Ralstonia mannitolilytica: Difference between revisions
No edit summary |
|||
| (65 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
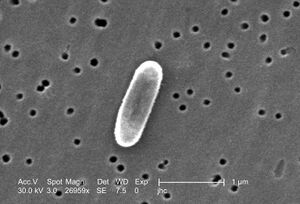
[[Image: | [[File:Enlarged view of Ralstonia mannitolilytica.jpeg|thumb|300px|right|Enlarged view of <i>Ralstonia mannitolilytica</i> bacterium. Image credit: Noble-Wang, J.(2005)]] | ||
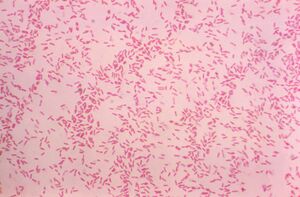
[[File:Gram-stained Ralstonia mannitolilytica.jpeg|thumb|300px|right|Photomicrograph of Gram-stained <i>Ralstonia mannitolilytica</i>. Image credit: Public Health Image Library (1977).]] | |||
| Line 8: | Line 9: | ||
Bacteria; Proteobacteria; Pseudomonadati; Pseudomonadota; Betaproteobacteria; Burkholderiales; Burkholderiaceae; Ralstonia. | Bacteria; Proteobacteria; Pseudomonadati; Pseudomonadota; Betaproteobacteria; Burkholderiales; Burkholderiaceae; Ralstonia; Ralstonia mannitolilytica. | ||
===Species=== | ===Species=== | ||
| Line 26: | Line 27: | ||
==Description and Significance== | ==Description and Significance== | ||
''Ralstonia mannitolilytica'' is a rod-shaped, Gram-negative bacterium found in soil and water. It can survive in many different environments thanks to its ability to use a variety of nutrients and metabolic processes [1]. This makes it useful for environmental cleanup, like breaking down pollutants such as oil spills and other bioremediation. It does so through the lipase it creates, which excels at breaking down the hydrocarbons in oil [2]. However, it is also considered an opportunistic pathogen, as it’s known to cause infections in people with weakened immune systems- specifically in hospitals. Its ability to stick to medical devices by forming biofilms and its natural resistance to many antibiotics and other disinfection practices make it difficult to manage in clinical settings. Infections from this are specifically found to be due to long term intravenous medical supplies like catheters [1]. Studying this bacterium helps us better understand how to manage its harmful effects while exploring its potential environmental benefits. | |||
==Genome Structure== | ==Genome Structure== | ||
The genome size of reference ''R. mannitolilytica'' strain SN82F48 is 5,072,301 bp. It has 2 circular chromosomes and a plasmid. Chromosome 1 is 3,503,899 bp, chromosome 2 is 1,377,860 bp, and plasmid pRMAN01 is 190,542 bp [3]. Carbapenem-resistant strain JARB-RN-0044 has one circular chromosome that is 3.5 Mb and a large replicon plasmid that is 1.5 Mb [4]. Another carbapenem-resistant strain, MRY14-0246, has a total genome size of 4,671,011 bp [5]. | |||
The GC percentage of all three strains are similar: SN82F48 has 65.5% GC, JARB-RN-0044 has 66.1% GC, and MRY14-0246 has 65.8%. Strain SN82F48 has | The GC percentage of all three strains are similar: SN82F48 has 65.5% GC, JARB-RN-0044 has 66.1% GC, and MRY14-0246 has 65.8% GC. Strain SN82F48 has 4,472 protein genes and 58 RNA genes, strain JARB-RN-0044 has 3,336 protein-coding genes on the chromosome and 1,390 on the replicon, and strain MRY14-0246 has 4,357 coding sequences [4, 5, 6]. Genome sequencing of JARB-RN-0044 reveals that genes involved in replication, recombination, repair, and metabolism are mostly found on the large chromosome, while genes involved in transcription and cell motility are mostly found on the replicon plasmid [4]. | ||
