Human Astrovirus: Difference between revisions
| (19 intermediate revisions by the same user not shown) | |||
| Line 28: | Line 28: | ||
==Description and Significance== | ==Description and Significance== | ||
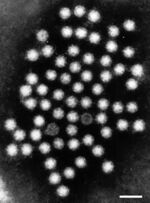
[[File:humanastro.jpg|thumb|right|150px|Electron Microscope Stain of Human Astrovirus at 50 nm stained using phosphotungstic acid. | [[File:humanastro.jpg|thumb|right|150px|Electron Microscope Stain of Human Astrovirus at 50 nm stained using phosphotungstic acid. From Bosch et al[1].]] | ||
Human Astrovirus (HAstV) is a species of the genera ''Mamastrovirus'', which are a group of enteric viruses known to be capable of causing mild symptomatic gastrointestinal infection. HAstV was first discovered as a causative agent for gastrointestinal infection among children under the age of 2. HAstV was first discovered in 1975, but the ''Astroviridae'' family was not established until 1995 [1]. In the early 2000s, three distinctive clades were discovered among the astrovirus species; VA1-VA3, VA2-VA4, and MLB [2]. | Human Astrovirus (HAstV) is a species of the genera ''Mamastrovirus'', which are a group of enteric viruses known to be capable of causing mild symptomatic gastrointestinal infection. HAstV was first discovered as a causative agent for gastrointestinal infection among children under the age of 2. HAstV was first discovered in 1975, but the ''Astroviridae'' family was not established until 1995 [1]. In the early 2000s, three distinctive clades were discovered among the astrovirus species; VA1-VA3, VA2-VA4, and MLB [2]. | ||
| Line 43: | Line 43: | ||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
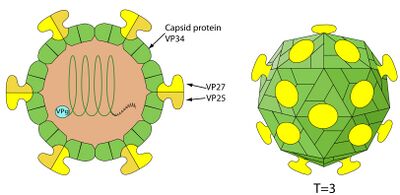
[[File:Astrostructure.jpg|thumb|left|400px|Structure of astrovirus with labeled protein components. From ViralZone [5].]] | |||
===Cell Structure=== | ===Cell Structure=== | ||
Human Astrovirus has a fairly simple structure, and one that is typical of other pathogenic viruses, such as SARS-CoV-2. It is a smooth, spherical, capsid-based virus that may have star-like projections [5]. The star-like projections are formed from a dimer of proteins contained within their genome. Only about 10% of replicated astroviruses have these star projections [1]; the other 90% have a more smooth, rounded surface. Astroviruses with spikes have a distinct pathogenic advantage in that they can use these spikes to attach to polysaccharides; upon attaching to the polysaccharide, receptor-mediated endocytosis allows the virus free passage into the cell [1]. The capsid is typically cleaved once outside the host cell to signify virus maturation. | Human Astrovirus has a fairly simple structure, and one that is typical of other pathogenic viruses, such as SARS-CoV-2. It is a smooth, spherical, capsid-based virus that may have star-like projections [5]. The star-like projections are formed from a dimer of proteins contained within their genome. Only about 10% of replicated astroviruses have these star projections [1]; the other 90% have a more smooth, rounded surface. Astroviruses with spikes have a distinct pathogenic advantage in that they can use these spikes to attach to polysaccharides; upon attaching to the polysaccharide, receptor-mediated endocytosis allows the virus free passage into the cell [1]. The capsid is typically cleaved once outside the host cell to signify virus maturation. | ||
===Metabolism=== | ===Metabolism=== | ||
Viruses have no cellular machinery and do not replicate on their own to survive. As such, they have no metabolism and do not uptake resources from their extraneous environment. Astroviruses instead make use of host cellular machinery, typically the rough endoplasmic reticulum, in order to replicate and continue to spread. | Viruses have no cellular machinery and do not replicate on their own to survive. As such, they have no metabolism and do not uptake resources from their extraneous environment. Astroviruses instead make use of host cellular machinery, typically ribosomes in the rough endoplasmic reticulum, in order to replicate and continue to spread. | ||
===Life Cycle=== | ===Life Cycle=== | ||
Human Astrovirus has a fairly typical life cycle for a virus. Upon entering a host cell through receptor-mediated endocytosis, the virus moves through the cytoplasm to the host cell's rough endoplasmic reticulum and uncoats its genetic material, using the cell's ribosomes to begin replicating its genome [1]. Non-structural proteins are created that allow the virus to replicate its genome quicker, and many copies of this genome are created. Following replication of the genome, assembly of the virus's genome into the capsid is performed, and the spikes are added to the capsid shell if still available. Immature virions, known as VP70 [1], are then released out of the cell, where a trypsin enzyme cleaves the capsid, signaling that the virus has fully matured. Notably, when immature virions exit the cell, there is no cell lysis. The reasoning behind the non-lytic release is unknown. | |||
==Ecology and Pathogenesis== | ==Ecology and Pathogenesis== | ||
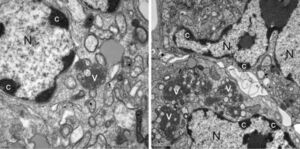
