Neisseria elongata: Difference between revisions
No edit summary |
No edit summary |
||
| (82 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
{{Biorealm Genus}} | {{Biorealm Genus}} | ||
==Classification== | ==Classification (1)== | ||
===Higher order taxa=== | ===Higher order taxa=== | ||
Bacteria; Proteobacteria; Betaproteobacteria; Neisseriales; Neisseriaceae; Neisseria | |||
===Species=== | ===Species=== | ||
| Line 14: | Line 15: | ||
|} | |} | ||
'' | ''Neisseria elongata'' | ||
===Subspecies=== | |||
<i>N. elongata</i> subsp. <i>elongata,</i> <i>N. elongata</i> subsp. <i>glycolytica,</i> <i>N. elongata</i> subsp. <i>nitroreducens</i> | |||
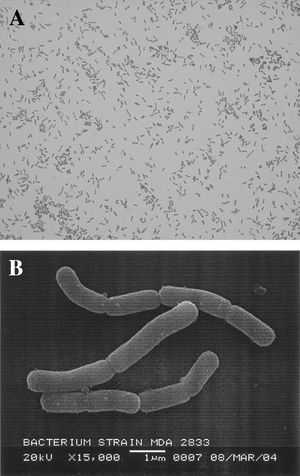
[[Image:Zjm0020661710002.jpg|thumb|Gram staining (magnification, ×1,000) (top) and electron microscopy (bar, 1 μm) (bottom) of MDA2833, a bacillary Neisseria sp. From the American Society of Microbiology (6)]] | |||
==Description and significance== | ==Description and significance== | ||
<i>Neisseria elongata</i>, formerly known as Centers for Disease Control (CDC) group M6, was described by Bovre and Holten in 1970 as a gram-negative, rod-shaped bacterium of the family Neisseriaceae, where it is found in the oral bacterial flora of the human pharynx and throat or in the blood of those infected (2). <i>N. elongata</i> consists of three subspecies, <i>N. elongata</i> subsp. <i>elongata,</i> <i>N. elongata</i> subsp. <i>glycolytica,</i> and <i>N. elongata</i> subsp. <i>nitroreducens,</i> in which are separated based on their biochemical differences (4). Although these subspecies of <i>N. elongata</i> were previously believed to be nonpathogenic to humans, recent case studies from patients suffering from endocarditis, have indicated that all three <i>N. elongata</i> subspecies are associated with human disease, particularly endocarditis and osteomyelitis (3). | |||
Although the <i>N. elongata</i> genome has not yet been sequenced, the importance of sequencing its genome will provide information on the three <i>N. elongata</i> subspecies that could possibly assist in distinguishing their pathogenic roles in endocarditis and osteomyelitis. Before the pathogenic roles of all three <i>N. elongata</i> subspecies were discovered, where <i>N. elongata</i> subsp. <i>nitroreducens</i> was the first subspecies to be discovered as pathogenic, <i>N. elongata</i> subsp. <i>elongata</i> and <i>N. elongata</i> subsp. <i>glycolytica</i> were considered just to be transient colonizers of the human upper respiratory tract and urogentical tract (4). Thus, the sequencing of their genomes could possibly provide further insight into the differences and similarities involved in the factors influencing the metabolism and virulent features of the three <i>N. elongata</i> subspecies. | |||
==Genome structure== | ==Genome structure== | ||
The genome of <i>N. elongata</i> has yet to be sequenced along with the type and number of chromosomes found in the organism. However, comparisons between other bacterial organisms and <i>N. elongata</i> have been made through DNA hybridization studies, where the results of the comparisons between the DNA sequences indicate how closely related or unrelated the two species are to one another. Many case studies have used this method of DNA hybridization between <i>N. elongata</i> and isolated strains from infected patients to identify and determine whether or not the isolated strain belongs to one of the <i>N. elongata</i> subspecies (6). All three <i>N. elongata</i> subspecies have G+C contents ranging from 55-58%, where their G+C contents can also be used in comparison against other possible <i>N. elongata</i> strains isolated from infected patients (4). Currently, the single most reliable technique used to identify <i>N. elongata</i> strains is through the use of 16S rRNA gene sequencing (5). | |||
==Cell structure and metabolism== | ==Cell structure and metabolism== | ||
