Cryphonectria parasitica: Difference between revisions
No edit summary |
|||
| (85 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Classification== | ==Classification== | ||
<b>By Jinghao Li</b> | |||
===Higher order taxa=== | ===Higher order taxa=== | ||
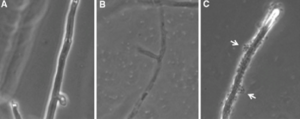
Domain: Fungus | [[Image:截屏2024-04-14 下午7.37.08.png|thumb|"Observing Cryphonectria parasitica hyphal cells under bright field microscopy after a 72-hour interaction with Lysobacter enzymogenes: (a) Untreated C. parasitica, and (b) C. parasitica treated with wildtype strain C3 of L. enzymogenes. Hyphal cell appearances are depicted."<ref>[https://link.springer.com/article/10.1007/s10482-013-9907-3 Patel, N. et al. (2013) 'Use of the tetrazolium salt MTT to measure cell viability effects of the bacterial antagonist Lysobacter enzymogenes on the filamentous fungus Cryphonectria parasitica,' Antonie Van Leeuwenhoek, 103(6), pp. 1271–1280. https://doi.org/10.1007/s10482-013-9907-3.]</ref>]] | ||
Kingdom: Fungus | Domain: Fungus, Kingdom: Fungus, Phylum: Ascomycetes, Class: Sordariomycete, Order: Diaporthales, Family: Cryphonectriaceae, Genus: Cryphonectria, Species: ''Cryphonectria parasitica'' <ref>[https://storymaps.arcgis.com/stories/0554468188c946399998fe14854fd8e3 Card, E.P.S. (2023) 'Cryphonectria parasitica,' ArcGIS StoryMaps, 10 October. https://storymaps.arcgis.com/stories/0554468188c946399998fe14854fd8e3.]</ref> | ||
Phylum: Ascomycetes | |||
Class: Sordariomycete | |||
Order: Diaporthales | |||
Family: Cryphonectriaceae | |||
Genus: Cryphonectria | |||
Species: ''Cryphonectria parasitica'' | |||
===Species=== | ===Species=== | ||
''Cryphonectria parasitica'' | ''Cryphonectria parasitica'' <ref>[https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=5116&lvl=3&lin=f&keep=1&srchmode=1&unlock Taxonomy (no date) Taxonomy browser (Cryphonectria parasitica). https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=5116&lvl=3&lin=f&keep=1&srchmode=1&unlock.]</ref> | ||
{| | {| | ||
| height="10" bgcolor="#FFDF95" | | | height="10" bgcolor="#FFDF95" | | ||
'''NCBI: [https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi]''' | '''NCBI: [https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=5116&lvl=3&lin=f&keep=1&srchmode=1&unlock Taxonomy]''' | ||
|} | |} | ||
==Description and significance== | |||
''Cryphonectria parasitica'' is a deadly fungus originally found on American chestnut trees outside the chestnut's native range. ''Cryphonectria parasitica'' was first observed in the New York City Zoo in 1904 and became known as the "chestnut blight." Originally taxonomically known as ''Diaporthe parasitica'', the fungus was later reclassified into the genus Endothia and finally named ''Cryphonectria parasitic''. This foreign pathogen brings deadly disaster. Its emergence has resulted in the American chestnut becoming the dominant species in the forest canopy of North America, while in Europe, some special chestnut populations such as Castanea sativa Mill. have brought near-extinction hazards. This pattern of introduced fungal pathogens wreaking havoc on tree species has persisted, with ash dieback (caused by ''Hymenoscyphus fraxineus'') in Europe being the most recent example (Gross et al. 2014). It is worth noting that chestnut wilt has attracted attention due to its hypovirulence phenomenon, in which viral infection weakens the virulence of the pathogen, providing a basis for biological control of the disease. In addition, conservation breeding efforts aim to restore the American chestnut to its status as an important forest species. <ref>[https://www.tandfonline.com/doi/full/10.1080/15572536.2003.11833253 Hoegger, P.J. et al. (2002b) 'Cryphonectria radicalis: rediscovery of a lost fungus,' Mycologia, 94(1), pp. 105–115. https://doi.org/10.1080/15572536.2003.11833253.]</ref> | |||
==Genome structure== | |||
[[Image:Genome cryp.webp|thumb|"This figure illustrates the chromosomal distribution of DNA methylation on scaffold 4. The density (A) and level (B) of DNA methylation are represented by green and red bars, respectively, indicating methylation on the Watson and Crick strands. Genic regions and transposable elements (TEs) are depicted by steel blue and purple bars, respectively, on the chromosome. Gray lines indicate CG contents. Arrows highlight regions where differences among strains are observed. It's noteworthy that densely methylated regions are predominantly situated around gene-poor areas of the chromosome."<ref>[https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2018.00103/full So, K.-K. et al. (2018) 'Global DNA Methylation in the Chestnut Blight Fungus Cryphonectria parasitica and Genome-Wide Changes in DNA Methylation Accompanied with Sectorization,' Frontiers in Plant Science, 9. https://doi.org/10.3389/fpls.2018.00103.[</ref>]] | |||
Both ''Cryphonectria parasitica'' mycoreoviruses (CpMYRV-1 and CpMYRV-2) consist of 11 genome segments categorized under Group 1. Despite sharing this structural similarity, they exhibit only around 29% amino acid sequence identity in the capping enzyme, affirming their classification as distinct species. <ref>[https://pubmed.ncbi.nlm.nih.gov/15483262/ Suzuki, N. et al. (2004b) 'Complete genome sequence of Mycoreovirus-1/Cp9B21, a member of a novel genus within the family Reoviridae, isolated from the chestnut blight fungus Cryphonectria parasitica,' Journal of General Virology, 85(11), pp. 3437–3448. https://doi.org/10.1099/vir.0.80293-0.]</ref> | |||
The complete genome of ''Cryphonectria parasitica'' mycoreovirus-1 (CpMRV-1) spans 23,436 base pairs (bp), with the length of individual segments varying between 732 bp and 4,127 bp. This results in a distinctive electrophoretic profile, exhibiting a pattern of 3, 3, 2, 3 segments when analyzed using either 11% polyacrylamide gel electrophoresis (PAGE) or 1% agarose gel electrophoresis (AGE). <ref>[https://apsjournals.apsnet.org/doi/10.1094/PHYTO-12-19-0478-A Crouch, J.A. et al. (2020) 'Genome Sequence of the Chestnut Blight Fungus Cryphonectria parasitica EP155: A Fundamental Resource for an Archetypical Invasive Plant Pathogen,' Phytopathology, 110(6), pp. 1180–1188. https://doi.org/10.1094/phyto-12-19-0478-a.]</ref> | |||
===''Cryphonectria parasitica'' strain EP155=== | |||
''Cryphonectria parasitica'' strain EP155, one of the strains causing damage to American chestnut trees, was originally isolated from chestnut canker. The genome sequencing and assembly process involved the use of Sanger sequencing protocols, resulting in a high-quality assembly of 26 scaffolds containing 33 contigs, spanning 43.9 Mb. The assembly was further refined using genetic linkage data, providing valuable insights into the organization of the genome at the chromosome level. Functional annotations of the genome revealed a total of 11,609 genes, with over 85% showing similarities to proteins from the NCBI nonredundant protein database. The genome also contains a significant number of genes involved in pathogenicity, secondary metabolite production, and cytochrome P450s. <ref>[https://mycocosm.jgi.doe.gov/Crypa2/Crypa2.home.html Home - Cryphonectria parasitica EP155 v2.0 (no date). https://mycocosm.jgi.doe.gov/Crypa2/Crypa2.home.html.]</ref> | |||
==Host range== | |||
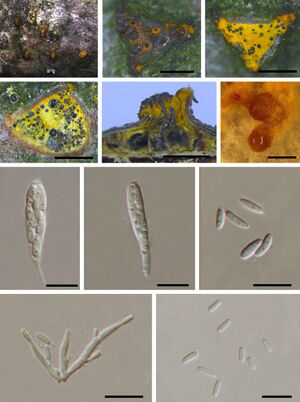
[[Image:89289123.jpeg|thumb|left|"Morphological characteristics of Cryphonectria neoparasitica isolated from Castanea mollissima (BJFC-S1435, holotype). (a) Overall appearance of ascostromata on a chestnut branch; (b, c) Ascostromata; (d) Cross-sectional view of ascostromata; (e) Longitudinal section through ascostromata; (f) Conidiomata cultivated on potato dextrose agar; (g, h) Asci; (i) Ascospores; (j) Conidiophores; (k) Conidia. Scale bars: b-f = 1 mm; e-h = 10 µm."<ref>[https://bsppjournals.onlinelibrary.wiley.com/doi/10.1111/ppa.13033 Jiang, N., Fan, X. and Tian, C. (2019) 'Identification and pathogenicity of Cryphonectriaceae species associated with chestnut canker in China,' Plant Pathology, 68(6), pp. 1132–1145. https://doi.org/10.1111/ppa.13033.]</ref>]] | |||
