared with the yeast Pityrosporum and aerobic staphylococci and micrococci on its surface [1]. Despite its name, and its colloquial associations, P. acnes is not solely associated with acne [2]; it is a normal resident of the skin microbiota in most people. However, it can contribute to the development of acne vulgaris, the most common form of acne, when factors such as excess sebum production, hormonal changes, and inflammation are present. Age-related differences are also noted in which lower levels of P. acnes are found in young children before they hit puberty. Acne is one of the most common skin diseases affecting more than 45 million people in the United States. In addition, in a clinical context, it is estimated that nearly 20% of visits to dermatologists are related to acne and the treatment of the condition [2]. Varying degrees of acne affects nearly all people between the ages of 15-17 with 15-20% of those cases being moderate to severe [3].
As for other opportunistic diseases, P. acnes is known to be involved in endocarditis, osteomyelitis [4][5] arthritis, and postoperative device infection as a result of the insertion of prosthetics and heart valves.
The genome of P. acnes is 2.5 Mb [6]. P. acnes has genes encoding metabolic enzymes allowing it to survive in microaerophilic conditions. When pathogenic, the evasion of host cells (epithelial cells) is characterized by the meditation of the bacterial surface proteins or adhesions recognizing the extracellular matrix (ECM) components. These components are the ideal target of use by many pathogens for tissue colonization. Other skin-associated bacteria such as those in the genera of Staphylococcus and Streptococcus express skin surface proteins known as MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) that bind to those EMCs. P. acnes is also able to perform this recognition process while adhering to the skin but also is able to promote more reaction by traveling deeper into the skin.
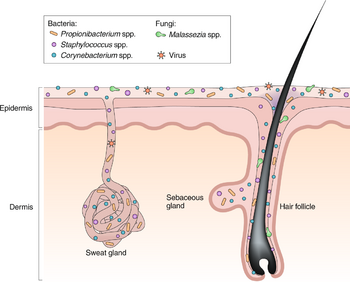
Skin Microbiome
Microbiomes, in general, serve a greater purpose than living organisms just existing in their habitat. Through a combination of commensal species of microbes and their interactions with their habitat, environments are formed where the host and bacteria can adapt and regulate processes either to their advantage or negative effects of competition. The skin microbiome works identically. There is mass variability in the skin microbiome. As for microbes involved, fungi, bacteria, viruses, and small arthropods contribute to this relationship [1].
In addition, the microbiome is much more complex than once thought. Past research has tended to focus only on pathogens and opportunistic pathogens rather than the entire spread of microbes in general (even “harmless” to human hosts). In addition to the variability of the microbes present in the skin microbiome, locations of the skin and their own environments are also variable from person to person. However, even in these differences, homeostasis between the microbiome and host is imperative for continued healthy interactions on the epithelium and to avoid the occurrence of disease. The skin ecosystem is continuously variable in humidity, temperature, pH, and composition of antimicrobial peptides and lipids [1]. In addition, the frequency of hair follicles can also determine the production of sebaceous materials and eccrine/apocrine glands. With this variety of environments, it establishes a separate niche for microbes to fill and thrive in. The abundance of certain bacteria is dependent on these niches [8].
Pathogenesis of P. acnes (Acne Vulgeris)
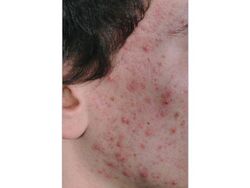
P. acnes is present on healthy skin and disrupted skin [9]. Therefore associated conditions cannot be classified as infectious diseases. However, pathogenisis of P. acnes does disrupt normal epithelium and its symptoms look to be treated by many. P. acnes is considered to contribute to the development of acne vulgaris, which can chronically affect 15% of people of all ages and at least 85% of teenagers in the United States [10] [11] . As for its role in the disruption of the epithelium is the onset of acne vulgeris. This is a condition where painful, red, and inflamed portions of the skin are infected by P. acnes. P. acnes only triggers the disease when it meets a favorable terrain, therefore the dense colonization of bacteria on the skin is necessary but not sufficient for pathogenesis. Research suggests that the density of bacteria has no effect on the frequency of acne vulgeris however there has been some evidence that certain strains of P. acnes can be more pathogenic when met with favorable conditions. However, it has been shown that there is a correlation between high sebum production and P. acnes density [9]. Regular colonization of P. acnes is quite beneficial to the skin microbiome as it can hydrolyze triglycerides and release free fatty acids to maintain acidic pH on the skin surface. This then helps down regulate the density of other pathogenic bacteria such as Streptococcus pyogenes and Staphylococcus aureus [6].