Novel class D β-lactamase blaOXA-1176 and blaOXA-1177 genes were found in the JARB-RN-0044 strain, while MRY14-0246 was found to have the novel class D β-lactamase genes OXA-443 and OXA-444. These D β-lactamase genes could be a contributing factor to carbapenem class antibiotic resistance in R. mannitolilytica, which makes infections difficult to treat | Novel class D β-lactamase blaOXA-1176 and blaOXA-1177 genes were found in the JARB-RN-0044 strain, while MRY14-0246 was found to have the novel class D β-lactamase genes OXA-443 and OXA-444. These D β-lactamase genes could be a contributing factor to carbapenem class antibiotic resistance in R. mannitolilytica, which makes infections difficult to treat [4,5]. Furthermore, MRY14-0246 does not have T3SS (Type III Secretion System) gene cluster, but does have the T6SS (Type VI Secretion System). In the plant pathogen ''Ralstonia solanacearum'', T6SS is known to deliver effectors to other organisms that cause cell death, which suggests that T6SS could be a factor in determining the virulence of a ''Ralstonia'' species [5]. | ||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
''Ralstonia mannitolilytica'' is a non-motile, rod-shaped bacilli bacteria with a | ''Ralstonia mannitolilytica'' is a non-motile, rod-shaped bacilli bacteria with a thin peptidoglycan layer, as it is gram-negative, and an outer membrane containing lipopolysaccharides [7]. The bacteria has pili present, which may be a contributing factor to its ability to form biofilms [8]. | ||
''Ralstonia mannitolilytica'' is a metabolically diverse bacteria | ''Ralstonia mannitolilytica'' is a metabolically diverse bacteria and gains energy through catabolic processes. It oxidizes ammonium to nitrite and can also breakdown carbohydrates like mannitol to gain energy [9]. Because ''Ralstonia mannitolilytica'' metabolizes mannitol, it produces polysaccharides [10]. | ||
It is aerobic, but can survive through fermentative processes when oxygen is low or absent [9]. | |||
''Ralstonia mannitolilytica'' originates from soil, water, and plant root settings [9]. Its diverse metabolism makes it hardy and able to survive in different environments. While great for its survival, in hospital settings, however, this makes it difficult to get rid of. | |||
This bacteria is an opportunistic pathogen, and can be particularly harmful to those who are immunocompromised [11]. Its ability to form biofilms, as well as documented cases of antibiotic resistance, and its relatively unknown existence, makes it difficult to diagnose and treat [9]. It commonly enters hospitals through contaminated water or equiptment, such as catheters [12]. In addition, ''Ralstonia mannitolilytica'' can form capsules, which adds to their ability to resist treatments, commonly those in the form of antibiotics [13]. In immunocompromised patients, it has been known to cause bacteremia, which are infections of the bloodstream, pneumonia, fever, osteomyelitis, and even death [14, 15]. | |||
==Ecology and Pathogenesis== | ==Ecology and Pathogenesis== | ||
''Ralstonia mannitolilytica'' is commonly found in aquatic environments, soil, and plants. It has been isolated from river water, sewage, and even medical devices that have been contaminated with the bacterium [12]. It is a facultative anaerobe that can grow with or without oxygen and has a diverse metabolism [16]. | |||
In the environment, ''Ralstonia mannitolilytica'' contributes to the breakdown of organic matter [17]. Through its ability to metabolize various organic substrates, such as mannitol, the bacterium can help recycle nutrients in the aquatic and soil environments it is commonly found in [17]. | |||
When it does get into hospital settings, an environment different from its usual, it can persist through the formation of capsules and biofilms[12]. This makes the bacteria particularly hard to get rid of, and its biofilm can protect it from antibiotics. Although the bacteria is resistant to many antibiotics, its infection can still be treated. Antibiotics including ceftazidime, ciprofloxacin, metronidazole work best while colistin shows the most resistance [21]. Targeting proteins may disrupt R. mannitolilytica's pathogenicity and virulence when antibiotics fail. LpxC is crucial for lipopolysaccharide biosynthesis, FepA is involved in iron uptake, and ExsA controls virulence factor expression [21]. | |||