''Cryphonectria parasitica'' primarily infects various species within the genus Castanea, including the American chestnut, European chestnut, Chinese chestnut (''C. mollissima Blume''), and Japanese chestnut (''C. crenata Siebold'' & ''Zucc.''), all of which belong to the family Fagaceae. <ref>[https://cdnsciencepub.com/doi/abs/10.1139/x92-075?journalCode=cjfr Anagnostakis, S.L. (1992) 'Measuring resistance of chestnut trees to chestnut blight,' Canadian Journal of Forest Research, 22(4), pp. 568–571. https://doi.org/10.1139/x92-075.]</ref> | |||
==Life Cycle== | |||
''Cryphonectria parasitica'' is the causative agent of chestnut blight. They use damage to tree trunks or branches to enter trees and become parasitic. Furthermore, recent studies have shown that ''Cryphonectria parasitica'' can infect abandoned galls of chestnut gall wasps, providing new pathways for pathogens to invade host tissues. Both sexual and asexual spores of ''Cryphonectria parasitica'' can cause infection. When the spores germinate, ulcers form. Later, ''Cryphonectria parasitica'' may produce spores on infected bark and recently dead chestnut wood. The fruiting body, called a stroma, is composed of sexual (ascotheca) and asexual (pycnidia) structures and develops in numerous yellow-orange to reddish-brown pustules. These structures range from 0.5 to 4 mm in diameter and up to 2.5 mm in height and are embedded in the bark, with both types of fruiting bodies often coexisting closely. <ref>[https://apsjournals.apsnet.org/doi/abs/10.1094/PHYTO-96-1337 Prospero, S. et al. (2006) 'Saprophytic Activity and Sporulation of Cryphonectria parasitica on Dead Chestnut Wood in Forests with Naturally Established Hypovirulence,' Phytopathology, 96(12), pp. 1337–1344. https://doi.org/10.1094/phyto-96-1337.] </ref> | |||
The complex life cycle and diverse reproductive structures of ''Cryphonectria parasitica'' facilitate its successful colonization and spread in chestnut populations. By exploiting a variety of entry points and employing sexual and asexual reproduction methods, ''Cryphonectria parasitica'' exhibits remarkable adaptability and resilience, posing an ongoing challenge to the management and conservation of chestnut trees. The emergence of ''Cryphonectria parasitica'' has had a great impact on different chestnut trees and even oak species in various regions, and even led to the extinction of chestnut trees in the United States for a certain period of time. It has had a very negative impact on agriculture and the economy. <ref>[https://www.sciencedirect.com/science/article/pii/S1087184509001352?via%3Dihub Milgroom, M.G. et al. (2009) 'Heterokaryons and parasexual recombinants of Cryphonectria parasitica in two clonal populations in southeastern Europe,' Fungal Genetics and Biology, 46(11), pp. 849–854. https://doi.org/10.1016/j.fgb.2009.07.007.]</ref> | |||
==Disease Symptoms== | |||
[[Image:Trunck.jpeg|thumb|"Chestnut blight, also known as canker, caused by the pathogen Cryphonectria parasitic."<ref>[https://www.invasive.org/browse/detail.cfm?imgnum=5050040 chestnut blight or canker (Cryphonectria parasitica) (2006). https://www.invasive.org/browse/detail.cfm?imgnum=5050040.]</ref>]] | |||
''Cryphonectria parasitica'' mainly infects the branches of trees, so ''Cryphonectria parasitica'' is classified as a bark pathogen. Symptom expression on susceptible hosts, including European and American chestnuts, varies depending on the virulence of the ''Cryphonectria parasitica'' strain and the age of the infected tree part. Virulent strains of ''Cryphonectria parasitica'' form necrotic lesions on the bark, known as cankers, that can rapidly kill smaller branches or twigs within months. But on thicker branches, it usually takes several years for the cankers to develop before death occurs. While red and orange colors form on young stems and branches, the color is less noticeable on thicker branches, making it more difficult to judge. <ref>[https://www.forestresearch.gov.uk/tools-and-resources/fthr/pest-and-disease-resources/sweet-chestnut-blight-cryphonectria-parasitica/ Sweet chestnut blight (Cryphonectria parasitica) - Forest Research (2024b). https://www.forestresearch.gov.uk/tools-and-resources/fthr/pest-and-disease-resources/sweet-chestnut-blight-cryphonectria-parasitica/.]</ref> | |||
Within the bark and cambium, the characteristic light brown fans of ''Cryphonectria parasitica'' mycelium will appear, a telltale sign of chestnut wilt infection. If the cambium is killed, the bark will sink inward, making the canker look sunken. Afterwards the tree dies and the leaves wilt and turn yellow or brown. <ref>[https://forestpathology.org/canker/chestnut-blight/ Chestnut Blight: an American Tragedy | Forest pathology (no date). https://forestpathology.org/canker/chestnut-blight/.]</ref> | |||
Unlike cankers caused by virulent strains, cankers caused by less virulent strains are usually non-fatal. Stromata harboring the fruiting bodies of ''Cryphonectria parasitica'' may develop on the surface of cankers, while symptoms on oaks consist of slowly developing, calling using cankers that typically do not lead to stem or branch death. <ref>[https://onlinelibrary.wiley.com/doi/full/10.1111/efp.12063 Bryner, S.F. et al. (2013) 'Informative value of canker morphology on the presence or absence of virus infection in chestnut blight cankers,' Forest Pathology, 43(6), pp. 496–504. https://doi.org/10.1111/efp.12063.]</ref> | |||
==Epidemiology== | |||
New populations of ''Cryphonectria parasitica'' usually result from the introduction of one or more genotypes. It is likely that new ''Cryphonectria parasitica'' populations in North America were accidentally introduced with infected plant material. North American ''Cryphonectria parasitica'' has the fastest growth rate at 20°C, while European chestnut ''Cryphonectria parasitica'' has the fastest growth rate at 27°C and is significantly slower below 20°C. Adequate moisture provided by humid air, rain or dew is also critical for the survival, development and spread of ''Cryphonectria parasitica'', for example, moist conditions trigger spore release. There is a seasonal pattern in European chestnut susceptibility to ''Cryphonectria parasitica'', with a peak in spring and summer and a decrease in autumn and winter, possibly due to changes in bark nutritional value and moisture content. <ref>[https://pubmed.ncbi.nlm.nih.gov/24118686/ Gross, A. et al. (2013) 'Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback,' Molecular Plant Pathology, 15(1), pp. 5–21. https://doi.org/10.1111/mpp.12073.]</ref> | |||
==History== | |||
Cryphonectria parasitica was first discovered in East Asia, specifically China, Japan, and Korea. However, in the 20th century it was inadvertently introduced to North America and Europe via infected chestnut plants. After its discovery in 1904, it spread rapidly throughout the American chestnut's native distribution range at a rate of more than 30 kilometers per year, covering approximately 3.6 million hectares in eastern North America. This resulted in the endangered chestnut tree in North America and it has a great impact on the economy and environment. | |||