Follicular Epithelium
The pilosebaceous unit is composed of three subunits: hair follicle, arrector pili muscle, and sebaceous gland [12]. The unit functions mainly as a form of protection against the external environment and aids in the dispersion of sweat. The shape of the hair follicle is also variable and can determine differences in the environments of the skin microbiome. The transformation of the pilosebaceous unit (follicle) into the primary acne lesion is known as “comedogensis” [13]. During this process, P. acenes can get trapped in layers of corneocytes and excess sebum which then in turn rapidly increases colonization and presence of the bacteria in the comedonal kernel. This irregular colonization then results in the formation of a microcomedone. These microcomedomes are invisible to the naked eye but can continue to develop into a mature comedome. These mature comedomes can be a closed structure (whitehead) or an open structure (blackhead). Closed comedomes cannot allow the release of cell debris, sebum, and excess P. acnes and its associated products. This clog then makes the closed comedones more prone to inflammation and rupture. In this inflamed acne vulgeris, comedones rupture displacing follicular material into the dermis that then are released onto the skin’s surface. The degree of damage can be classified as papules (least severe), pustules, or nodules (most severe).
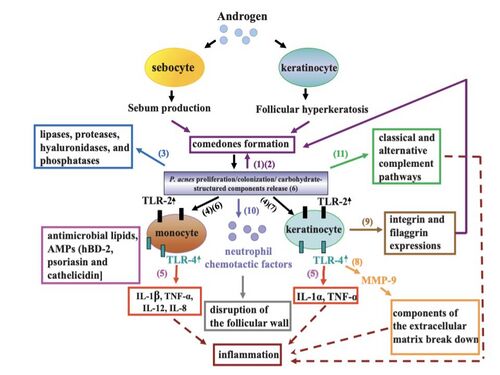
P. acnes byproducts and inflammatory responses
Substances produced by the trapped P. acnes tend to be the main reason for the rupture and inflammation of closed comedones [14]. The bacteria secrete many polypeptides. Many of these polypeptides can be classified as extracellular enzymes such as proteases, hyaluronidases, and neuraminidase. These polypeptides play a role in destabilizing the epithelium, resulting in the possible burst and the continued formation of acne vulgeris.
There are multiple ways in which this bacteria can harness the pathogenesis of the follicular epithelium and therefore trigger an inflammatory response. P. acnes produces pro inflammatory cytokine inducing factors. P. acnes is a known inducer of Th1 immune response cells [15] and of natural killer (NK) cells [16]. CD4+ T cells and macrophages infiltrate sebaceous follicles that contain trapped P. acnes. This increases the expression of inflammation response by the immune system.
P. acnes can activate the classical and alternative complement pathways of the innate immune system[17] [18]. In turn, inflammatory responses are deployed by the immune system to attack this infectious bacteria. This inflammation is mainly due to the violent neutrophils of the immune system engulfing P. acnes into the sebaceous follicle and then release hydrolases that can then damage the follicular wall. In addition to the inflammation caused by the immune system, P. acnes also releases lipases, proteases, and hyaluronidases that can add to epithelial injury.
The toll-like receptor 2 pathway is also important to the pathogenesis of P. acnes. TLR2 is expressed on the cell surface of macrophages that are called upon by the immune system in acne lesions. P. acnes may trigger some sort of inflammatory cytokine response in activation once the TLR2 pathway is initiated. This interaction can lead to the upregulation of cytokine expression in sebaceous glands. However, these cytokines are also always present in these tissues even in the absence of these influences (i.e. pathogenic bacteria and inflammation).
Public Health Implications
Antibiotic Resistance
The overuse and misuse of antibiotics have led to the widespread development of resistance to antimicrobial therapy by many pathogens including P. acnes. Antibiotic resistance is a very important public health concern, if not maybe, the most important topic. Antimicrobial resistance has resulted from an association between poor therapeutic response and antibiotic resistance. It is estimated that P. acnes antibiotic resistance has grown from an average of 20% being resistant to some form of antibiotic in 1978 to approximately 62% in 1996 [19]. The most common resistance was seen of the drugs: erythromycin, clindamycin, tetracycline, doxycycline, and trimethoprim however, resistance to minocycline was rare [19].