Various reports from around the globe have shown that this bacterium can serve as an opportunistic pathogen. It has been documented to have caused bacteremia, which are bloodstream infections, endocarditis, pulmonary cavitation, and more [14, 15]. These opportunistic pathogens are typically found in immunocompromised patients creating both localized and systemic infections. Common patient symptoms include a high-grade fever, abdominal pain, and tachycardia [20]. A report found that the bacteria infects cerebral spinal fluid and the central nervous system, causing meningitis[20]. | |||
The virulence factors that make ''Ralstonnia mannitolilytica'' so dangerous in humans include its ability to form biofilms, which protect it from antibiotics and the host's immune system, its ability to form capsules, which further allows it to evade immune cells, and its ability to produce endotoxins, which can cause sepsis [18]. Additionally, lipopolysaccharides (LPS), extracellular enzymes, and siderophores are virulence factors [19]. These virulence factors contribute to the bacterium's ability to incite immune responses, invade and destroy host tissues using protease and lipase, and acquire essential nutrients like iron using hemolysin [19]. | |||
Prevention of infection by Ralstonnia mannitolilytica can be taken by updating policies and regulations that pertain to the microbiological safety of aqueous solutions and devices used in clinical practice. It is also essential that sufficient sterilization of equipment is maintained and microbiological techniques are enhanced to attain an accurate diagnosis of the underlying organism. | |||
==References== | ==References== | ||
[1] Ryan MP, Adley CC. 2014. ''Ralstonia'' spp." Emerging global opportunistic pathogens. ''Eur J Clin Microbiol Infect Dis.'' 33(3):291-304. SpringerLink. | |||
[2] Abdulkareem RS, Al-Ezee AMM, Musafer Hk. 2024. New bioremediation by lipase purified from ''Ralstonia mannitolilytica'' for petroleum hydrocarbons. ''Samarra J Pure Apple Sci.'' 6(1):148-164. https://doi.org/10.54153/sjpas.2024.v6i1.656. | |||
[3] National Center for Biotechnology Information. 2015. ''Ralstonia mannitolilytica'' genome assemby ASM95413v2. https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_000954135.1/. | |||
[4] Hayashi W, Kaiju H, Kayama S, Yu L, Zuo H, Sugawara Y, Azuma K, Takahashi A, Hata Y, Sugai M. Complete sequence of carbapenem-resistant ''Ralstonia mannitolilytica'' clinical isolate co-producing novel class D β-lacatmase OXA-1176 and OXA-1177 in Japan. ''Am Soc Microbiol.'' 12(4):e03919-23. | |||
[5] Suzuki M, Nishio H, Asagoe K, Kida K, Suzuki S, Matsui M, Shibayama K. 2015. Genome sequence of a carbapenem-resistant strain of ''Ralstonia mannitolilytica.'' ''Am Soc Microbiol.'' 3(3):e00405-15. | |||
[6] Kyoto Encyclopedia of Genes and Genomes. 2015. ''Ralstonia mannitolilytica.'' https:www.genome.jp./kegg-bin/show_organism?org=rmn. | |||
[7] Boattini M, Bianco G, Biancone L, Cavallo R, Costa C. 2018. ''Ralstonia mannitolilytica'' bacteraemia: a case report and literature review. ''Le Infezioni in Medicina.'' 26(4):374-378. | |||
[8] Basso M, Venditti C, Raponi G, Navazio AS, Alessandri F, Giombini E, Nisii C, Di Caro A, Venditti M. 2019. A case of persistant bacteraemia by ''Ralstonia mannitolilytica'' and ''Ralstonia pickettii'' in an intensive care unit. ''Infect Drug Resist'' 12:2391-2395. https://doi.org/10.2147/IDR.S206492. | |||
[9] Siddiqui T, Patel SS, Sinha R, Ghoshal U, Sahu C. 2022. ''Ralstonia mannitolilytica'': an emerging multidrug-resistant oppportunistic pathogen in tertiary care hospital setting. ''Access Microbiol'' 4(5):acmi000367. Published 2022 May 31. https://doi.org/10.1099/acmi.0.000367. | |||