In Europe, the pathogen was officially detected in 1938 near Genoa, Italy's main international port. Genetic studies suggest that North America may have been the original source of introduction to Italy. By 1950, chestnut wilt had become widespread in Italy's main chestnut-growing areas and quickly spread to neighboring France. Between the 1920s and 1950s, Europe imported large quantities of chestnut trees from Asia and the United States, which may have facilitated the spread of C. parasitica. Ironically, the purpose of many of these imports was to obtain chestnut trees that were resistant to ink disease, the most serious chestnut disease before the arrival of chestnut wilt. <ref>[https://pubmed.ncbi.nlm.nih.gov/22548317/ Dutech, C. et al. (2012) 'The chestnut blight fungus world tour: successive introduction events from diverse origins in an invasive plant fungal pathogen,' Molecular Ecology, 21(16), pp. 3931–3946. https://doi.org/10.1111/j.1365-294x.2012.05575.x.]</ref> | |||
==Vegetative incompatibility== | |||
[[Image:123123123.jpeg|thumb|left|"Exploring the influence of vegetative incompatibility (VI) on secondary metabolites in Cryphonectria parasitica sheds light on fungal adaptation and ecological interactions." <ref>[https://www.sciencedirect.com/science/article/pii/S1087184521001171 Witte, T.E. et al. (2021b) 'A metabolomic study of vegetative incompatibility in Cryphonectria parasitica,' Fungal Genetics and Biology (Print), 157, p. 103633. https://doi.org/10.1016/j.fgb.2021.103633.]</ref>]] | |||
Vegetative incompatibility is a common phenomenon in fungi that serves as a defense mechanism against cytoplasm-borne diseases by hindering the formation of stable hyphal fusion and cytoplasmic exchange between individuals. In ''Cryphonectria parasitica'', the vegetative incompatibility system is of particular interest because it limits the horizontal transmission of attenuated virulence mycoviruses. <ref>[https://www.sciencedirect.com/science/article/pii/S1087184521001171 Witte, T.E. et al. (2021) 'A metabolomic study of vegetative incompatibility in Cryphonectria parasitica,' Fungal Genetics and Biology (Print), 157, p. 103633. https://doi.org/10.1016/j.fgb.2021.103633.]</ref> | |||
==Disease management== | |||
In order to reduce the introduction and spread of ''Cryphonectria parasitica'', the world has strictly managed the export and import of chestnuts and established relevant laws for management. In Europe, the EPPO continues to recommend that ''Cryphonectria parasitica'' be regulated as an A2 quarantine organism, which represents a pathogen that is indigenous to the EPPO region. Chestnut and oak plants intended for cultivation can only be transported within Europe and must be accompanied by a legal plant passport to prove that the plants come from a parasite-free area and have not been exposed to pathogens during transportation. The beginning of the last complete vegetation cycle. Despite these measures, however, quarantine regulations have not completely prevented the spread of the pathogen, particularly due to the challenges posed by asymptomatically infected plants evading visual inspection. <ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6638123/ EPPO (2005) EPPO Standards – Diagnostic protocols for regulated pests – PM7/45 Cryphonectria parasitica. EPPO Bull. 35, 295–298.]</ref> | |||
And if ''Cryphonectria parasitica'' is discovered in a new area, people usually take the most direct methods such as fire and felling to eliminate the pathogen. However, these efforts have largely failed due to the difficulty of locating and eliminating all sources of inoculum, especially in wild forests. It can be eradicated in artificial breeding environments because chestnut trees that are not infected with C. parasitica can be isolated from chestnut trees that are infected with ''Cryphonectria parasitica''. Also, plantations can reduce the chance of ''Cryphonectria parasitica'' infection by reducing surface wounds on chestnut trees. For the eradication of ''Cryphonectria parasitica'', chemical means are not the preferred method. First, many countries often restrict or ban the use of chemicals in forests. Second, fungicides may exhibit phytotoxic effects or promote the development of resistance. Moreover, this is likely to turn some ''Cryphonectria parasitica'' into new drug-resistant strains that can resist the toxicity of fungicides. Furthermore, transposable elements (TEs) were found to constitute approximately 14% of the C. parasitica EP155 genome, with class I retrotransposons being the most abundant group. The presence of TEs, including intact coding sequences, suggests a dynamic genome with potential implications for genome evolution and adaptation. <ref>[https://www.cabidigitallibrary.org/doi/10.1079/9781780640402.0318 Prospero, S. and Rigling, D. (2013) 'Chestnut blight.,' in CABI eBooks, pp. 318–339. https://doi.org/10.1079/9781780640402.0318.]</ref> <ref>[https://link.springer.com/article/10.1007/bf03356540?utm_source=getftr&utm_medium=getftr&utm_campaign=getftr_pilot Trapiello, E., González-Varela, G. and González, A.J. (2015) 'Chestnut Blight Control by Agrochemicals in Castanea sativa under Managed Conditions,' Journal of Plant Diseases and Protection, 122(3), pp. 120–124. https://doi.org/10.1007/bf03356540.]</ref> | |||
==Hypovirulence== | |||
Hypovirulence in ''Cryphonectria parasitica'' is a fascinating example of natural disease control facilitated by mycoviruses. Among these strains, especially Hypovirulence viruses such as CHV-1, CHV-2, CHV-3 and CHV-4 infect fungal hosts and induce hypo virulence phenotype, reducing its pathogenicity. Hypovirulence is not only low toxicity among fungi, but also lower toxicity of spores and reduced infectivity. <ref>[https://linkinghub.elsevier.com/retrieve/pii/S0065352704630077 Hillman, B.I. and Suzuki, N. (2004) 'Viruses of the Chestnut Blight Fungus, Cryphonectria parasitica,' in Advances in virus research, pp. 423–472. https://doi.org/10.1016/s0065-3527(04)63007-7.]</ref> <ref>[https://www.jstor.org/stable/41998375 'VIRUS-INDUCED HYPOVIRULENCE IN CRYPHONECTRIA PARASITICA: STILL AN UNRESOLVED CONUNDRUM on JSTOR' (no date) www.jstor.org [Preprint]. https://www.jstor.org/stable/41998375.]</ref> | |||
Interestingly, low viral transmission was limited to nutritionally incompatible ''Cryphonectria parasitica'' strains. In Europe, hypovirulence occurs naturally, such as the multiple genetic subtypes of CHV-1 found in Europe. However, in some other areas where chestnut blight is endemic, CHV-1 or other hypovirulence strains have not been found. Outside Europe, CHV-1 strains have also been found in Asia, but in North America, CHV-1 has not been found. However, parts of North America have been artificially released with CHV-1 strains for biological control. <ref>[https://apsjournals.apsnet.org/doi/10.1094/PHYTO-96-1337 Prospero, S. et al. (2006b) 'Saprophytic Activity and Sporulation of Cryphonectria parasitica on Dead Chestnut Wood in Forests with Naturally Established Hypovirulence,' Phytopathology, 96(12), pp. 1337–1344. https://doi.org/10.1094/phyto-96-1337.]</ref> | |||
As mentioned before, other strains such as CHV-2 and CHV-3 also induce a hypovirulent phenotype in ''Cryphonectria parasitica'', but CHV-4 strains do not cause obvious symptoms. <ref>[https://www.sciencedirect.com/science/article/abs/pii/S0065352704630077?via%3Dihub Hillman, B.I. and Suzuki, N. (2004b) 'Viruses of the Chestnut Blight Fungus, Cryphonectria parasitica,' in Advances in virus research, pp. 423–472. https://doi.org/10.1016/s0065-3527(04)63007-7.]</ref> | |||
===How to use Hypovirulence to control chestnut blight=== | |||