P. acnes resistance genes have been tied to mutations in 16s and 23s mRNA. This is especially alarming since these genes are closely tied to prokaryotic phylogeny and therefore might be extremely heritable. However, the mechanisms of this antibiotic resistance are a knowledge gap. These strains have been noticed and isolated from Europe, Australia, the United States, and Japan [20].
Epidemiology
Not much is known about the epidemiology of acne vulgaris. Clinical and histological features are well defined however much less appears to be written about its epidemiology. This is quite strange because acne is a condition that is defined colloquially as a disease and is almost always present in the teenage years [21].
Diet
Historically, dietary advice was very common when discussing acenes treatments. Early studies hinted that patients with acne had skewed glucose tolerance and altered carbohydrate metabolism. Therefore, patients were advised to avoid excessive carbohydrates and sugary goods [22]. On the other hand, later studies have found that students who consumed a high carbohydrate diet had no flare-ups over time. Granted, this specific study had a small sample size and no control group [23]. However, an additional comprehensive review of seven studies found no clear positive evidence that any dietary components increase acne risk [24].What needs to be defined however is if acne levels and dietary makeup are being demonstrated based on acne occurrence as opposed to food that could influence the severity of disease flares of those already predisposed to higher levels of acne.
Stress and Picking
Stress is perceived to be a major factor in exacerbating acne vulgaris [25]. Relaxation training and stress management techniques found that people with acne had massive improvements in severity [26].On the other hand, stress and anxiety increase with the severity of acne, unfortunately adding to the vicious cycle of stressing, picking, and the worsening of acne vulgaris. Stressful events are also triggers in rates of acne vulgaris.
Acne Vulgeris Treatments
Contributing factors of the pathogenesis of acne vulgaris by P. acnes are hyperkeratinization and obstruction of the follicular epithelium. In addition, stimulated sebum production and inflammation are targets and reasons for complications of effective new treatments [21].
Topical or combined agents are used with the majority of patients with mild to moderate acne. Combination treatment is beneficial to target multiple causes and sites where acne vulgaris pathogenizes. Systemic therapy can be used for severe cases with nodules, cysts, or even scarring. Our current understanding of the pathophysiology of the inflammatory scene supports some sort of combination therapy aimed at eradicating pathogenic P. acnes and normalizing abnormal makeup of the follicular glands (such as an increase in the production of sebaceous oils). This will then eliminate an environment where P. acnes will proliferate extensively and then cause infection. In turn, this will downregulate the creation of the microcomedone which leads to acne lesions and mature comedones [27].
Topical/Systemic Antibiotics
This is one of the most common acne treatments. It takes a relatively long time to reduce P. acnes populations. They also do not address other causative factors of acne. However, there is an increase in antibiotic resistant strains for P. acnes, and new better treatments are needed. Antibiotics used currently and that were more effective in the past have been ampicillin, clindamycin, erythromycin, tetracycline, doxycycline, nadifloxacin, ofloxacin, and tetracycline [28].The most potent agents are erythromycin and clindamycin. Antibiotics target and suppress the growth of P. acnes and can be direct suppressors of inflammation. Specifically speaking, tetracycline and erythromycin can cause a decrease in neutrophil chemotaxis and the production of chemotactic factors [29].
Side effects of antibiotic usage are gastrointestinal pain, bone and tooth pigmentation, urticarial reactions, vertigo-like symptoms, and deposition of carbofuran-like pigment of the skin [30].In addition, doxycycline therapy can result in photosensitivity reactions [31]. These side effects are related to any antibiotic usage and are not isolated to usage in an acene-targeting sense. In addition, antibiotic resistance is a result of overuse of antibiotics, so careful prescription is needed to avoid overuse and misuse of this drug. Antibiotics should be used in combination with a topical retinoid always. If long-term usage is needed, then benzoyl peroxide should be used in combination to reduce the emergence of antibiotic resistance [32].
Oral/Topical Isotretinoin
Isotretinoin is a vitamin A-derived retinoid. Its most common form on the market was Accutane but since has been discontinued in September 2023. It has been cited with severe side effects including: elevated serum triglyceride levels, acute pancreatitis, hepatotoxicity, clinical depression, and birth defects in pregnant women. The use of this treatment was initially approved in the United States in 1982 and is known for its teratogenicity (causing birth defects) [33] indicating that it was not an appropriate treatment choice for acne patients even at that time.
