Naegleria fowleri aka "Brain Eating Ameoba": Difference between revisions
No edit summary |
No edit summary |
||
| (30 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Introduction== | ==Introduction== | ||
[[file:Aem.01866-20-f0001 (1).jpeg|thumb|200px|left|Figure 1. Schematic representation of amoeba diversity. (A) Amoebae are spread in several supergroups, including Amoebozoa, Rhizaria, Excavata, Heterokonta, Alveolata, Opisthokonta, and other ungrouped species. Amoebozoa (black) is the only group that solely consists of amoebae. The tree topology is from previous classifications (3, 139). (B) Amoebae show considerable variation in their sizes.<ref name = "Shi"></ref>.]] | |||
Ameoba are single celled organisms that are found in every major eukaryotic lineage. They are eukaryotic organisms that are defined by their lack of cell walls and the presence of pseudopods. Pseudopods are temporary extensions of the cytoplasm that are facilitated by microfilaments in the cytoplasm, which allow amoeba to be motile. <br><ref>[https://books.google.com/books?hl=en&lr=&id=J0vniMFGPukC&oi=fnd&pg=PP1&dq=amoeba+biology&ots=senLYlfMKm&sig=FAN0EtEvU8AgBwSaQ9-T_ECNqLA#v=onepage&q=amoeba%20biology&f=false Jeon K, editor. The biology of amoeba. Elsevier; 2012 Dec 2.]</ref> | |||
They live in the water, soil, and air of a number of diverse ecosystems. They are often predators that consume fungi and bacteria and aid with the recycling of nutrients. They feed via phagocytosis, engulfing smaller particles and organisms into their cytoplasm using their pseudopods. To regulate their internal environment, amoebas have vacuoles that regulate osmosis.<br><br> | |||
Amoeba can have vastly different structures, which is determined by the actin filaments in the cytoplasm. It is these filaments that control the structure of the pseudopods.<br><ref name="Shi">[https://academic.oup.com/femspd/article/51/2/243/888715 Shi Y, Queller DC, Tian Y, Zhang S, Yan Q, He Z, He Z, Wu C, Wang C, Shu L.2021.The Ecology and Evolution of Amoeba-Bacterium Interactions. Appl Environ Microbiol87:e01866-20.https://doi.org/10.1128/AEM.01866-20]</ref> | |||
Amoeba are divided into two main groups: free-living and non-free-living, some of which are pathogenic to humans. One of the most pathogenic species of amoeba is <i>Naegleria fowleri</i>, which belongs to the <i>Naegleria</i> genus. Fortunately only one,<i>Naegleria fowleri</i>, out of 30 species in this genus can infect humans. | |||
<br> | |||
<br> <br> | <br> <br> | ||
| Line 19: | Line 19: | ||
<br><i>Naegleria fowleri</i> is a highly dangerous ameoba that can infect humans and cause death within 7 to 10 days of infection. This amoeba attacks brain tissue and causes brain swelling; an infection known as primary amebic meningoencephalitis (PAM).<br> | <br><i>Naegleria fowleri</i> is a highly dangerous ameoba that can infect humans and cause death within 7 to 10 days of infection. This amoeba attacks brain tissue and causes brain swelling; an infection known as primary amebic meningoencephalitis (PAM).<br> | ||
<i>N. fowleri</i> survives best in warm water like hot springs, ponds, and poorly chlorinated pools. They cannot survive in salt water. These are heat loving amoeba and can survive in temperatures up to | <i>N. fowleri</i> survives best in warm water like hot springs, ponds, and poorly chlorinated pools. They cannot survive in salt water. These are heat loving amoeba and can survive in temperatures up to 46° Celsius, making them ideal to adapt to internal body temperatures of humans and other animals.<br><ref name="Abdul"></ref> | ||
<i>Naegleria fowleri</i> has three different lifeforms — trophozoite, flagellate, and cyst. They are often in their cyst form when environmental conditions are not ideal. This form has an endocyst wall to protect against large changes in pH or temperatures. The flagellate form is pear shaped with two flagella. The trophozoite form is infectious to humans and is when the amoeba eats (via trogocytosis and phagocytosis) and replicates (via binary fission).This form has the characteristic pseudopods, which are used to classify amoeba.<br> | <i>Naegleria fowleri</i> has three different lifeforms — trophozoite, flagellate, and cyst. They are often in their cyst form when environmental conditions are not ideal. This form has an endocyst wall to protect against large changes in pH or temperatures. The flagellate form is pear shaped with two flagella. The trophozoite form is infectious to humans and is when the amoeba eats (via trogocytosis and phagocytosis) and replicates (via binary fission).This form has the characteristic pseudopods, which are used to classify amoeba.<br><ref name="Francine">[Francine Marciano-Cabral, Guy A. Cabral, The immune response to Naegleria fowleri amebae and pathogenesis of infection, FEMS Immunology & Medical Microbiology, Volume 51, Issue 2, November 2007, Pages 243–259, https://doi.org/10.1111/j.1574-695X.2007.00332.x]</ref> | ||