Introducing Hypovirulence, especially CHV-1, into areas where ''Cryphonectria parasitica'' is endemic can effectively control chestnut blight. This process is divided into five steps in total. First, we need to determine the vegetable compatibility (VC) type. Ideally, the strain causing chestnut blight and the Hypovirulence strain used should have the same or similar VC type. If different VC types are found, we can use a mixture of multiple different VC types of Hypovirulence. Second, an in vitro transmission assay is performed. Researchers can do this by spreading strains of Hypovirulence into virulent strains of the target VC type in the laboratory. This method ensures that Hypovirulence strains can grow normally in the wild. After that, the institute needs to carry out canker treatment. This method will be simpler. As we mentioned in the previous section, ''Cryphonectria parasitica'' is mainly infected from wounds on broken branches. So, the method is to inject the Hypovirulence strain into the cankers of infected chestnut trees. After the first three steps are completed, scientists still need to evaluate the trees for treatment. After at least one growth cycle, scientists can assess how successful the treatment was. In this process, not only can the extent of cankers after inoculation with the Hypovirulence strain be assessed, but ''Cryphonectria parasitica'' can also be re-isolated from the treated cankers to verify the presence of Hypovirulence. The final step is to determine the final treatment opportunity. During the growing season, approximately April to October, vaccination with the Hypovirulence strain during this time is most effective in treating canker. In this way, the artificial introduction of Hypovirulence can effectively weaken the chestnut wilt fungus, stop the spread of canker, and ultimately help control the disease in affected chestnut stands. <ref>[https://apsjournals.apsnet.org/doi/10.1094/PHYTO.2000.90.7.730 Robin, C., Anziani, C. and Cortesi, P. (2000) 'Relationship Between Biological Control, Incidence of Hypovirulence, and Diversity of Vegetative Compatibility Types of Cryphonectria parasitica in France,' Phytopathology, 90(7), pp. 730–737. https://doi.org/10.1094/phyto.2000.90.7.730.]</ref> | |||
===Restoration efforts for American chestnut trees=== | |||
Of paramount importance to plans for the revival of the American chestnut tree is the importance of seedling quality and the role it plays in successful establishment. Larger seedlings tend to have an advantage in growth and competitiveness, but smaller seedlings may be better able to withstand stress initially. However, the long-term growth and survival benefits of larger seedlings are significantly greater than those of smaller seedlings. In addition, many problems often occur during the transplantation process. For example, seedlings often suffer from planting shock due to damage to their root systems, or they are eaten by animals such as deer, causing damage to the trunk or roots. These factors may hinder the early growth of chestnut trees. To mitigate planting shock, it is crucial to minimize root damage and protect seedlings from nibblers during the transplanting process. Rapid recovery from planting stress is critical, which emphasizes the importance of selecting seedlings with traits conducive to rapid recovery. And some advanced breeding materials such as BC3F3 bring hope to the recovery of chestnut trees in the United States. These hybrid seedlings showed traits more similar to American chestnut than to Chinese chestnut (''Castanea mollissima''), a tree frequently used in blight resistance breeding programs in previous studies. <ref>[https://www.fs.usda.gov/research/treesearch/42728 Burhans, B. and Hebard, F.V. (2012) Restoring the American chestnut tree. https://www.fs.usda.gov/research/treesearch/42728.]</ref> | |||
==Evolution== | |||
[[Image:Chestnut tree.png|thumb|"The comparison between two Petri dishes containing cultures of the hypovirulent strain of the fungus Cryphonectria parasitica isolate RT 1-2. On the left side is a Petri dish with unamended potato dextrose agar (PDA), while on the right side is a Petri dish with PDA amended with strawberry extract. Both cultures have been incubated for 10 days at a temperature of 20°C."<ref>[https://www.researchgate.net/publication/265443381_Strawberry_Amendment_to_Potato_Dextrose_Agar_to_Increase_Conidiation_in_Cryphonectria_parasitica Double, M.L. and Marshall, M.R. (2014) 'STRAWBERRY AMENDMENT TO POTATO DEXTROSE AGAR TO INCREASE CONIDIATION IN CRYPHONECTRIA PARASITICA,' Acta Horticulturae, (1019), pp. 81–84. https://doi.org/10.17660/actahortic.2014.1019.12.]</ref>]] | |||
The evolutionary dynamics within the tri-trophic pathosystem of tree-fungus-hypovirus, particularly in introduced ranges of ''Cryphonectria parasitica'', are complex and challenging to predict. Understanding the interactions and co-evolution between Hypovirulence and fungi has implications for biological control systems. Research shows that hypovirulence virulence varies widely even within local populations, which represents the possibility of evolution. Specific regions of the viral genome associated with these variants have been identified through genome comparison and mutation analysis. Compared with other strains, CHV-1 strains evolve significantly faster than other strains, which means that CHV-1 has great potential to become a strain for treating chestnut blight. <ref>[https://onlinelibrary.wiley.com/doi/10.1002/ece3.429 Bryner, S.F., Rigling, D. and Brunner, P.C. (2012) 'Invasion history and demographic pattern of Cryphonectria hypovirus 1 across European populations of the chestnut blight fungus,' Ecology and Evolution, 2(12), pp. 3227–3241. https://doi.org/10.1002/ece3.429.]</ref> | |||
Environmental factors such as temperature and humidity also play a role in virulence, suggesting an interaction between genotype and genotype and environment. Contrary to the hypothesis that fungal viruses would evolve toward lower virulence in populations with increasingly restrictive horizontal transmission barriers, studies have not shown such a trend. In fact, there's evidence of a positive association between hypovirus virulence and transmission across vegetative incompatibility barriers. The reason for this may be that virulent hypoviruses may interfere with the nutritional incompatibility response induced during hyphal fusion of incompatible fungal strains. Furthermore, ecological factors may contribute to the persistence of viral hypoviruses in C. parasitica populations. Unlike hyperviruses, hypoviruses can survive and reproduce asexually on dead chestnut wood, serving as a reservoir for the Hypovirulence inoculum. <ref>[https://www.journals.uchicago.edu/doi/10.1086/657620 Bryner, S.F. and Rigling, D. (2011) 'Temperature‐Dependent Genotype‐by‐Genotype Interaction between a Pathogenic Fungus and Its Hyperparasitic Virus,' the American Naturalist, 177(1), pp. 65–74. https://doi.org/10.1086/657620.]</ref> | |||
Uncoupling of saprophytic and parasitic potential in ''Cryphonectria parasitica'' species with low viral infection may contribute to the observed changes in low viral virulence. Despite these complexities, further investigation of the evolutionary dynamics of fungal-hypovirus interactions is critical for the effective understanding and management of chestnut blight. <ref>[https://onlinelibrary.wiley.com/doi/10.1111/j.1558-5646.2012.01637.x Bryner, S.F. and Rigling, D. (2012) 'VIRULENCE NOT ONLY COSTS BUT ALSO BENEFITS THE TRANSMISSION OF a FUNGAL VIRUS,' Evolution, 66(8), pp. 2540–2550. https://doi.org/10.1111/j.1558-5646.2012.01637.x.]</ref> | |||
==Resistance Breeding== | |||