[10] Papagrigorakis E, Vavourakis M, Vlachos C, Zachariou D, Galanis A, Marougklianis V, Polyzois V, Pneumaticos S. 2022. Osteomyelitis caused by ''Ralstonia mannitolilytica'', a rate opportunistic pathogen. ''Cureus'' 14(4):e24151. https://doi.org/10.7759/cureus.24151. PMID: 35586345; PMCID: PMC9109608. | |||
[11] Carreira M, Gomes C, Silva M, Duro R. 2020. ''Ralstonia mannitolilytica'' endocarditis: A case report. ''IDCases'' 22:e01003. https://doi.org/10.1016/j.idcr.2020.e01003. | |||
[12] Lim CTS, Lee SE. 2017. A rare case of ''Ralstonia mannitolilytica'': an emerging multidrug-resistant opportunistic pathogen in a tertiary care hospital setting. ''Access Microbiol'' 4(5):acmi000367. https://doi.org/10.1099/acmi.0.000367. PMID: 16003352; PMCID: PMC9394538 | |||
[13] Xie P, Liu J, Wu L, Liu D. 2021. Pulmonary cavitation: A hint of ''Ralstonia mannitolilytica'' infectionn through imaging. ''Am J Respir Crit Care Med'' 203(12):e947-e949. htpps://doi/orgg/10.1164/rccm.202009-3616IM. PubMed: 33535022. | |||
[14] Kim G, Yoo RN, So H, Lee JY, Kim MN, Kim SH, Jhang WK, Park SJ, Lee J. 2023. Clinical manifestation of ''Ralstonia mannitolilytica'' infection in pediatric patients and epidemiological investigation of outbreaks. ''J Korean Med Sci'' 38(33):e252. https://doi.org/10.3346/jkms.2023.38.e252. | |||
[15] Said M, van Hougenhouck-Tulleken W, Naidoo R, Mbelle N, Ismail F. Outbreak of ''Ralstonia mannitolilytica'' bacteraemia in patients undergoing haemodialysis at a tertiary hospital in Pretoria, South Africa. ''Antimicrob Resist Infect Control'' 9:117. https://doi.org/10.1186/s13756-020-00772-6. | |||
[16] Jeonbuk National University Medical School, Microbiological Laboratory. 2017. ''Ralstonia mannitolilytica'' GMB0650. Available from: https://www.gmbank.org/product-page/gmb0650. | |||
[17] Wang Z, Luo W, Cheng S, Zhang H, Zong J, Zhang Z. 2023. ''Ralstonia solanacearum''- A soil borne hidden enemy of plants: Research development in management strategies, their action mechanism and challenges. ''Front Plant Sci'' 14:1141902. https://doi.org/10.3389/fpls.2023.1141902. | |||
[18] Liu CX, Yan C, Zhang P, Li FQ, Yang JH, Li XY. 2016. ''Ralstonia mannitolilytica''-induced septicemia and homology analysis in infected patients: 3 case reports. ''Jundishapur J Microbiol'' 9(7):e34373. https://doi.org/10.5812/jjm.34373. PMID: 27679705: PMCID: PMC5035395 | |||
[19] Al-Abdullah, N., & Amir, Y. N. 2023. Inauguration the enigma: Ralstonia mannitolilytica septicemia – clinical journey, multidimensional investigation, and paradigm- shifting research insights. Biosciences Biotechnology Research Asia. 20(3), 865-873. doi:https://doi.org/10.13005/bbra/3138 | |||
[20] Aldhafeeri, W. F., Habalrih, F., Al Omar, A. H., Altamimi, A. A., Alshahrani, M. S., Abdullah, J., Alrabie, A., & Shah, S. 2022. Postoperative cerebrospinal fluid infection by Ralstonia mannitolilytica: Two case reports and a literature review. Surgical neurology international. 13(602). https://doi.org/10.25259/SNI_ | |||
[21] Alshuhri, S., Alosaimi, A., Alnafee, K., Alkahtany,J., Alhamami, S., et al. 2024. Ralstonia mannitolilytica infection in a tertiary care center: An outbreak report. American Journal of Infection Control. 0196-6553. https://doi.org/10.1016/j.ajic.2024.09.019. | |||
==Author== | ==Author== | ||
Page authored by Clarice Fahrenkrug, Elinor Morkos, Halie Grenier | Page authored by Clarice Fahrenkrug, Elinor Morkos, Randie Dillon, & Halie Grenier, students of Prof. Bradley Tolar at UNC Wilmington. | ||
<!-- Do not remove this line-->[[Category:Pages edited by students of Bradley Tolar at UNC Wilmington]] | <!-- Do not remove this line-->[[Category:Pages edited by students of Bradley Tolar at UNC Wilmington]] | ||
Latest revision as of 02:51, 11 December 2024
Classification
Bacteria; Proteobacteria; Pseudomonadati; Pseudomonadota; Betaproteobacteria; Burkholderiales; Burkholderiaceae; Ralstonia; Ralstonia mannitolilytica.