Benzoyl Peroxide
Benzoyl peroxide is an alternative to antibiotics in the treatment of acne vulgaris. It is widely used due to its efficacy and favorable tolerability profile (very low amounts of adverse effects). Benzoyl peroxide contains mediums that have antibacterial, anti-inflammatory keratolytic, and wound healing properties [34]. Combination therapies including benzoyl peroxide are also very beneficial at targeting P. acnes in a pathogenic state. It is a powerful oxidizing agent derived from chlorohydroxyquinoline, a by-product of coal tar [35]. The compound is highly lipophilic and easily able to penetrate and enter the pilosebaceous follicle [36]. With antibiotic-resistant bacteria more prevalent, benzoyl peroxide is an effective alternative to antibiotic monotherapy in the treatment of mild to moderate acne vulgaris.
Oral Contraceptives
Hormonal therapy is also employed as acne therapy. When treating acne, estrogen from the hormone treatment is beneficial to clearing acne, whereas the progestin-only pill tends to exacerbate acne. Oral contraceptives reduce acne lesions by supplying estrogen and increasing the sex hormone-binding globulin (SHBG). This in turn decreases free testosterone and suppresses the overall production of androgen [37]. This process decreases sebum production and hair growth [38]. Patients who do not qualify for the use of oral contraceptives as a form of acne therapy include: men, people with thromboembolic disorders, severe uncontrolled hypertension, migraine with focal neurological symptoms, some malignancies, pregnancy, and heavy smokers older than 35 years old [39].
Corticosteroids
Topical corticosteroids can be used in certain conditions such as to treat very inflammatory acne. However, the treatment period can only be very short. It can also be used for the initial treatment of inflammatory manifestations and aggravation of acne [40]. The vehicle of the steroids can be in the form of a topical ointment as it increases the potency of the drug [41]. Despite the legal obligation to report to regulatory agencies the observed adverse effects and drug reactions, the clinical practice of reporting is most certainly poor and incomplete. It is suggested that moderate to severe side effects will not really be reported to authorities. However, life threatening effects will be. The available databases tend to suggest this. However, there are reports of long-term use of this chemical in more local adverse events rather than systemic reactions [42]. These have been characterized by decreasing efficacy due to the tissue treated now becoming less sensitive (tachyphylaxis) [43].
Acne Vaccine Treatments
The majority of acne treatments are isolated to topical/systemic antibiotics, oral/topical isotretinoin, chemicals like benzoyl peroxide, oral contraceptives, and corticosteroids. A possible P. acnes vaccine may be in development using a multifaceted approach of traditional immunological and biochemical antigen discovery strategies as well as genomic. Vaccines against P. acnes could be quite clinically valuable. It has been proved that G-coated bacteria are present in comedones of acne patients showing that antibodies produced from immunization should be able to reach P. acnes [2]. These antibodies would be able to target secretory virulence factor-induced inflammation rather than specific bacteria themselves. This would be beneficial to keep the healthy microbiome in order and only respond to bacteria releasing toxins in it virulent state, keeping high levels of inflammation at bay.
Conclusion
Conditions caused by the pathogenesis of P. acnes are rarely lethal. However, I find the behavior surrounding the treatment of conditions such as acne vulgeris quite interesting, especially in the United States. It is easily understood that the cosmetic associations with or with not having acne would be treated. However, to the extent of such therapies as isotretinoin derived therapeutics are incredibly interesting and worth studying. This question begins to creep into a psychological discipline that is worth researching.