Breeding efforts to resist chestnut blight rely primarily on genetic resistance discovered in Asian management. The representative species is the Chinese chestnut tree. In previous studies, it was found that the Chinese chestnut tree has a stronger ability to resist withering than the Japanese chestnut tree. Based on these findings, the American Chestnut Foundation launched a major breeding program aimed at restoring the American chestnut's status as a forest tree. By combining ''Cryphonectria parasitica''-resistant species with native American chestnut trees over three successive generations, the laboratory obtained good results, showing that backcrossed chestnuts had higher survival rates and lower morbidity. but. Before conducting large-scale experiments, it is still necessary to determine whether the new chestnut trees generated from the hybrid are immune to other viruses and whether they can grow normally in different environments. In many European countries, new strains obtained by successfully crossing European chestnut trees and Asian chestnut trees have good resistance to chestnut blight. These hybrids also tend to be less susceptible to ink disease and chestnut gall wasp, making them increasingly popular for nut production. As another way to help plants resist viruses, genetic modification can also resist chestnut blight. By integrating wheat's oxalate oxidase gene into the chestnut genome, chestnut trees with enhanced resistance to blight disease were developed. This gene targets the degradation of oxalic acid, a toxin produced by ''Cryphonectria parasitica'' during the infection process. These diverse methods of fighting chestnut blight can effectively help control the development of chestnut blight in Europe and America in the future, restore chestnut tree populations in different regions, and improve local economies. <ref>[https://www.sciencedirect.com/science/article/pii/S0378112705007632?pes=vor Diskin, M., Steiner, K.C. and Hebard, F.V. (2006) 'Recovery of American chestnut characteristics following hybridization and backcross breeding to restore blight-ravaged Castanea dentata,' Forest Ecology and Management, 223(1–3), pp. 439–447. https://doi.org/10.1016/j.foreco.2005.12.022.] </ref> | |||
==References== | |||
Latest revision as of 18:45, 15 April 2024
Classification
By Jinghao Li
Higher order taxa
Domain: Fungus, Kingdom: Fungus, Phylum: Ascomycetes, Class: Sordariomycete, Order: Diaporthales, Family: Cryphonectriaceae, Genus: Cryphonectria, Species: Cryphonectria parasitica [2]
Species
Cryphonectria parasitica [3]
|
NCBI: Taxonomy |
Description and significance
Cryphonectria parasitica is a deadly fungus originally found on American chestnut trees outside the chestnut's native range. Cryphonectria parasitica was first observed in the New York City Zoo in 1904 and became known as the "chestnut blight." Originally taxonomically known as Diaporthe parasitica, the fungus was later reclassified into the genus Endothia and finally named Cryphonectria parasitic. This foreign pathogen brings deadly disaster. Its emergence has resulted in the American chestnut becoming the dominant species in the forest canopy of North America, while in Europe, some special chestnut populations such as Castanea sativa Mill. have brought near-extinction hazards. This pattern of introduced fungal pathogens wreaking havoc on tree species has persisted, with ash dieback (caused by Hymenoscyphus fraxineus) in Europe being the most recent example (Gross et al. 2014). It is worth noting that chestnut wilt has attracted attention due to its hypovirulence phenomenon, in which viral infection weakens the virulence of the pathogen, providing a basis for biological control of the disease. In addition, conservation breeding efforts aim to restore the American chestnut to its status as an important forest species. [4]
Genome structure

Both Cryphonectria parasitica mycoreoviruses (CpMYRV-1 and CpMYRV-2) consist of 11 genome segments categorized under Group 1. Despite sharing this structural similarity, they exhibit only around 29% amino acid sequence identity in the capping enzyme, affirming their classification as distinct species. [6]
The complete genome of Cryphonectria parasitica mycoreovirus-1 (CpMRV-1) spans 23,436 base pairs (bp), with the length of individual segments varying between 732 bp and 4,127 bp. This results in a distinctive electrophoretic profile, exhibiting a pattern of 3, 3, 2, 3 segments when analyzed using either 11% polyacrylamide gel electrophoresis (PAGE) or 1% agarose gel electrophoresis (AGE). [7]
Cryphonectria parasitica strain EP155
Cryphonectria parasitica strain EP155, one of the strains causing damage to American chestnut trees, was originally isolated from chestnut canker. The genome sequencing and assembly process involved the use of Sanger sequencing protocols, resulting in a high-quality assembly of 26 scaffolds containing 33 contigs, spanning 43.9 Mb. The assembly was further refined using genetic linkage data, providing valuable insights into the organization of the genome at the chromosome level. Functional annotations of the genome revealed a total of 11,609 genes, with over 85% showing similarities to proteins from the NCBI nonredundant protein database. The genome also contains a significant number of genes involved in pathogenicity, secondary metabolite production, and cytochrome P450s. [8]
Host range
Cryphonectria parasitica primarily infects various species within the genus Castanea, including the American chestnut, European chestnut, Chinese chestnut (C. mollissima Blume), and Japanese chestnut (C. crenata Siebold & Zucc.), all of which belong to the family Fagaceae. [10]
Life Cycle
Cryphonectria parasitica is the causative agent of chestnut blight. They use damage to tree trunks or branches to enter trees and become parasitic. Furthermore, recent studies have shown that Cryphonectria parasitica can infect abandoned galls of chestnut gall wasps, providing new pathways for pathogens to invade host tissues. Both sexual and asexual spores of Cryphonectria parasitica can cause infection. When the spores germinate, ulcers form. Later, Cryphonectria parasitica may produce spores on infected bark and recently dead chestnut wood. The fruiting body, called a stroma, is composed of sexual (ascotheca) and asexual (pycnidia) structures and develops in numerous yellow-orange to reddish-brown pustules. These structures range from 0.5 to 4 mm in diameter and up to 2.5 mm in height and are embedded in the bark, with both types of fruiting bodies often coexisting closely. [11]
The complex life cycle and diverse reproductive structures of Cryphonectria parasitica facilitate its successful colonization and spread in chestnut populations. By exploiting a variety of entry points and employing sexual and asexual reproduction methods, Cryphonectria parasitica exhibits remarkable adaptability and resilience, posing an ongoing challenge to the management and conservation of chestnut trees. The emergence of Cryphonectria parasitica has had a great impact on different chestnut trees and even oak species in various regions, and even led to the extinction of chestnut trees in the United States for a certain period of time. It has had a very negative impact on agriculture and the economy. [12]
Disease Symptoms

Cryphonectria parasitica mainly infects the branches of trees, so Cryphonectria parasitica is classified as a bark pathogen. Symptom expression on susceptible hosts, including European and American chestnuts, varies depending on the virulence of the Cryphonectria parasitica strain and the age of the infected tree part. Virulent strains of Cryphonectria parasitica form necrotic lesions on the bark, known as cankers, that can rapidly kill smaller branches or twigs within months. But on thicker branches, it usually takes several years for the cankers to develop before death occurs. While red and orange colors form on young stems and branches, the color is less noticeable on thicker branches, making it more difficult to judge. [14]