Species
|
NCBI: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=105219 |
Ralstonia mannitolilytica
Description and Significance
Ralstonia mannitolilytica is a rod-shaped, Gram-negative bacterium found in soil and water. It can survive in many different environments thanks to its ability to use a variety of nutrients and metabolic processes [1]. This makes it useful for environmental cleanup, like breaking down pollutants such as oil spills and other bioremediation. It does so through the lipase it creates, which excels at breaking down the hydrocarbons in oil [2]. However, it is also considered an opportunistic pathogen, as it’s known to cause infections in people with weakened immune systems- specifically in hospitals. Its ability to stick to medical devices by forming biofilms and its natural resistance to many antibiotics and other disinfection practices make it difficult to manage in clinical settings. Infections from this are specifically found to be due to long term intravenous medical supplies like catheters [1]. Studying this bacterium helps us better understand how to manage its harmful effects while exploring its potential environmental benefits.
Genome Structure
The genome size of reference R. mannitolilytica strain SN82F48 is 5,072,301 bp. It has 2 circular chromosomes and a plasmid. Chromosome 1 is 3,503,899 bp, chromosome 2 is 1,377,860 bp, and plasmid pRMAN01 is 190,542 bp [3]. Carbapenem-resistant strain JARB-RN-0044 has one circular chromosome that is 3.5 Mb and a large replicon plasmid that is 1.5 Mb [4]. Another carbapenem-resistant strain, MRY14-0246, has a total genome size of 4,671,011 bp [5].
The GC percentage of all three strains are similar: SN82F48 has 65.5% GC, JARB-RN-0044 has 66.1% GC, and MRY14-0246 has 65.8% GC. Strain SN82F48 has 4,472 protein genes and 58 RNA genes, strain JARB-RN-0044 has 3,336 protein-coding genes on the chromosome and 1,390 on the replicon, and strain MRY14-0246 has 4,357 coding sequences [4, 5, 6]. Genome sequencing of JARB-RN-0044 reveals that genes involved in replication, recombination, repair, and metabolism are mostly found on the large chromosome, while genes involved in transcription and cell motility are mostly found on the replicon plasmid [4].
Novel class D β-lactamase blaOXA-1176 and blaOXA-1177 genes were found in the JARB-RN-0044 strain, while MRY14-0246 was found to have the novel class D β-lactamase genes OXA-443 and OXA-444. These D β-lactamase genes could be a contributing factor to carbapenem class antibiotic resistance in R. mannitolilytica, which makes infections difficult to treat [4,5]. Furthermore, MRY14-0246 does not have T3SS (Type III Secretion System) gene cluster, but does have the T6SS (Type VI Secretion System). In the plant pathogen Ralstonia solanacearum, T6SS is known to deliver effectors to other organisms that cause cell death, which suggests that T6SS could be a factor in determining the virulence of a Ralstonia species [5].
Cell Structure, Metabolism and Life Cycle
Ralstonia mannitolilytica is a non-motile, rod-shaped bacilli bacteria with a thin peptidoglycan layer, as it is gram-negative, and an outer membrane containing lipopolysaccharides [7]. The bacteria has pili present, which may be a contributing factor to its ability to form biofilms [8].
Ralstonia mannitolilytica is a metabolically diverse bacteria and gains energy through catabolic processes. It oxidizes ammonium to nitrite and can also breakdown carbohydrates like mannitol to gain energy [9]. Because Ralstonia mannitolilytica metabolizes mannitol, it produces polysaccharides [10]. It is aerobic, but can survive through fermentative processes when oxygen is low or absent [9].
Ralstonia mannitolilytica originates from soil, water, and plant root settings [9]. Its diverse metabolism makes it hardy and able to survive in different environments. While great for its survival, in hospital settings, however, this makes it difficult to get rid of.
This bacteria is an opportunistic pathogen, and can be particularly harmful to those who are immunocompromised [11]. Its ability to form biofilms, as well as documented cases of antibiotic resistance, and its relatively unknown existence, makes it difficult to diagnose and treat [9]. It commonly enters hospitals through contaminated water or equiptment, such as catheters [12]. In addition, Ralstonia mannitolilytica can form capsules, which adds to their ability to resist treatments, commonly those in the form of antibiotics [13]. In immunocompromised patients, it has been known to cause bacteremia, which are infections of the bloodstream, pneumonia, fever, osteomyelitis, and even death [14, 15].
Ecology and Pathogenesis
Ralstonia mannitolilytica is commonly found in aquatic environments, soil, and plants. It has been isolated from river water, sewage, and even medical devices that have been contaminated with the bacterium [12]. It is a facultative anaerobe that can grow with or without oxygen and has a diverse metabolism [16].