References
- ↑ 1.0 1.1 1.2 Leyden, J. J. (1997). Propionibacterium acnes colonization in acne and non-acne.Journal of Investigative Dermatology, 3(108), 379. From https://www.infona.pl/resource/bwmeta1.element.elsevier-c3dc00b6-a2eb-39e6-b2e6-bdac77b2d48c/
- ↑ 2.0 2.1 2.2 Bhatia, A., Maisonneuve, J. F., & Persing, D. H. (2004, June). Propionibacterium acnes and chronic diseases. In The Infectious Etiology of Chronic Diseases: Defining the Relationship, Enhancing the Research, and Mitigating the Effects: Workshop Summary., Knobler, SL et al.(eds.)(pp. 74-80). From https://www.ncbi.nlm.nih.gov/books/NBK83685/#/
- ↑ Law, M. P. M., Chuh, A. A. T., Lee, A., & Molinari, N. (2010). Acne prevalence and beyond: acne disability and its predictive factors among Chinese late adolescents in Hong Kong. Clinical and experimental dermatology, 35(1), 16-21. From https://academic.oup.com/ced/article-abstract/35/1/16/6622092/
- ↑ Jakab, E., Zbinden, R., Gubler, J., Ruef, C., Von Graevenitz, A., & Krause, M. (1996). Severe infections caused by Propionibacterium acnes: an underestimated pathogen in late postoperative infections. The Yale journal of biology and medicine, 69(6), 477. From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2589039//
- ↑ Söderquist, B., Holmberg, A., & Unemo, M. (2010). Propionibacterium acnes as an etiological agent of arthroplastic and osteosynthetic infections–two cases with specific clinical presentation including formation of draining fistulae. Anaerobe, 16(3), 304-306. From https://www.sciencedirect.com/science/article/pii/S107599640900153X/
- ↑ 6.0 6.1 6.2 Liu, J., Cheng, A., Bangayan, N. J., Barnard, E., Curd, E., Craft, N., & Li, H. (2014). Draft genome sequences of Propionibacterium acnes type strain ATCC6919 and antibiotic-resistant strain HL411PA1. Genome announcements, 2(4), 10-1128. From https://journals.asm.org/doi/full/10.1128/genomea.00740-14/
- ↑ Schmidt, C. (2020). Out of your skin. Nat. Biotechnol, 38(4), 392-397. From https://www.nature.com/articles/s41587-020-0473-8/ Out of your skin/
- ↑ Grange, P. A., Raingeaud, J., Morelle, W., Marcelin, A. G., Calvez, V., & Dupin, N. (2017). Characterization of a Propionibacterium acnes surface protein as a fibrinogen-binding protein. Scientific Reports, 7(1), 6428. From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5527093//
- ↑ 9.0 9.1 Dessinioti, C., & Katsambas, A. D. (2010). The role of Propionibacterium acnes in acne pathogenesis: facts and controversies. Clinics in dermatology, 28(1), 2-7. https://www-sciencedirect-com.libproxy.kenyon.edu/science/article/pii/S0738081X09000583?via%3Dihub/
- ↑ Baldwin, H. E., Friedlander, S. F., Eichenfield, L. F., Mancini, A. J., & Yan, A. C. (2011, September). The effects of culture, skin color, and other nonclinical issues on acne treatment. In Seminars in cutaneous medicine and surgery (Vol. 30, No. 3 Suppl, pp. S12-5). From https://europepmc.org/article/med/21943562/
- ↑ Tom, W. L., & Fallon Friedlander, S. (2008). Acne through the ages: case-based observations through childhood and adolescence. Clinical pediatrics, 47(7), 639-651. From https://journals.sagepub.com/doi/abs/10.1177/0009922808315444/
- ↑ McManus, L. M., & Mitchell, R. N. (2014). Pathobiology of human disease: a dynamic encyclopedia of disease mechanisms. Elsevier. From https://www.sciencedirect.com/referencework/9780123864574/pathobiology-of-human-disease/
- ↑ Plevig, G., & Kligman, A. M. (2000). Acne and rosacea. 3rd. From https://scholar.google.com/scholar hl=en&as_sdt=0%2C36&q=Plewig+G%2C+Kligman+AM%2C+editors.+Acne+and+Rosacea.+3rd+ed.+New+York%3A+Springer-Verlag%3B+2000.+744+pages.&btnG=/
- ↑ Noble, W. C. (1984). Skin microbiology: coming of age. Journal of Medical Microbiology, 17(1), 1-12. From https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=7d4f8e02f93f69ba000282cc340cd2a16fe0078b/