[[File:Astroinfection.jpg|thumb|right|300px|Astrovirus infection of human cells. Mass of condensed chromatin (label C) indicate a cell's nuclei has been infected by the virus. From Bosch et al[1].]] | |||
Human Astrovirus strains have been determined to be an opportunistic pathogen; although they can infect any human, they are most often symptomatic in infants, the elderly, and the immunocompromised [3]. Most healthy adults never prevent with symptoms even when infected, due to adaptive immunity. Astrovirus has been theorized to be an immune system "primer"; a mild infection in children allows the immune system to develop antibodies and strengthen itself without putting the host in major danger. Changes in pathology and the effects of astrovirus on human cells are not well documented. A single case involving a immunocompromised child was determined to have resulted in blunting of villi in the intestines, irregular epithelial cell lining, and inflammatory cells in the connective tissue of the mucosa of the intestines [1]. The virus has proven itself to be difficult to cleanse from most environments; as previously mentioned, the virus survives well in both warm and cold water and is not deactivated by traditional antiseptics, making it especially prevalent in hospitals. It has also been noted that HAstV is often a co-pathogen with other enteric pathogens, meaning it has no problem subsisting amongst other competitors. | |||
HAstV's pathogenesis may differ between the classical serotypes (HAstV1) and other mutated serotypes (VA1). Notably, non-lytic release resulted in increased membrane permeability in classical serotypes, but not in the mutated serotypes. Additionally, an outbreak in 2008 of the VA1 variant was associated with encephalitis and viremia (viruses in the bloodstream), which likely resulted in increased pathogenesis [4]. In classical serotypes, blunting of the villi of the intestines by HAstV leads to shedding of epithelial cells containing the virus, which then exit the host upon defecation. The virus then subsist in the feces, and if the feces is submerged in water, HAstV can spread and contaminate the water source. Non-lytic release of the cells allows the virus to remain untouched until the appropriate moment for pathogenesis occurs. | |||
==References== | ==References== | ||
[ | [1] [https://pubmed.ncbi.nlm.nih.gov/25278582/ Bosch, A., Pinto, R. M., & Guix, S. (2014). Human Astroviruses. ''American Society for Microbiology'', ''27''(4), 1048–1074.]<br> | ||
[2] [http://www.antimicrobe.org/v37.asp Bass, D. M., & Greenberg, H. B. (n.d.). ''Astroviruses''. AntiMicrobe.]<br> | |||
[3] [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7148626/ Moser, L., & Schultz-Cherry, S. (2008). Astroviruses. ''Encyclopedia of Virology'', ''3'', 204–210. https://doi.org/10.1016/b978-012374410-4.00348-4]<br> | |||
[4] [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7997325/ Hargest, V., Davis, A. E., Tan, S., Cortez, V., & Schultz-Cherry, S. (2021). Human Astroviruses: A Tale of Two Strains. ''Viruses'', ''13''(3), 376. https://doi.org/10.3390/v13030376]<br> | |||
[5] [https://viralzone.expasy.org/281.html?outline=all_by_species Swiss Institute of Bioinformatics. (n.d.). ''Mamastrovirus''. ViralZone.]<br> | |||
==Author== | ==Author== | ||
Latest revision as of 16:07, 23 April 2024
Classification
Higher Order Taxonomy
Virus
Realm: Riboviria
Kingdom: Orthornavirae
Phylum: Pisuviricota
Class: Stelpaviricetes
Order: Stellavirales
Family: Astroviridae
Genus: Mamastrovirus
Species
Human Astrovirus 1-8
Description and Significance
Human Astrovirus (HAstV) is a species of the genera Mamastrovirus, which are a group of enteric viruses known to be capable of causing mild symptomatic gastrointestinal infection. HAstV was first discovered as a causative agent for gastrointestinal infection among children under the age of 2. HAstV was first discovered in 1975, but the Astroviridae family was not established until 1995 [1]. In the early 2000s, three distinctive clades were discovered among the astrovirus species; VA1-VA3, VA2-VA4, and MLB [2].
Among other viruses, HAstV is notable for its resilience outside of a host environment. It has been determined that HAstV is resistant to inactivation by alcohols (including the common antiseptic ethanol), bleach, detergents, and to mild UV or heat treatments, along with being capable of staying alive in water for up to 90 days [3]. This makes HAstV commonplace in human environments, and as such HAstV has been found to responsible for roughly 4.0-8.6% of all diarrhea-related symptoms in young children [2]. Symptoms are relatively mild compared to other pathogens such as norovirus and rotavirus. Milder symptoms and the lack of prevalence in healthy young adults suggests that adaptive immunity is often developed in youth following a first encounter with HAstV [3].
Additionally of note are a few isolated cases associated with the HAstV serotype VA1. In 10 distinct cases, infection of the central nervous system has been noted, along with a lack of gastroenteric infection. Over half of the resulting cases were fatal [4]. It is unknown by what mechanisms these infections occur, although mink and ovine astrovirus infections have also resulted in CNS disease.