[[NF photo.jpeg | [[file:NF photo.jpeg|thumb|300px|right|Figure 1.Depiction of <i>Naegleria fowleri</i> in its three states of transformation. (a) Transmission electron micrograph (TEM) of trophozoites illustrating the prominent nucleus with a centrally located electron-dense nucleolus. (b) Scanning electron micrograph (SEM) of trophozoites exhibiting ‘food-cups’ (arrow). (c) SEM of a cyst. (d) Light micrograph of a flagellate with the characteristic two flagella. (e) TEM of flagellate illustrating one of the flagella (arrow). The scale bars represent 10µm for (a–d) and 2µm for (e).<ref name="Francine"></ref>. ]] | ||
<br>There are a few other pathogenic amoeba that cause diseases in the central nervous system (CNS) such as <i>Balamuthia mandrillaris</i>, <i>Sappinia diploidea</i>, and <i>Acanthamoeba</i>. Infections from <i>Balamuthia mandrillaris</i> and <i>Acanthameoba</i> are fatal, while infection from <i>Sappinia diploidea</i> is non-fatal. | <br>There are a few other pathogenic amoeba that cause diseases in the central nervous system (CNS) such as <i>Balamuthia mandrillaris</i>, <i>Sappinia diploidea</i>, and <i>Acanthamoeba</i>. Infections from <i>Balamuthia mandrillaris</i> and <i>Acanthameoba</i> are fatal, while infection from <i>Sappinia diploidea</i> is non-fatal.<ref>[https://www.sciencedirect.com/science/article/pii/S0020751904001365?via%3Dihub Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol. 2004 Aug;34(9):1001-27. doi: 10.1016/j.ijpara.2004.06.004. PMID: 15313128.]</ref> | ||
| Line 34: | Line 33: | ||
==Method of Invasion== | ==Method of Invasion== | ||
<br><i>Naegleria fowleri</i> enters the human body via the nasal cavity, when contaminated water is inhaled. However, no damage is done to the nasal cavity. Instead most of the damage is found in the olfactory bulb and other regions of the brain, which the trophozoite gains access to by attaching to the nasal mucosa and then traveling through the cribriform plate. It is clear that there is some signal in the brain that the <i>N. fowleri</i> are targeting, but because there are no suitable animal models, discovering what is providing the N. fowleri with this ‘cerebral roadmap’ has been difficult. However, progress has been made identifying acetylcholine as a possible signaling protein. Research done by Abdul Mannan Baig (2016) has discovered homology between acetylcholine and a G-protein coupled receptor on <i>N. fowleri</i>. It could be acetylcholine and other growth promoting chemicals that are signaling the amoeba to target the brain. More research will need to be done to confirm these results. | <br><i>Naegleria fowleri</i> enters the human body via the nasal cavity, when contaminated water is inhaled. However, no damage is done to the nasal cavity. Instead most of the damage is found in the olfactory bulb and other regions of the brain, which the trophozoite gains access to by attaching to the nasal mucosa and then traveling through the cribriform plate. It is clear that there is some signal in the brain that the <i>N. fowleri</i> are targeting, but because there are no suitable animal models, discovering what is providing the N. fowleri with this ‘cerebral roadmap’ has been difficult. However, progress has been made identifying acetylcholine as a possible signaling protein. Research done by Abdul Mannan Baig (2016) has discovered homology between acetylcholine and a G-protein coupled receptor on <i>N. fowleri</i>. It could be acetylcholine and other growth promoting chemicals that are signaling the amoeba to target the brain. More research will need to be done to confirm these results.<ref name="Abdul">[https://pubs.acs.org/doi/epdf/10.1021/acschemneuro.6b00197?ref=article_openPDF Abdul Mannan Baig Primary Amoebic Meningoencephalitis: Neurochemotaxis and Neurotropic Preferences of Naegleria fowleriACS Chemical Neuroscience 2016 7 (8), 1026-1029 DOI: 10.1021/acschemneuro.6b00197]</ref> | ||