- ↑ Sugisaki, H., Yamanaka, K., Kakeda, M., Kitagawa, H., Tanaka, K., Watanabe, K., ... & Mizutani, H. (2009). Increased interferon-γ, interleukin-12p40 and IL-8 production in Propionibacterium acnes-treated peripheral blood mononuclear cells from patient with acne vulgaris: Host response but not bacterial species is the determinant factor of the disease. Journal of dermatological science, 55(1), 47-52. From https://www.sciencedirect.com/science/article/pii/S0923181109000863/
- ↑ Miyata, H., Himeno, K., & Nomoto, K. (1983). Mechanisms of the potentiation of specific antitumor immunity by intratumor injection of Corynebacterium parvum. Cancer research, 43(10), 4670-4675. From https://aacrjournals.org/cancerres/article/43/10/4670/486780/Mechanisms-of-the-Potentiation-of-Specific/
- ↑ Webster, G. F., Leyden, J. J., Norman, M. E., & Nilsson, U. R. (1978). Complement activation in acne vulgaris: in vitro studies with Propionibacterium acnes and Propionibacterium granulosum. Infection and Immunity, 22(2), 523-529. From https://journals.asm.org/doi/abs/10.1128/iai.22.2.523-529.1978/
- ↑ Kim, J. Acne vaccines: therapeutic option for the treatment of acne vulgaris? J. Invest. Dermatol., 2008, 128(10), 2353-2354. From https://www.sciencedirect.com/science/article/pii/S0022202X15336411/
- ↑ 19.0 19.1 Cooper, A. J. (1998). Systematic review of Propionibacterium acnes resistance to systemic antibiotics. Medical journal of Australia, 169(5), 259-261. From https://onlinelibrary.wiley.com/doi/abs/10.5694/j.1326-5377.1998.tb140250.x/
- ↑ Ross, J. I., Snelling, A. M., Eady, E. A., Cove, J. H., Cunliffe, W. J., Leyden, J. J., ... & Oshima, S. (2001). Phenotypic and genotypic characterization of antibiotic‐resistant Propionibacterium acnes isolated from acne patients attending dermatology clinics in Europe, the USA, Japan and Australia. British Journal of Dermatology, 144(2), 339-346. From https://academic.oup.com/bjd/article-abstract/144/2/339/6690999/
- ↑ 21.0 21.1 21.2 Bhate, K., & Williams, H. C. (2013). Epidemiology of acne vulgaris. British Journal of Dermatology, 168(3), 474-485. From https://onlinelibrary.wiley.com/doi/epdf/10.1111/bjd.12149/
- ↑ CAMPBELL, G. G. (1931). The relation of sugar intolerance to certain diseases of the skin. British Journal of Dermatology, 43(6), 297-304. From https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2133.1931.tb09505.x/
- ↑ Taylor, S. C., Cook-Bolden, F., Rahman, Z., & Strachan, D. (2002). Acne vulgaris in skin of color. Journal of the American Academy of Dermatology, 46(2), S98-S106. From https://www.sciencedirect.com/science/article/pii/S0190962202107407/
- ↑ Magin, P., Pond, D., Smith, W., & Watson, A. (2005). A systematic review of the evidence for ‘myths and misconceptions’ in acne management: diet, face-washing and sunlight. Family practice, 22(1), 62-70. From https://academic.oup.com/fampra/article/22/1/62/440463/
- ↑ Poli, F., Dreno, B., & Verschoore, M. (2001). An epidemiological study of acne in female adults: results of a survey conducted in France. Journal of the European Academy of Dermatology and Venereology, 15(6), 541-545. From https://onlinelibrary.wiley.com/doi/full/10.1046/j.1468-3083.2001.00357.x/
- ↑ Hughes, H., Lawlis, G. F., Brown, B. W., & Fulton Jr, J. E. (1983). Treatment of acne vulgaris by biofeedback relaxation and cognitive imagery. Journal of Psychosomatic Research, 27(3), 185-191. From https://www.sciencedirect.com/science/article/pii/0022399983900211/
- ↑ Leyden, J. J. (1998). Topical treatment of acne vulgaris: retinoids and cutaneous irritation. Journal of the American Academy of Dermatology, 38(4), S1-S4. From https://www.jaad.org/article/S0190-9622(98)70138-0/fulltext/
- ↑ KUROKAWA, I., NISHIJIMA, S., & KAWABATA, S. (1999). Antimicrobial susceptibility of Propionibacterium acnes isolated from acne vulgaris. European Journal of Dermatology, 9(1), 25-8. From https://www.jle.com/en/revues/ejd/e docs/antimicrobial_susceptibility_of_propionibacterium_acnes_isolated_from_acne_vulgaris_100287/article.phtml?cle_doc=000187BF/