Genome Structure
Human Astrovirus has a single stranded, RNA-based genome, as is typical of many viruses. The genome is approximately 6.8kb long, and contains three main open reading frames (ORFs), known as ORF1a, ORF1b, and ORF2. ORF1a and ORF1b overlap one another by roughly 70 nucleotides, but the function of the five encoded peptides is relatively unknown. ORF2 is more well understood. ORF2 is roughly 2.4 kb long, and contains subgenomic RNA (sgRNA). The sgRNA allows for the encoding of multiple proteins over a single strand of RNA, and allows the genome to be more easily compressed. It is believed that ORF2 encodes for the capsid protein, and sgRNA allows for heavily increased expression of the capsid protein later in the replication cycle to accommodate for creation of new viruses [2].
The G+C content of the genome has not been analyzed, nor has it been sequenced and compared to other viruses. Since HAstV generates nucleotide mutations at a rate of 3.7 x 10^-3 substitutions per site per year [1] and astroviruses are prevalent on a global scale, it is difficult to develop a definitive genome when so many different serotypes exist.
Cell Structure, Metabolism and Life Cycle
Cell Structure
Human Astrovirus has a fairly simple structure, and one that is typical of other pathogenic viruses, such as SARS-CoV-2. It is a smooth, spherical, capsid-based virus that may have star-like projections [5]. The star-like projections are formed from a dimer of proteins contained within their genome. Only about 10% of replicated astroviruses have these star projections [1]; the other 90% have a more smooth, rounded surface. Astroviruses with spikes have a distinct pathogenic advantage in that they can use these spikes to attach to polysaccharides; upon attaching to the polysaccharide, receptor-mediated endocytosis allows the virus free passage into the cell [1]. The capsid is typically cleaved once outside the host cell to signify virus maturation.
Metabolism
Viruses have no cellular machinery and do not replicate on their own to survive. As such, they have no metabolism and do not uptake resources from their extraneous environment. Astroviruses instead make use of host cellular machinery, typically ribosomes in the rough endoplasmic reticulum, in order to replicate and continue to spread.
Life Cycle
Human Astrovirus has a fairly typical life cycle for a virus. Upon entering a host cell through receptor-mediated endocytosis, the virus moves through the cytoplasm to the host cell's rough endoplasmic reticulum and uncoats its genetic material, using the cell's ribosomes to begin replicating its genome [1]. Non-structural proteins are created that allow the virus to replicate its genome quicker, and many copies of this genome are created. Following replication of the genome, assembly of the virus's genome into the capsid is performed, and the spikes are added to the capsid shell if still available. Immature virions, known as VP70 [1], are then released out of the cell, where a trypsin enzyme cleaves the capsid, signaling that the virus has fully matured. Notably, when immature virions exit the cell, there is no cell lysis. The reasoning behind the non-lytic release is unknown.
Ecology and Pathogenesis
Human Astrovirus strains have been determined to be an opportunistic pathogen; although they can infect any human, they are most often symptomatic in infants, the elderly, and the immunocompromised [3]. Most healthy adults never prevent with symptoms even when infected, due to adaptive immunity. Astrovirus has been theorized to be an immune system "primer"; a mild infection in children allows the immune system to develop antibodies and strengthen itself without putting the host in major danger. Changes in pathology and the effects of astrovirus on human cells are not well documented. A single case involving a immunocompromised child was determined to have resulted in blunting of villi in the intestines, irregular epithelial cell lining, and inflammatory cells in the connective tissue of the mucosa of the intestines [1]. The virus has proven itself to be difficult to cleanse from most environments; as previously mentioned, the virus survives well in both warm and cold water and is not deactivated by traditional antiseptics, making it especially prevalent in hospitals. It has also been noted that HAstV is often a co-pathogen with other enteric pathogens, meaning it has no problem subsisting amongst other competitors.
HAstV's pathogenesis may differ between the classical serotypes (HAstV1) and other mutated serotypes (VA1). Notably, non-lytic release resulted in increased membrane permeability in classical serotypes, but not in the mutated serotypes. Additionally, an outbreak in 2008 of the VA1 variant was associated with encephalitis and viremia (viruses in the bloodstream), which likely resulted in increased pathogenesis [4]. In classical serotypes, blunting of the villi of the intestines by HAstV leads to shedding of epithelial cells containing the virus, which then exit the host upon defecation. The virus then subsist in the feces, and if the feces is submerged in water, HAstV can spread and contaminate the water source. Non-lytic release of the cells allows the virus to remain untouched until the appropriate moment for pathogenesis occurs.
References
[2] Bass, D. M., & Greenberg, H. B. (n.d.). Astroviruses. AntiMicrobe.
[5] Swiss Institute of Bioinformatics. (n.d.). Mamastrovirus. ViralZone.
Author
Page authored by Ethan Haggin and Kasia Jackowski, students of Prof. Jay Lennon at Indiana University.