Roseburia intestinalis: Difference between revisions
No edit summary |
No edit summary |
||
| (20 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
==Taxonomic Classification== | ==Taxonomic Classification== | ||
[[File:Scanning-electron-micrograph-of-Roseburia-intestinalis-sp-nov-L1-82-T-showing-a_(1).png|thumb|Scanning electron micrograph taken of ''Roseburia intestinalis'' [4]]] | |||
Domain: '''Bacteria''' | Domain: '''Bacteria''' | ||
| Line 15: | Line 17: | ||
===Species=== | ===Species=== | ||
{| | |||
| height="10" bgcolor="#E39FF6" | | |||
'''NCBI: ''R. intestinalis'' Taxonomy [https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=536231]''' | |||
|} | |||
| Line 20: | Line 26: | ||
Other species in '''Roseburia''' genus: | Other species in '''Roseburia''' genus: | ||
''Roseburia hominis; Roseburia inulinivorans; Roseburia faecis; Roseburia cecicola'' | ''Roseburia hominis; Roseburia inulinivorans; Roseburia faecis; Roseburia cecicola'' [7] | ||
==Description and Significance== | ==Description and Significance== | ||
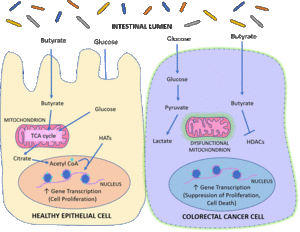
''Roseburia intestinalis'' is a major inhabitant of the human gut microbiome, making up 2.3% of the entire gut microbiome and up to 20% of the bacteria found in the colorectal region [ | ''Roseburia intestinalis'' is a major inhabitant of the human gut microbiome, making up 2.3% of the entire gut microbiome and up to 20% of the bacteria found in the colorectal region [11]. ''R. intestinalis'' is a butyrate producer, a short-chain fatty acid that provides an energy source for colon epithelial cells to break down dietary fiber. The main reason for it’s ability to produce butyrate is from the enzyme Butyryl-CoA:acetate CoA transferase, which can transform acetate into butyrate. Butyrate is also known to suppress colon cancer, as it induces histone acetylation on the epithelial cells [1]. Butyrate in healthy colon cells will feed them through beta-oxidation, which is what most healthy epithelial cells prefer. It acts as a natural histone deacetylase enzyme inhibitor, which are enzymes known to lead to oncogene expression and is a factor that leads to colon cancer. As ''R. intestinalis'' acts as a natural inhibitor to these HDACs, cancer therapies involving ''R. intestinalis'' is on the rise in research. Lack of butyrate production has been associated with diseases such as inflammatory bowel disease and Type 2 diabetes [11]. Live Biotherapeutics Drug Discovery has announced ''R. intestinalis'' and the rest of its genus as a Next Generation Probiotic for people with digestive issues to restore their gut health, as butyrate production is a big reason inflammation can be suppressed in the gut [12]. | ||
==Genome Structure== | ==Genome Structure== | ||
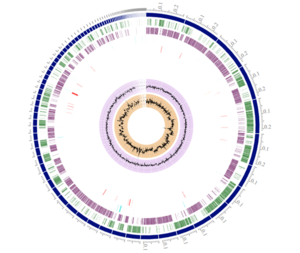
[[File:Rosie Genome.png|thumb|right|Complete genome of the ''R. intestinalis'' strain L1-82 [5]]] | |||
Genome size: 4.5 Mbp | Genome size: 4.5 Mbp | ||
One circular chromosome | One circular chromosome | ||
G+C Content is known to be 42.6% [ | 4,193 genes including: | ||
* 34 antibiotic resistance genes | |||
* 1 virulence factor gene | |||
::- gene known to be obtained by the organism ''Streptococcus pneumoniae'' but not sure when the encounter was nor how it affects the bacterium [5,6] | |||
* 175 essential genes | |||
G+C Content is known to be 42.6% [10] | |||
Two known prophages are found in the commonly researched ''R. intestinalis'' strain L1-82, Jekyll and Shimadzu that help the bacteria gain host-phage resistance via horizontal gene transfer [2]. | Two known prophages are found in the commonly researched ''R. intestinalis'' strain L1-82, Jekyll and Shimadzu that help the bacteria gain host-phage resistance via horizontal gene transfer [2]. | ||
| Line 37: | Line 53: | ||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
[[File:Diagram Colon.jpg|thumb|left| Diagram of the colon [3]]] | |||
* Gram-positive | |||
* Non-spore forming | |||
* Curved rod shape | |||
* Anaerobe | |||
* Mesophilic | |||
* Favors pH of 6.5-7.5 | |||
* Motile; has a flagella | |||
''Roseburia intestinalis'''s flagella acts as its form of motion to get through the colon mucus layer where most of the butyrate is found and able to interact with the epithelial cells. ''R. intestinalis'' also has the ability to ferment arabinose, cellobiose, fructose, maltose, and melibiose as it is a saccharolytic organism [11]. Because the favored environment of ''R. intestinalis'' is the anaerobic colon and is saccharolytic, butyrate most likely comes from the fermentation of these sugars which then undergoes beta-oxidation to make energy for the healthy epithelial cells. | |||
==Ecology and Pathogenesis== | ==Ecology and Pathogenesis== | ||