- ↑ Sykes Jr, N. L., & Webster, G. F. (1994). Acne: a review of optimum treatment. Drugs, 48(1), 59-70. From https://link.springer.com/article/10.2165/00003495-199448010-00006/
- ↑ CUNLIFFE, W. J., GROSSHANS, E., BELAICH, S., MEYNADIER, J., ALIREZAI, M., & THOMAS, L. (1998). A comparison of the efficacy and safety of lymecycline and minocycline in patients with moderately severe acne vulgaris. European Journal of Dermatology, 8(3), 161-6. From https://www.jle.com/en/revues/ejd/e-docs/a_comparison_of_the_efficacy_and_safety_of_lymecycline_and_minocycline_in_patients_with_moderately_severe_acne_vulgaris_100541/article.phtml/
- ↑ Leyden, J. J. (1997). Topical treatment for acne vulgaris. N Engl J Med, 336, 1156-1162. From https://scholar.google.com/scholar?hl=en&as_sdt=0%2C36&q=Leyden+JJ.+Topical+treatment+for+acne+vulgaris.+N+Engl+J+Med+1997%3B+336%3A+1156+%E2%80%93+1162.&btnG=/
- ↑ Ellis, C. N., Leyden, J., Katz, H. I., Goldfarb, M. T., Hickman, J., Jones, T. M., & Tschen, E. (2001). Therapeutic studies with a new combination benzoyl peroxide/clindamycin topical gel in acne vulgaris. Cutis, 67(2 Suppl), 13-20. From https://europepmc.org/article/med/11236210/
- ↑ Bauer, L. B., Ornelas, J. N., Elston, D. M., & Alikhan, A. (2016). Isotretinoin: controversies, facts, and recommendations. Expert review of clinical pharmacology, 9(11), 1435-1442. From https://www.tandfonline.com/doi/abs/10.1080/17512433.2016.1213629/
- ↑ Merker, P. C. (2002). Benzoyl peroxide: a history of early research and researchers. International journal of dermatology, 41(3). From https://eds.p.ebscohost.com/eds/detail/detail?vid=0&sid=9ff8152d-d475-442b-94d5-1587efe839a0%40redis&bdata=JnNpdGU9ZWRzLWxpdmU%3d#AN=6697926&db=aph/
- ↑ Pace, W. E. (1965). A benzoyl peroxide-sulfur cream for acne vulgaris. Canadian Medical Association Journal, 93(6), 252. From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1928665//
- ↑ Ibbotson, S. H., Lambert, C. R., Moran, M. N., Lynch, M. C., & Kochevar, I. E. (1998). Benzoyl peroxide increases UVA-induced plasma membrane damage and lipid oxidation in murine leukemia L1210 cells. Journal of investigative dermatology, 110(1), 79-83. From https://www.sciencedirect.com/science/article/pii/S0022202X15373711/
- ↑ Haider, A., & Shaw, J. C. (2004). Treatment of acne vulgaris. Jama, 292(6), 726-735. From https://jamanetwork.com/journals/jama/article-abstract/199214/
- ↑ Murphy, A. A., Cropp, C. S., Smith, B. S., Burkman, R. T., & Zacur, H. A. (1990). Effect of low-dose oral contraceptive on gonadotropins, androgens, and sex hormone binding globulin in nonhirsute women. Fertility and sterility, 53(1), 35-39. From https://www.sciencedirect.com/science/article/pii/S0015028216532129/
- ↑ Rich, P. (2008). Hormonal contraceptives for acne management. Cutis, 81(1), 13. From https://cdn.mdedge.com/files/s3fs-public/issues/articles/081010000s.pdf#page=15/
- ↑ Gollnick, H. P., & Krautheim, A. (2003). Topical treatment in acne: current status and future aspects. Dermatology, 206(1), 29-36. From https://karger.com/drm/article-abstract/206/1/29/111131/Topical-Treatment-in-Acne-Current-Status-and/
- ↑ Ayres, P. J. W., & Hooper, G. (1978). Assessment of the skin penetration properties of different carrier vehicles for topically applied cortisol. British Journal of Dermatology, 99(3), 307-317. From https://academic.oup.com/bjd/article-abstract/99/3/307/6683247/
- ↑ Robertson, D. B., & Maibach, H. I. (1982). Topical corticosteroids. International journal of dermatology, 21(2), 59-67. From https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-4362.1982.tb00498.x/
- ↑ du Vivier, A. (1976). Tachyphylaxis to topically applied steroids. Archives of Dermatology, 112(9), 1245-1248. From https://jamanetwork.com/journals/jamadermatology/article-abstract/536218/
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski,at Kenyon College,2024