Within the bark and cambium, the characteristic light brown fans of Cryphonectria parasitica mycelium will appear, a telltale sign of chestnut wilt infection. If the cambium is killed, the bark will sink inward, making the canker look sunken. Afterwards the tree dies and the leaves wilt and turn yellow or brown. [15]
Unlike cankers caused by virulent strains, cankers caused by less virulent strains are usually non-fatal. Stromata harboring the fruiting bodies of Cryphonectria parasitica may develop on the surface of cankers, while symptoms on oaks consist of slowly developing, calling using cankers that typically do not lead to stem or branch death. [16]
Epidemiology
New populations of Cryphonectria parasitica usually result from the introduction of one or more genotypes. It is likely that new Cryphonectria parasitica populations in North America were accidentally introduced with infected plant material. North American Cryphonectria parasitica has the fastest growth rate at 20°C, while European chestnut Cryphonectria parasitica has the fastest growth rate at 27°C and is significantly slower below 20°C. Adequate moisture provided by humid air, rain or dew is also critical for the survival, development and spread of Cryphonectria parasitica, for example, moist conditions trigger spore release. There is a seasonal pattern in European chestnut susceptibility to Cryphonectria parasitica, with a peak in spring and summer and a decrease in autumn and winter, possibly due to changes in bark nutritional value and moisture content. [17]
History
Cryphonectria parasitica was first discovered in East Asia, specifically China, Japan, and Korea. However, in the 20th century it was inadvertently introduced to North America and Europe via infected chestnut plants. After its discovery in 1904, it spread rapidly throughout the American chestnut's native distribution range at a rate of more than 30 kilometers per year, covering approximately 3.6 million hectares in eastern North America. This resulted in the endangered chestnut tree in North America and it has a great impact on the economy and environment. In Europe, the pathogen was officially detected in 1938 near Genoa, Italy's main international port. Genetic studies suggest that North America may have been the original source of introduction to Italy. By 1950, chestnut wilt had become widespread in Italy's main chestnut-growing areas and quickly spread to neighboring France. Between the 1920s and 1950s, Europe imported large quantities of chestnut trees from Asia and the United States, which may have facilitated the spread of C. parasitica. Ironically, the purpose of many of these imports was to obtain chestnut trees that were resistant to ink disease, the most serious chestnut disease before the arrival of chestnut wilt. [18]
Vegetative incompatibility
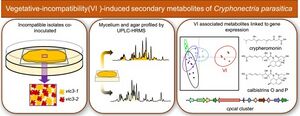
Vegetative incompatibility is a common phenomenon in fungi that serves as a defense mechanism against cytoplasm-borne diseases by hindering the formation of stable hyphal fusion and cytoplasmic exchange between individuals. In Cryphonectria parasitica, the vegetative incompatibility system is of particular interest because it limits the horizontal transmission of attenuated virulence mycoviruses. [20]
Disease management
In order to reduce the introduction and spread of Cryphonectria parasitica, the world has strictly managed the export and import of chestnuts and established relevant laws for management. In Europe, the EPPO continues to recommend that Cryphonectria parasitica be regulated as an A2 quarantine organism, which represents a pathogen that is indigenous to the EPPO region. Chestnut and oak plants intended for cultivation can only be transported within Europe and must be accompanied by a legal plant passport to prove that the plants come from a parasite-free area and have not been exposed to pathogens during transportation. The beginning of the last complete vegetation cycle. Despite these measures, however, quarantine regulations have not completely prevented the spread of the pathogen, particularly due to the challenges posed by asymptomatically infected plants evading visual inspection. [21]
And if Cryphonectria parasitica is discovered in a new area, people usually take the most direct methods such as fire and felling to eliminate the pathogen. However, these efforts have largely failed due to the difficulty of locating and eliminating all sources of inoculum, especially in wild forests. It can be eradicated in artificial breeding environments because chestnut trees that are not infected with C. parasitica can be isolated from chestnut trees that are infected with Cryphonectria parasitica. Also, plantations can reduce the chance of Cryphonectria parasitica infection by reducing surface wounds on chestnut trees. For the eradication of Cryphonectria parasitica, chemical means are not the preferred method. First, many countries often restrict or ban the use of chemicals in forests. Second, fungicides may exhibit phytotoxic effects or promote the development of resistance. Moreover, this is likely to turn some Cryphonectria parasitica into new drug-resistant strains that can resist the toxicity of fungicides. Furthermore, transposable elements (TEs) were found to constitute approximately 14% of the C. parasitica EP155 genome, with class I retrotransposons being the most abundant group. The presence of TEs, including intact coding sequences, suggests a dynamic genome with potential implications for genome evolution and adaptation. [22] [23]
Hypovirulence
Hypovirulence in Cryphonectria parasitica is a fascinating example of natural disease control facilitated by mycoviruses. Among these strains, especially Hypovirulence viruses such as CHV-1, CHV-2, CHV-3 and CHV-4 infect fungal hosts and induce hypo virulence phenotype, reducing its pathogenicity. Hypovirulence is not only low toxicity among fungi, but also lower toxicity of spores and reduced infectivity. [24] [25]
Interestingly, low viral transmission was limited to nutritionally incompatible Cryphonectria parasitica strains. In Europe, hypovirulence occurs naturally, such as the multiple genetic subtypes of CHV-1 found in Europe. However, in some other areas where chestnut blight is endemic, CHV-1 or other hypovirulence strains have not been found. Outside Europe, CHV-1 strains have also been found in Asia, but in North America, CHV-1 has not been found. However, parts of North America have been artificially released with CHV-1 strains for biological control. [26]
As mentioned before, other strains such as CHV-2 and CHV-3 also induce a hypovirulent phenotype in Cryphonectria parasitica, but CHV-4 strains do not cause obvious symptoms. [27]
How to use Hypovirulence to control chestnut blight