The discovery of ''R. intestinalis'' came from Sylvia Duncan's team sampling of human infant feces along with the rest of its genus to study the symbiosis of butyrate producing bacteria and the human gut [4]. The human gut, specifically the colon, has created a space for ''R. intestinalis'' to have a symbiotic relationship producing butyrate to feed the human colon cells and getting nutrients from them as well. It is also important in breaking down the plant cell wall with its ability in fermenting beta-mannan and xylan sugars [11]. | |||
As this is a known beneficial bacteria for humans, the only pathogenic trait is when there is not enough bacterial cells found in the gut microbiome. Low ''R. intestinalis'' rates lead to low butyrate production and increases the chances of colorectal cancer, type 2 diabetes, and inflammatory bowel disease [11]. | |||
This pathogenic trait is able to be controlled by the possibility of being a probiotic for the public to use and treat patients with IBD and type 2 diabetes as the butyrate production is known to have insulin resistance and anti-inflammatory properties [1]. | |||
The possibility of R. intestinalis becoming a cancer therapy is becoming a reality, as butyrate is a natural inhibitor of histone deacetylase enzymes to decrease oncogene expression and essentially starve the colorectal cancer cells [8]. | |||
[[File:Butyrate in the Colon.gif|thumb|center| Example of butyrate being an HDAC inhibitor in a colorectal cancer cell [9]]] | |||
==References== | ==References== | ||
| Line 49: | Line 83: | ||
[2] [https://www.nature.com/articles/s41396-019-0566-x Cornuault JK, Moncaut E, Loux V, Mathieu A, Sokol H, Petit MA, De Paepe M. The enemy from within: a prophage of Roseburia intestinalis systematically turns lytic in the mouse gut, driving bacterial adaptation by CRISPR spacer acquisition. ISME J. 2020 Mar;14(3):771-787.] | [2] [https://www.nature.com/articles/s41396-019-0566-x Cornuault JK, Moncaut E, Loux V, Mathieu A, Sokol H, Petit MA, De Paepe M. The enemy from within: a prophage of Roseburia intestinalis systematically turns lytic in the mouse gut, driving bacterial adaptation by CRISPR spacer acquisition. ISME J. 2020 Mar;14(3):771-787.] | ||
[3] [https://www.microbiologyresearch.org/content/journal/ijsem/10.1099/00207713-52-5-1615 Duncan, S. H., Hold, G. L., Barcenilla, A., Stewart, C. S., & Flint, H. J. (2002, September 1). Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human faeces. microbiologyresearch.org.] | [3] [https://www.dreamstime.com/stock-illustration-large-intestine-human-anatomy-isolated-white-background-medical-illustration-labeled-diagram-image47483240 Diagram of the colon photo] | ||
[4] [https://www.microbiologyresearch.org/content/journal/ijsem/10.1099/00207713-52-5-1615 Duncan, S. H., Hold, G. L., Barcenilla, A., Stewart, C. S., & Flint, H. J. (2002, September 1). Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human faeces. microbiologyresearch.org.] | |||
[5] [https://www.bv-brc.org/view/Genome/536231.5#view_tab=overview Genome information about ''Roseburia intestinalis''] | |||
[6] [https://pubmed.ncbi.nlm.nih.gov/12207705/ Hava D., Camilli A. Large-scale identification of serotype 4 ''Streptococcus pneumoniae'' virulence factors. Mol Microbiol. 2002 Sep;45(5):1389-406. PMID: 12207705; PMCID: PMC2788772.] | |||
[7] [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7478625/ Hillman, E. T., Kozik, A. J., Hooker, C. A., Burnett, J. L., Heo, Y., Kiesel, V. A., Nevins, C. J., Oshiro, J. M. K. I., Robins, M. M., Thakkar, R. D., Wu, S. T., & Lindemann, S. R. (2020, July). Comparative genomics of the genus roseburia reveals divergent biosynthetic pathways that may influence colonic competition among species. Microbial genomics.] | |||
[8] [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8620738/ King J, Patel M, Chandrasekaran S. Metabolism, HDACs, and HDAC Inhibitors: A Systems Biology Perspective. Metabolites. 2021 Nov 20;11(11):792. doi: 10.3390/metabo11110792. PMID: 34822450; PMCID: PMC8620738.] | |||
[9] [https://www.lucymailing.com/scfas-part-2-the-benefits-of-butyrate/ Mailing, L. The Benefits of Butyrate. (2017)] | |||
[ | [10] [https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_900537995.1/ NCBI genome for ''Roseburia intestinalis''] | ||
[ | [11] [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8647967/ Nie, K., Ma, K., Luo, W., Shen, Z., Yang, Z., Xiao, M., Tong, T., Yang, Y., & Wang, X. (2021, November 22). Roseburia intestinalis: A beneficial gut organism from the discoveries in genus and species. Frontiers in cellular and infection microbiology.] | ||
[ | [12] [https://live-biotherapeutic.creative-biolabs.com/roseburia-spp.htm Roseburia spp. as next generation probiotics. Live Biotherapeutic. (n.d.).] | ||
==Author== | ==Author== | ||
Latest revision as of 02:25, 25 April 2024