<br>Symptoms for PAM are very similar to that of bacterial meningitis, so often a PAM diagnosis is not given until after death. The initial symptoms are fever, vomiting, altered sense of smell, and severe headaches, that progress into more violent symptoms such as seizures, stiff neck, coma, hallucinations, and apprehension. People with PAM die an average of 5 days after symptoms begin. | <br>Symptoms for PAM are very similar to that of bacterial meningitis, so often a PAM diagnosis is not given until after death. The initial symptoms are fever, vomiting, altered sense of smell, and severe headaches, that progress into more violent symptoms such as seizures, stiff neck, coma, hallucinations, and apprehension. People with PAM die an average of 5 days after symptoms begin.<ref>[https://www.cdc.gov/naegleria/media/pdfs/naegleria_factsheet508c.pdf Facts about Naegleria fowleri and Primary Amebic Meningoencephalitis CDC CS 332678-A July 11, 2022]</ref> | ||
<br>Most of the people who contract PAM are engaging in water related sports, though a few cases have been reported from people who use improperly sanitized nasal rinses. | <br>Most of the people who contract PAM are engaging in water related sports, though a few cases have been reported from people who use improperly sanitized nasal rinses. | ||
==Immune Response== | ==Immune Response== | ||
[[File:BrainNF.jpeg|thumb|300px|left|Figure 3Light microscopy histopathology of (B6C3)F1 mice inoculated intranasally with Naegleria fowleri. (a, b) Whole-brain sagital sections. (a) Uninfected mouse. (b) Infected mouse at 10 days postnasal instillation with trophozoites. Note the massive neuropathology (arrow) in the olfactory area and frontal lobe of the brain. (c) A focal lesion (arrow) in the striatum (st) of a mouse at 4 days postintranasal instillation with trophozoites. (d) Extensive accumulation of mononuclear and polymorphonuclear cells in striatum of mouse at 7 days postintranasal instillation with trophozoites. Note the numerous trophozoites admixed with the mononuclear and polymorphonuclear cells. All sections were stained with hematoxylin and eosin. The bars designate 2 mm for (a) and (b) and 10 µm for (b) and (c). am, amygdala; cb, cerebellum; ctx, cerebral cortex; gp, globus pallidus; hi, hippocampus; ob, olfactory bulb; th, thalamus; st, striatum.<ref name="Francine"></ref>.]] | |||
<br>Once the <i>Naegleria fowleri</i> has entered the brain, the trophozoites cut off and ingest various vital cells such as nerve cells. This damages the brain tissue and causes hemorrhaging, which triggers an inflammatory response, causing further damage to the brain. The innate immunity response involves activation of the complement, neutrophils, and macrophages. Unfortunately, it appears that <i>N. fowleri</i> has begun to acquire immunity to the body’s natural responses, such as avoidance of complement lysis. | <br>Once the <i>Naegleria fowleri</i> has entered the brain, the trophozoites cut off and ingest various vital cells such as nerve cells. This damages the brain tissue and causes hemorrhaging, which triggers an inflammatory response, causing further damage to the brain. The innate immunity response involves activation of the complement, neutrophils, and macrophages. Unfortunately, it appears that <i>N. fowleri</i> has begun to acquire immunity to the body’s natural responses, such as avoidance of complement lysis. | ||
The key to surviving infection by <i>Naegleria fowleri</i> is early identification and quick treatment. While there is no official treatment for <i>Naegleria fowleri</i>, a combination of Amphotericin B with rifampin and other antifungal compounds | The key to surviving infection by <i>Naegleria fowleri</i> is early identification and quick treatment. While there is no official treatment for <i>Naegleria fowleri</i>, a combination of Amphotericin B with rifampin and other antifungal compounds have been used to treat PAM in a few select cases <ref>[https://www.nejm.org/doi/full/10.1056/NEJM198202113060607 James S. Seidel and Paul Harmatz and G. S. Visvesvara and Arthur Cohen and Jack Edwards and Jerrold TurnerSuccessful Treatment of Primary Amebic MeningoencephalitisNew England Journal of Medicine 306 6 346-348 1982 10.1056/NEJM198202113060607]</ref>. Although there is no perfect model organism that can be studied to confirm processes of <i>Naegleria fowleri</i> in humans, research is being done on animals, such as mice, to study the overall progression of the infection and possible experimental treatment methods. The goal of these studies are to gain a better overall understanding of the infection so that doctors can identify and hopefully find ways of slowing the infection. | ||