Introducing Hypovirulence, especially CHV-1, into areas where Cryphonectria parasitica is endemic can effectively control chestnut blight. This process is divided into five steps in total. First, we need to determine the vegetable compatibility (VC) type. Ideally, the strain causing chestnut blight and the Hypovirulence strain used should have the same or similar VC type. If different VC types are found, we can use a mixture of multiple different VC types of Hypovirulence. Second, an in vitro transmission assay is performed. Researchers can do this by spreading strains of Hypovirulence into virulent strains of the target VC type in the laboratory. This method ensures that Hypovirulence strains can grow normally in the wild. After that, the institute needs to carry out canker treatment. This method will be simpler. As we mentioned in the previous section, Cryphonectria parasitica is mainly infected from wounds on broken branches. So, the method is to inject the Hypovirulence strain into the cankers of infected chestnut trees. After the first three steps are completed, scientists still need to evaluate the trees for treatment. After at least one growth cycle, scientists can assess how successful the treatment was. In this process, not only can the extent of cankers after inoculation with the Hypovirulence strain be assessed, but Cryphonectria parasitica can also be re-isolated from the treated cankers to verify the presence of Hypovirulence. The final step is to determine the final treatment opportunity. During the growing season, approximately April to October, vaccination with the Hypovirulence strain during this time is most effective in treating canker. In this way, the artificial introduction of Hypovirulence can effectively weaken the chestnut wilt fungus, stop the spread of canker, and ultimately help control the disease in affected chestnut stands. [28]
Restoration efforts for American chestnut trees
Of paramount importance to plans for the revival of the American chestnut tree is the importance of seedling quality and the role it plays in successful establishment. Larger seedlings tend to have an advantage in growth and competitiveness, but smaller seedlings may be better able to withstand stress initially. However, the long-term growth and survival benefits of larger seedlings are significantly greater than those of smaller seedlings. In addition, many problems often occur during the transplantation process. For example, seedlings often suffer from planting shock due to damage to their root systems, or they are eaten by animals such as deer, causing damage to the trunk or roots. These factors may hinder the early growth of chestnut trees. To mitigate planting shock, it is crucial to minimize root damage and protect seedlings from nibblers during the transplanting process. Rapid recovery from planting stress is critical, which emphasizes the importance of selecting seedlings with traits conducive to rapid recovery. And some advanced breeding materials such as BC3F3 bring hope to the recovery of chestnut trees in the United States. These hybrid seedlings showed traits more similar to American chestnut than to Chinese chestnut (Castanea mollissima), a tree frequently used in blight resistance breeding programs in previous studies. [29]
Evolution

The evolutionary dynamics within the tri-trophic pathosystem of tree-fungus-hypovirus, particularly in introduced ranges of Cryphonectria parasitica, are complex and challenging to predict. Understanding the interactions and co-evolution between Hypovirulence and fungi has implications for biological control systems. Research shows that hypovirulence virulence varies widely even within local populations, which represents the possibility of evolution. Specific regions of the viral genome associated with these variants have been identified through genome comparison and mutation analysis. Compared with other strains, CHV-1 strains evolve significantly faster than other strains, which means that CHV-1 has great potential to become a strain for treating chestnut blight. [31]
Environmental factors such as temperature and humidity also play a role in virulence, suggesting an interaction between genotype and genotype and environment. Contrary to the hypothesis that fungal viruses would evolve toward lower virulence in populations with increasingly restrictive horizontal transmission barriers, studies have not shown such a trend. In fact, there's evidence of a positive association between hypovirus virulence and transmission across vegetative incompatibility barriers. The reason for this may be that virulent hypoviruses may interfere with the nutritional incompatibility response induced during hyphal fusion of incompatible fungal strains. Furthermore, ecological factors may contribute to the persistence of viral hypoviruses in C. parasitica populations. Unlike hyperviruses, hypoviruses can survive and reproduce asexually on dead chestnut wood, serving as a reservoir for the Hypovirulence inoculum. [32]
Uncoupling of saprophytic and parasitic potential in Cryphonectria parasitica species with low viral infection may contribute to the observed changes in low viral virulence. Despite these complexities, further investigation of the evolutionary dynamics of fungal-hypovirus interactions is critical for the effective understanding and management of chestnut blight. [33]
Resistance Breeding
Breeding efforts to resist chestnut blight rely primarily on genetic resistance discovered in Asian management. The representative species is the Chinese chestnut tree. In previous studies, it was found that the Chinese chestnut tree has a stronger ability to resist withering than the Japanese chestnut tree. Based on these findings, the American Chestnut Foundation launched a major breeding program aimed at restoring the American chestnut's status as a forest tree. By combining Cryphonectria parasitica-resistant species with native American chestnut trees over three successive generations, the laboratory obtained good results, showing that backcrossed chestnuts had higher survival rates and lower morbidity. but. Before conducting large-scale experiments, it is still necessary to determine whether the new chestnut trees generated from the hybrid are immune to other viruses and whether they can grow normally in different environments. In many European countries, new strains obtained by successfully crossing European chestnut trees and Asian chestnut trees have good resistance to chestnut blight. These hybrids also tend to be less susceptible to ink disease and chestnut gall wasp, making them increasingly popular for nut production. As another way to help plants resist viruses, genetic modification can also resist chestnut blight. By integrating wheat's oxalate oxidase gene into the chestnut genome, chestnut trees with enhanced resistance to blight disease were developed. This gene targets the degradation of oxalic acid, a toxin produced by Cryphonectria parasitica during the infection process. These diverse methods of fighting chestnut blight can effectively help control the development of chestnut blight in Europe and America in the future, restore chestnut tree populations in different regions, and improve local economies. [34]
References
- ↑ Patel, N. et al. (2013) 'Use of the tetrazolium salt MTT to measure cell viability effects of the bacterial antagonist Lysobacter enzymogenes on the filamentous fungus Cryphonectria parasitica,' Antonie Van Leeuwenhoek, 103(6), pp. 1271–1280. https://doi.org/10.1007/s10482-013-9907-3.
- ↑ Card, E.P.S. (2023) 'Cryphonectria parasitica,' ArcGIS StoryMaps, 10 October. https://storymaps.arcgis.com/stories/0554468188c946399998fe14854fd8e3.
- ↑ Taxonomy (no date) Taxonomy browser (Cryphonectria parasitica). https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=5116&lvl=3&lin=f&keep=1&srchmode=1&unlock.
- ↑ Hoegger, P.J. et al. (2002b) 'Cryphonectria radicalis: rediscovery of a lost fungus,' Mycologia, 94(1), pp. 105–115. https://doi.org/10.1080/15572536.2003.11833253.
- ↑ [https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2018.00103/full So, K.-K. et al. (2018) 'Global DNA Methylation in the Chestnut Blight Fungus Cryphonectria parasitica and Genome-Wide Changes in DNA Methylation Accompanied with Sectorization,' Frontiers in Plant Science, 9. https://doi.org/10.3389/fpls.2018.00103.[
- ↑ Suzuki, N. et al. (2004b) 'Complete genome sequence of Mycoreovirus-1/Cp9B21, a member of a novel genus within the family Reoviridae, isolated from the chestnut blight fungus Cryphonectria parasitica,' Journal of General Virology, 85(11), pp. 3437–3448. https://doi.org/10.1099/vir.0.80293-0.