Taxonomic Classification
Domain: Bacteria
Phylum: Bacillota
Class: Clostridia
Order: Lachnospirales
Family: Lachnospiraceae
Genus: Roseburia
Species
|
NCBI: R. intestinalis Taxonomy [1] |
Roseburia intestinalis
Other species in Roseburia genus: Roseburia hominis; Roseburia inulinivorans; Roseburia faecis; Roseburia cecicola [7]
Description and Significance
Roseburia intestinalis is a major inhabitant of the human gut microbiome, making up 2.3% of the entire gut microbiome and up to 20% of the bacteria found in the colorectal region [11]. R. intestinalis is a butyrate producer, a short-chain fatty acid that provides an energy source for colon epithelial cells to break down dietary fiber. The main reason for it’s ability to produce butyrate is from the enzyme Butyryl-CoA:acetate CoA transferase, which can transform acetate into butyrate. Butyrate is also known to suppress colon cancer, as it induces histone acetylation on the epithelial cells [1]. Butyrate in healthy colon cells will feed them through beta-oxidation, which is what most healthy epithelial cells prefer. It acts as a natural histone deacetylase enzyme inhibitor, which are enzymes known to lead to oncogene expression and is a factor that leads to colon cancer. As R. intestinalis acts as a natural inhibitor to these HDACs, cancer therapies involving R. intestinalis is on the rise in research. Lack of butyrate production has been associated with diseases such as inflammatory bowel disease and Type 2 diabetes [11]. Live Biotherapeutics Drug Discovery has announced R. intestinalis and the rest of its genus as a Next Generation Probiotic for people with digestive issues to restore their gut health, as butyrate production is a big reason inflammation can be suppressed in the gut [12].
Genome Structure
Genome size: 4.5 Mbp
One circular chromosome
4,193 genes including:
- 34 antibiotic resistance genes
- 1 virulence factor gene
- - gene known to be obtained by the organism Streptococcus pneumoniae but not sure when the encounter was nor how it affects the bacterium [5,6]
- 175 essential genes
G+C Content is known to be 42.6% [10]
Two known prophages are found in the commonly researched R. intestinalis strain L1-82, Jekyll and Shimadzu that help the bacteria gain host-phage resistance via horizontal gene transfer [2].
Cell Structure, Metabolism and Life Cycle
- Gram-positive
- Non-spore forming
- Curved rod shape
- Anaerobe
- Mesophilic
- Favors pH of 6.5-7.5
- Motile; has a flagella
Roseburia intestinalis's flagella acts as its form of motion to get through the colon mucus layer where most of the butyrate is found and able to interact with the epithelial cells. R. intestinalis also has the ability to ferment arabinose, cellobiose, fructose, maltose, and melibiose as it is a saccharolytic organism [11]. Because the favored environment of R. intestinalis is the anaerobic colon and is saccharolytic, butyrate most likely comes from the fermentation of these sugars which then undergoes beta-oxidation to make energy for the healthy epithelial cells.
Ecology and Pathogenesis
The discovery of R. intestinalis came from Sylvia Duncan's team sampling of human infant feces along with the rest of its genus to study the symbiosis of butyrate producing bacteria and the human gut [4]. The human gut, specifically the colon, has created a space for R. intestinalis to have a symbiotic relationship producing butyrate to feed the human colon cells and getting nutrients from them as well. It is also important in breaking down the plant cell wall with its ability in fermenting beta-mannan and xylan sugars [11].
As this is a known beneficial bacteria for humans, the only pathogenic trait is when there is not enough bacterial cells found in the gut microbiome. Low R. intestinalis rates lead to low butyrate production and increases the chances of colorectal cancer, type 2 diabetes, and inflammatory bowel disease [11].
This pathogenic trait is able to be controlled by the possibility of being a probiotic for the public to use and treat patients with IBD and type 2 diabetes as the butyrate production is known to have insulin resistance and anti-inflammatory properties [1].
The possibility of R. intestinalis becoming a cancer therapy is becoming a reality, as butyrate is a natural inhibitor of histone deacetylase enzymes to decrease oncogene expression and essentially starve the colorectal cancer cells [8].
References
[3] Diagram of the colon photo
[5] Genome information about Roseburia intestinalis
[9] Mailing, L. The Benefits of Butyrate. (2017)
[10] NCBI genome for Roseburia intestinalis
[12] Roseburia spp. as next generation probiotics. Live Biotherapeutic. (n.d.).
Author
Page authored by Brianna Ritchey and Fernando Santos, students of Prof. Jay Lennon at Indiana University.