One study | One study that was observing possible genetic differences between pathogenic and non-pathogenic <i>Naegleria</i> found that there were two proteins: high mobility group protein and the 26s proteasome subunit were responsible for increasing pathogenicity of <i>N. fowleri</i>.<ref>[https://www.sciencedirect.com/science/article/pii/S093247390470053X Ho-Cheol Yun, Soon-Jung Park, Hyun-Hee Kong, Dong-Il Chung, | ||
Isolation of genes induced in Naegleria fowleriduring mouse brain passage European Journal of Protistology Volume 38, Issue 2 2002 Pages 105-111 ISSN 0932-4739 https://doi.org/10.1078/0932-4739-00859.]</ref><ref name = "Francine"></ref> | |||
==Survivors of <i>Naegleria fowleri</i>== | ==Survivors of <i>Naegleria fowleri</i>== | ||
<br>From 2012 to 2021, there have been a total of 31 cases of infection by Naegleria fowleri with only 3 survivors. | <br>From 2012 to 2021, there have been a total of 31 cases of infection by <i>Naegleria fowleri</i> with only 3 survivors. | ||
<br>One such case occured in 2017 to a 12 year old girl in Arkansas who was admitted to the hospital with a high fever, trouble waking up, vomiting, and trouble opening her eyes. A spinal tap was done to rule out meningitis and then <i>Naegleria fowleri</i> was identified to be the cause of the symptoms. She was quickly treated with a round of antibiotics and antifungals: Amphotericin B, Rifampin, Fluconazole, Dexamethasone, and Azithromycin. She was also placed on a catheter to reduce swelling in her brain. Even on this treatment, | <br>One such case occured in 2017 to a 12 year old girl in Arkansas who was admitted to the hospital with a high fever, trouble waking up, vomiting, and trouble opening her eyes. A spinal tap was done to rule out meningitis and then <i>Naegleria fowleri</i> was identified to be the cause of the symptoms. She was quickly treated with a round of antibiotics and antifungals: Amphotericin B, Rifampin, Fluconazole, Dexamethasone, and Azithromycin. She was also placed on a catheter to reduce swelling in her brain. Even on this treatment, her health continued to rapidly deteriorate. In response, she was given Miltefosine and her body temperature was lowered to prevent further brain damage by swelling. She remained in the ICU for 18 days under therapeutic hypothermia before CFS samples could be taken and it was verified that there was no remaining Naegleria fowleri left in her system. While the girl did sustain some brain damage, she was able to make a full neurologic recovery and was able to walk unassisted three months after the infection.<ref>[https://www.sciencedirect.com/science/article/pii/S1477893916302125 Travis W. Heggie Thomas Küpper Surviving Naegleria fowleri infections: A successful case report and novel therapeutic approach Travel Medicine and Infectious Disease Volume 16 2017 Pages 49-51 ISSN 1477-8939]</ref> | ||
Latest revision as of 19:12, 14 December 2024
Introduction
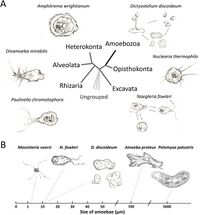
Ameoba are single celled organisms that are found in every major eukaryotic lineage. They are eukaryotic organisms that are defined by their lack of cell walls and the presence of pseudopods. Pseudopods are temporary extensions of the cytoplasm that are facilitated by microfilaments in the cytoplasm, which allow amoeba to be motile.
[2]
They live in the water, soil, and air of a number of diverse ecosystems. They are often predators that consume fungi and bacteria and aid with the recycling of nutrients. They feed via phagocytosis, engulfing smaller particles and organisms into their cytoplasm using their pseudopods. To regulate their internal environment, amoebas have vacuoles that regulate osmosis.