- ↑ Crouch, J.A. et al. (2020) 'Genome Sequence of the Chestnut Blight Fungus Cryphonectria parasitica EP155: A Fundamental Resource for an Archetypical Invasive Plant Pathogen,' Phytopathology, 110(6), pp. 1180–1188. https://doi.org/10.1094/phyto-12-19-0478-a.
- ↑ Home - Cryphonectria parasitica EP155 v2.0 (no date). https://mycocosm.jgi.doe.gov/Crypa2/Crypa2.home.html.
- ↑ Jiang, N., Fan, X. and Tian, C. (2019) 'Identification and pathogenicity of Cryphonectriaceae species associated with chestnut canker in China,' Plant Pathology, 68(6), pp. 1132–1145. https://doi.org/10.1111/ppa.13033.
- ↑ Anagnostakis, S.L. (1992) 'Measuring resistance of chestnut trees to chestnut blight,' Canadian Journal of Forest Research, 22(4), pp. 568–571. https://doi.org/10.1139/x92-075.
- ↑ Prospero, S. et al. (2006) 'Saprophytic Activity and Sporulation of Cryphonectria parasitica on Dead Chestnut Wood in Forests with Naturally Established Hypovirulence,' Phytopathology, 96(12), pp. 1337–1344. https://doi.org/10.1094/phyto-96-1337.
- ↑ Milgroom, M.G. et al. (2009) 'Heterokaryons and parasexual recombinants of Cryphonectria parasitica in two clonal populations in southeastern Europe,' Fungal Genetics and Biology, 46(11), pp. 849–854. https://doi.org/10.1016/j.fgb.2009.07.007.
- ↑ chestnut blight or canker (Cryphonectria parasitica) (2006). https://www.invasive.org/browse/detail.cfm?imgnum=5050040.
- ↑ Sweet chestnut blight (Cryphonectria parasitica) - Forest Research (2024b). https://www.forestresearch.gov.uk/tools-and-resources/fthr/pest-and-disease-resources/sweet-chestnut-blight-cryphonectria-parasitica/.
- ↑ Chestnut Blight: an American Tragedy | Forest pathology (no date). https://forestpathology.org/canker/chestnut-blight/.
- ↑ Bryner, S.F. et al. (2013) 'Informative value of canker morphology on the presence or absence of virus infection in chestnut blight cankers,' Forest Pathology, 43(6), pp. 496–504. https://doi.org/10.1111/efp.12063.
- ↑ Gross, A. et al. (2013) 'Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback,' Molecular Plant Pathology, 15(1), pp. 5–21. https://doi.org/10.1111/mpp.12073.
- ↑ Dutech, C. et al. (2012) 'The chestnut blight fungus world tour: successive introduction events from diverse origins in an invasive plant fungal pathogen,' Molecular Ecology, 21(16), pp. 3931–3946. https://doi.org/10.1111/j.1365-294x.2012.05575.x.
- ↑ Witte, T.E. et al. (2021b) 'A metabolomic study of vegetative incompatibility in Cryphonectria parasitica,' Fungal Genetics and Biology (Print), 157, p. 103633. https://doi.org/10.1016/j.fgb.2021.103633.
- ↑ Witte, T.E. et al. (2021) 'A metabolomic study of vegetative incompatibility in Cryphonectria parasitica,' Fungal Genetics and Biology (Print), 157, p. 103633. https://doi.org/10.1016/j.fgb.2021.103633.
- ↑ EPPO (2005) EPPO Standards – Diagnostic protocols for regulated pests – PM7/45 Cryphonectria parasitica. EPPO Bull. 35, 295–298.
- ↑ Prospero, S. and Rigling, D. (2013) 'Chestnut blight.,' in CABI eBooks, pp. 318–339. https://doi.org/10.1079/9781780640402.0318.
- ↑ Trapiello, E., González-Varela, G. and González, A.J. (2015) 'Chestnut Blight Control by Agrochemicals in Castanea sativa under Managed Conditions,' Journal of Plant Diseases and Protection, 122(3), pp. 120–124. https://doi.org/10.1007/bf03356540.
- ↑ Hillman, B.I. and Suzuki, N. (2004) 'Viruses of the Chestnut Blight Fungus, Cryphonectria parasitica,' in Advances in virus research, pp. 423–472. https://doi.org/10.1016/s0065-3527(04)63007-7.
- ↑ 'VIRUS-INDUCED HYPOVIRULENCE IN CRYPHONECTRIA PARASITICA: STILL AN UNRESOLVED CONUNDRUM on JSTOR' (no date) www.jstor.org [Preprint. https://www.jstor.org/stable/41998375.]
- ↑ Prospero, S. et al. (2006b) 'Saprophytic Activity and Sporulation of Cryphonectria parasitica on Dead Chestnut Wood in Forests with Naturally Established Hypovirulence,' Phytopathology, 96(12), pp. 1337–1344. https://doi.org/10.1094/phyto-96-1337.
- ↑ Hillman, B.I. and Suzuki, N. (2004b) 'Viruses of the Chestnut Blight Fungus, Cryphonectria parasitica,' in Advances in virus research, pp. 423–472. https://doi.org/10.1016/s0065-3527(04)63007-7.
- ↑ Robin, C., Anziani, C. and Cortesi, P. (2000) 'Relationship Between Biological Control, Incidence of Hypovirulence, and Diversity of Vegetative Compatibility Types of Cryphonectria parasitica in France,' Phytopathology, 90(7), pp. 730–737. https://doi.org/10.1094/phyto.2000.90.7.730.
- ↑ Burhans, B. and Hebard, F.V. (2012) Restoring the American chestnut tree. https://www.fs.usda.gov/research/treesearch/42728.
- ↑ Double, M.L. and Marshall, M.R. (2014) 'STRAWBERRY AMENDMENT TO POTATO DEXTROSE AGAR TO INCREASE CONIDIATION IN CRYPHONECTRIA PARASITICA,' Acta Horticulturae, (1019), pp. 81–84. https://doi.org/10.17660/actahortic.2014.1019.12.
- ↑ Bryner, S.F., Rigling, D. and Brunner, P.C. (2012) 'Invasion history and demographic pattern of Cryphonectria hypovirus 1 across European populations of the chestnut blight fungus,' Ecology and Evolution, 2(12), pp. 3227–3241. https://doi.org/10.1002/ece3.429.
- ↑ Bryner, S.F. and Rigling, D. (2011) 'Temperature‐Dependent Genotype‐by‐Genotype Interaction between a Pathogenic Fungus and Its Hyperparasitic Virus,' the American Naturalist, 177(1), pp. 65–74. https://doi.org/10.1086/657620.
- ↑ Bryner, S.F. and Rigling, D. (2012) 'VIRULENCE NOT ONLY COSTS BUT ALSO BENEFITS THE TRANSMISSION OF a FUNGAL VIRUS,' Evolution, 66(8), pp. 2540–2550. https://doi.org/10.1111/j.1558-5646.2012.01637.x.
- ↑ Diskin, M., Steiner, K.C. and Hebard, F.V. (2006) 'Recovery of American chestnut characteristics following hybridization and backcross breeding to restore blight-ravaged Castanea dentata,' Forest Ecology and Management, 223(1–3), pp. 439–447. https://doi.org/10.1016/j.foreco.2005.12.022.