In the environment, Ralstonia mannitolilytica contributes to the breakdown of organic matter [17]. Through its ability to metabolize various organic substrates, such as mannitol, the bacterium can help recycle nutrients in the aquatic and soil environments it is commonly found in [17].
When it does get into hospital settings, an environment different from its usual, it can persist through the formation of capsules and biofilms[12]. This makes the bacteria particularly hard to get rid of, and its biofilm can protect it from antibiotics. Although the bacteria is resistant to many antibiotics, its infection can still be treated. Antibiotics including ceftazidime, ciprofloxacin, metronidazole work best while colistin shows the most resistance [21]. Targeting proteins may disrupt R. mannitolilytica's pathogenicity and virulence when antibiotics fail. LpxC is crucial for lipopolysaccharide biosynthesis, FepA is involved in iron uptake, and ExsA controls virulence factor expression [21].
Various reports from around the globe have shown that this bacterium can serve as an opportunistic pathogen. It has been documented to have caused bacteremia, which are bloodstream infections, endocarditis, pulmonary cavitation, and more [14, 15]. These opportunistic pathogens are typically found in immunocompromised patients creating both localized and systemic infections. Common patient symptoms include a high-grade fever, abdominal pain, and tachycardia [20]. A report found that the bacteria infects cerebral spinal fluid and the central nervous system, causing meningitis[20].
The virulence factors that make Ralstonnia mannitolilytica so dangerous in humans include its ability to form biofilms, which protect it from antibiotics and the host's immune system, its ability to form capsules, which further allows it to evade immune cells, and its ability to produce endotoxins, which can cause sepsis [18]. Additionally, lipopolysaccharides (LPS), extracellular enzymes, and siderophores are virulence factors [19]. These virulence factors contribute to the bacterium's ability to incite immune responses, invade and destroy host tissues using protease and lipase, and acquire essential nutrients like iron using hemolysin [19].
Prevention of infection by Ralstonnia mannitolilytica can be taken by updating policies and regulations that pertain to the microbiological safety of aqueous solutions and devices used in clinical practice. It is also essential that sufficient sterilization of equipment is maintained and microbiological techniques are enhanced to attain an accurate diagnosis of the underlying organism.
References
[1] Ryan MP, Adley CC. 2014. Ralstonia spp." Emerging global opportunistic pathogens. Eur J Clin Microbiol Infect Dis. 33(3):291-304. SpringerLink.
[2] Abdulkareem RS, Al-Ezee AMM, Musafer Hk. 2024. New bioremediation by lipase purified from Ralstonia mannitolilytica for petroleum hydrocarbons. Samarra J Pure Apple Sci. 6(1):148-164. https://doi.org/10.54153/sjpas.2024.v6i1.656.
[3] National Center for Biotechnology Information. 2015. Ralstonia mannitolilytica genome assemby ASM95413v2. https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_000954135.1/.
[4] Hayashi W, Kaiju H, Kayama S, Yu L, Zuo H, Sugawara Y, Azuma K, Takahashi A, Hata Y, Sugai M. Complete sequence of carbapenem-resistant Ralstonia mannitolilytica clinical isolate co-producing novel class D β-lacatmase OXA-1176 and OXA-1177 in Japan. Am Soc Microbiol. 12(4):e03919-23.
[5] Suzuki M, Nishio H, Asagoe K, Kida K, Suzuki S, Matsui M, Shibayama K. 2015. Genome sequence of a carbapenem-resistant strain of Ralstonia mannitolilytica. Am Soc Microbiol. 3(3):e00405-15.
[6] Kyoto Encyclopedia of Genes and Genomes. 2015. Ralstonia mannitolilytica. https:www.genome.jp./kegg-bin/show_organism?org=rmn.
[7] Boattini M, Bianco G, Biancone L, Cavallo R, Costa C. 2018. Ralstonia mannitolilytica bacteraemia: a case report and literature review. Le Infezioni in Medicina. 26(4):374-378.
[8] Basso M, Venditti C, Raponi G, Navazio AS, Alessandri F, Giombini E, Nisii C, Di Caro A, Venditti M. 2019. A case of persistant bacteraemia by Ralstonia mannitolilytica and Ralstonia pickettii in an intensive care unit. Infect Drug Resist 12:2391-2395. https://doi.org/10.2147/IDR.S206492.