Amoeba can have vastly different structures, which is determined by the actin filaments in the cytoplasm. It is these filaments that control the structure of the pseudopods.
[1]
Amoeba are divided into two main groups: free-living and non-free-living, some of which are pathogenic to humans. One of the most pathogenic species of amoeba is Naegleria fowleri, which belongs to the Naegleria genus. Fortunately only one,Naegleria fowleri, out of 30 species in this genus can infect humans.
Pathogenic Amoebas
Naegleria fowleri is a highly dangerous ameoba that can infect humans and cause death within 7 to 10 days of infection. This amoeba attacks brain tissue and causes brain swelling; an infection known as primary amebic meningoencephalitis (PAM).
N. fowleri survives best in warm water like hot springs, ponds, and poorly chlorinated pools. They cannot survive in salt water. These are heat loving amoeba and can survive in temperatures up to 46° Celsius, making them ideal to adapt to internal body temperatures of humans and other animals.
[3]
Naegleria fowleri has three different lifeforms — trophozoite, flagellate, and cyst. They are often in their cyst form when environmental conditions are not ideal. This form has an endocyst wall to protect against large changes in pH or temperatures. The flagellate form is pear shaped with two flagella. The trophozoite form is infectious to humans and is when the amoeba eats (via trogocytosis and phagocytosis) and replicates (via binary fission).This form has the characteristic pseudopods, which are used to classify amoeba.
[4]
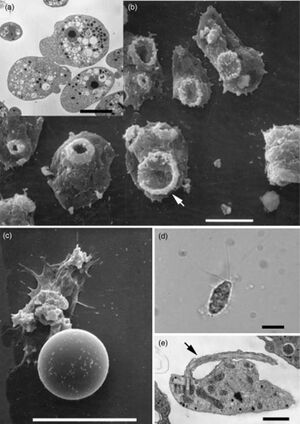
There are a few other pathogenic amoeba that cause diseases in the central nervous system (CNS) such as Balamuthia mandrillaris, Sappinia diploidea, and Acanthamoeba. Infections from Balamuthia mandrillaris and Acanthameoba are fatal, while infection from Sappinia diploidea is non-fatal.[5]
Method of Invasion
Naegleria fowleri enters the human body via the nasal cavity, when contaminated water is inhaled. However, no damage is done to the nasal cavity. Instead most of the damage is found in the olfactory bulb and other regions of the brain, which the trophozoite gains access to by attaching to the nasal mucosa and then traveling through the cribriform plate. It is clear that there is some signal in the brain that the N. fowleri are targeting, but because there are no suitable animal models, discovering what is providing the N. fowleri with this ‘cerebral roadmap’ has been difficult. However, progress has been made identifying acetylcholine as a possible signaling protein. Research done by Abdul Mannan Baig (2016) has discovered homology between acetylcholine and a G-protein coupled receptor on N. fowleri. It could be acetylcholine and other growth promoting chemicals that are signaling the amoeba to target the brain. More research will need to be done to confirm these results.[3]
Symptoms for PAM are very similar to that of bacterial meningitis, so often a PAM diagnosis is not given until after death. The initial symptoms are fever, vomiting, altered sense of smell, and severe headaches, that progress into more violent symptoms such as seizures, stiff neck, coma, hallucinations, and apprehension. People with PAM die an average of 5 days after symptoms begin.[6]
Most of the people who contract PAM are engaging in water related sports, though a few cases have been reported from people who use improperly sanitized nasal rinses.
Immune Response
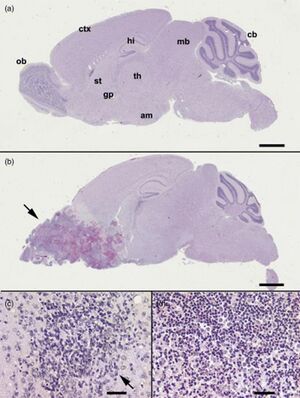
Once the Naegleria fowleri has entered the brain, the trophozoites cut off and ingest various vital cells such as nerve cells. This damages the brain tissue and causes hemorrhaging, which triggers an inflammatory response, causing further damage to the brain. The innate immunity response involves activation of the complement, neutrophils, and macrophages. Unfortunately, it appears that N. fowleri has begun to acquire immunity to the body’s natural responses, such as avoidance of complement lysis.