[9] Siddiqui T, Patel SS, Sinha R, Ghoshal U, Sahu C. 2022. Ralstonia mannitolilytica: an emerging multidrug-resistant oppportunistic pathogen in tertiary care hospital setting. Access Microbiol 4(5):acmi000367. Published 2022 May 31. https://doi.org/10.1099/acmi.0.000367.
[10] Papagrigorakis E, Vavourakis M, Vlachos C, Zachariou D, Galanis A, Marougklianis V, Polyzois V, Pneumaticos S. 2022. Osteomyelitis caused by Ralstonia mannitolilytica, a rate opportunistic pathogen. Cureus 14(4):e24151. https://doi.org/10.7759/cureus.24151. PMID: 35586345; PMCID: PMC9109608.
[11] Carreira M, Gomes C, Silva M, Duro R. 2020. Ralstonia mannitolilytica endocarditis: A case report. IDCases 22:e01003. https://doi.org/10.1016/j.idcr.2020.e01003.
[12] Lim CTS, Lee SE. 2017. A rare case of Ralstonia mannitolilytica: an emerging multidrug-resistant opportunistic pathogen in a tertiary care hospital setting. Access Microbiol 4(5):acmi000367. https://doi.org/10.1099/acmi.0.000367. PMID: 16003352; PMCID: PMC9394538
[13] Xie P, Liu J, Wu L, Liu D. 2021. Pulmonary cavitation: A hint of Ralstonia mannitolilytica infectionn through imaging. Am J Respir Crit Care Med 203(12):e947-e949. htpps://doi/orgg/10.1164/rccm.202009-3616IM. PubMed: 33535022.
[14] Kim G, Yoo RN, So H, Lee JY, Kim MN, Kim SH, Jhang WK, Park SJ, Lee J. 2023. Clinical manifestation of Ralstonia mannitolilytica infection in pediatric patients and epidemiological investigation of outbreaks. J Korean Med Sci 38(33):e252. https://doi.org/10.3346/jkms.2023.38.e252.
[15] Said M, van Hougenhouck-Tulleken W, Naidoo R, Mbelle N, Ismail F. Outbreak of Ralstonia mannitolilytica bacteraemia in patients undergoing haemodialysis at a tertiary hospital in Pretoria, South Africa. Antimicrob Resist Infect Control 9:117. https://doi.org/10.1186/s13756-020-00772-6.
[16] Jeonbuk National University Medical School, Microbiological Laboratory. 2017. Ralstonia mannitolilytica GMB0650. Available from: https://www.gmbank.org/product-page/gmb0650.
[17] Wang Z, Luo W, Cheng S, Zhang H, Zong J, Zhang Z. 2023. Ralstonia solanacearum- A soil borne hidden enemy of plants: Research development in management strategies, their action mechanism and challenges. Front Plant Sci 14:1141902. https://doi.org/10.3389/fpls.2023.1141902.
[18] Liu CX, Yan C, Zhang P, Li FQ, Yang JH, Li XY. 2016. Ralstonia mannitolilytica-induced septicemia and homology analysis in infected patients: 3 case reports. Jundishapur J Microbiol 9(7):e34373. https://doi.org/10.5812/jjm.34373. PMID: 27679705: PMCID: PMC5035395
[19] Al-Abdullah, N., & Amir, Y. N. 2023. Inauguration the enigma: Ralstonia mannitolilytica septicemia – clinical journey, multidimensional investigation, and paradigm- shifting research insights. Biosciences Biotechnology Research Asia. 20(3), 865-873. doi:https://doi.org/10.13005/bbra/3138
[20] Aldhafeeri, W. F., Habalrih, F., Al Omar, A. H., Altamimi, A. A., Alshahrani, M. S., Abdullah, J., Alrabie, A., & Shah, S. 2022. Postoperative cerebrospinal fluid infection by Ralstonia mannitolilytica: Two case reports and a literature review. Surgical neurology international. 13(602). https://doi.org/10.25259/SNI_
[21] Alshuhri, S., Alosaimi, A., Alnafee, K., Alkahtany,J., Alhamami, S., et al. 2024. Ralstonia mannitolilytica infection in a tertiary care center: An outbreak report. American Journal of Infection Control. 0196-6553. https://doi.org/10.1016/j.ajic.2024.09.019.
Author
Page authored by Clarice Fahrenkrug, Elinor Morkos, Randie Dillon, & Halie Grenier, students of Prof. Bradley Tolar at UNC Wilmington.