The key to surviving infection by Naegleria fowleri is early identification and quick treatment. While there is no official treatment for Naegleria fowleri, a combination of Amphotericin B with rifampin and other antifungal compounds have been used to treat PAM in a few select cases [7]. Although there is no perfect model organism that can be studied to confirm processes of Naegleria fowleri in humans, research is being done on animals, such as mice, to study the overall progression of the infection and possible experimental treatment methods. The goal of these studies are to gain a better overall understanding of the infection so that doctors can identify and hopefully find ways of slowing the infection.
One study that was observing possible genetic differences between pathogenic and non-pathogenic Naegleria found that there were two proteins: high mobility group protein and the 26s proteasome subunit were responsible for increasing pathogenicity of N. fowleri.[8][4]
Survivors of Naegleria fowleri
From 2012 to 2021, there have been a total of 31 cases of infection by Naegleria fowleri with only 3 survivors.
One such case occured in 2017 to a 12 year old girl in Arkansas who was admitted to the hospital with a high fever, trouble waking up, vomiting, and trouble opening her eyes. A spinal tap was done to rule out meningitis and then Naegleria fowleri was identified to be the cause of the symptoms. She was quickly treated with a round of antibiotics and antifungals: Amphotericin B, Rifampin, Fluconazole, Dexamethasone, and Azithromycin. She was also placed on a catheter to reduce swelling in her brain. Even on this treatment, her health continued to rapidly deteriorate. In response, she was given Miltefosine and her body temperature was lowered to prevent further brain damage by swelling. She remained in the ICU for 18 days under therapeutic hypothermia before CFS samples could be taken and it was verified that there was no remaining Naegleria fowleri left in her system. While the girl did sustain some brain damage, she was able to make a full neurologic recovery and was able to walk unassisted three months after the infection.[9]
References
- ↑ 1.0 1.1 Shi Y, Queller DC, Tian Y, Zhang S, Yan Q, He Z, He Z, Wu C, Wang C, Shu L.2021.The Ecology and Evolution of Amoeba-Bacterium Interactions. Appl Environ Microbiol87:e01866-20.https://doi.org/10.1128/AEM.01866-20
- ↑ Jeon K, editor. The biology of amoeba. Elsevier; 2012 Dec 2.
- ↑ 3.0 3.1 Abdul Mannan Baig Primary Amoebic Meningoencephalitis: Neurochemotaxis and Neurotropic Preferences of Naegleria fowleriACS Chemical Neuroscience 2016 7 (8), 1026-1029 DOI: 10.1021/acschemneuro.6b00197
- ↑ 4.0 4.1 4.2 4.3 [Francine Marciano-Cabral, Guy A. Cabral, The immune response to Naegleria fowleri amebae and pathogenesis of infection, FEMS Immunology & Medical Microbiology, Volume 51, Issue 2, November 2007, Pages 243–259, https://doi.org/10.1111/j.1574-695X.2007.00332.x]
- ↑ Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol. 2004 Aug;34(9):1001-27. doi: 10.1016/j.ijpara.2004.06.004. PMID: 15313128.
- ↑ Facts about Naegleria fowleri and Primary Amebic Meningoencephalitis CDC CS 332678-A July 11, 2022
- ↑ James S. Seidel and Paul Harmatz and G. S. Visvesvara and Arthur Cohen and Jack Edwards and Jerrold TurnerSuccessful Treatment of Primary Amebic MeningoencephalitisNew England Journal of Medicine 306 6 346-348 1982 10.1056/NEJM198202113060607
- ↑ [https://www.sciencedirect.com/science/article/pii/S093247390470053X Ho-Cheol Yun, Soon-Jung Park, Hyun-Hee Kong, Dong-Il Chung, Isolation of genes induced in Naegleria fowleriduring mouse brain passage European Journal of Protistology Volume 38, Issue 2 2002 Pages 105-111 ISSN 0932-4739 https://doi.org/10.1078/0932-4739-00859.]
- ↑ Travis W. Heggie Thomas Küpper Surviving Naegleria fowleri infections: A successful case report and novel therapeutic approach Travel Medicine and Infectious Disease Volume 16 2017 Pages 49-51 ISSN 1477-8939
Edited by [Cameron McCaleb], student of Joan Slonczewski for BIOL 116, 2024, Kenyon College.