<i>N. elongata</i> is a nonmotile, aerobic, catalase-negative, asaccharolytic, oxidase-positive, urea-negative, gram-negative coccobacilli with an optimal growth temperature at 35 degrees Celsius (5). Before the isolation and characterization of <i>Neisseria bacilliformis,</i> the second and more recent bacilliary <i>Neisseria</i> species that was isolated from human infections, <i>N. elongata</i> was the only bacillary <i>Neisseria</i> species derived from humans. The rod-like shape of <i>N. elongata</i> makes it unique from the other <i>Neisseria</i> species, which are either cocci or diplococci (6). Thus, it is critical to avoid classifying <i>N. elongata</i> strains based on the diplococcal morphology typically associated with the majority of the <i>Neisseria</i> species. | |||
The classification of <i>N. elongata</i> into three different subspecies, <i>N. elongata</i> subsp. <i>elongata,</i> <i>N. elongata</i> subsp. <i>glycolytica,</i> and <i>N. elongata</i> subsp. <i>nitroreducens,</i> are based on the biochemical differences between each subspecies. <i>N. elongata</i> subsp. <i>nitroreducens</i> are different from <i>N. elongata</i> subsp. <i>elongata</i> and <i>N. elongata</i> subsp. <i>glycolytica</i> in its ability to reduce nitrate (7). <i>N. elongata</i> subsp. <i>glycolytica</i> differs from the other subspecies based on its feature of testing positive for catalase, an enzyme involved in the decomposition of toxic hydrogen peroxide to water and oxygen (5). <i>N. elongata</i> subsp. <i>elongata</i> differs from the other two subspecies due to its inability to produce acid from D-glucose (4). | |||
Being strictly aerobic, <i>N. elongata</i> requires oxygen to oxidize substrates in order to obtain energy. The ability of <i>N. elongata</i> subsp. <i>glycolytica</i> and <i>N. elongata</i> subsp. <i>nitroreducens</i> to produce acid from D-glucose is another method of energy acquisition, as an accumulation of small amounts of acidic intermediates are formed when glucose is broken down (4). | |||
== | ==Ecology and Pathology== | ||
<i>N. elongata</i> is found in the bacterial flora of the human pharynx, respiratory secretions, and blood (3, 8). Cases of <i>N. elongata</i> infections have been reported in the USA, Europe, Canada, Australia, and in the UK (3); the first diagnosis of <i>N. elongata</i> subsp. <i>nitroreducens</i> associated endocarditis was reported in New Zealand in 1978 (7). There has been no extensive research performed on the interactions of <i>N. elongata</i> with other organisms that live in its environment. | |||
Through reports of case studies on patients from whom the <i>N. elongata</i> subspecies were isolated and characterized, it is indicated that <i>N. elongata</i> are capable of producing systemic diseases, such as endocarditis, septicemia, and osteomyelitis (8). <i>N. elongata</i> has only been found in humans, where risk factors for developing <i>N. elongata</i> infections are believed to include dental manipulations and/or a previous history of valvular heart disease, such as endocarditis, valve damage, or rheumatic fever (5). Studies have shown that <i>N. elgonata</i> is fully sensitive to the antibiotics amoxicillin, gentamicin, cefuroxime, and ciprofloxacin, yet having reduced sensitivity to penicillin and trimethoprim. However, endocarditis caused by <i>N. elongata</i> infections typically require early surgical invention as treatment with antibiotics alone may not be sufficient (3). | |||
Although <i>N. elongata</i> infection cases were documented beginning in 1978 (7), the cases of <i>N. elongata</i> infections are uncommon in comparison to other species in the same <i>Neisseria</i> genus, particularly <i>N. gonorrhoeae</i> and <i>N. menigitidis</i>, which have caused between an estimated 500,000 to 1 million cases of septicemia and meningitis each year (9). Thus, there is a lack of extensive research that has been performed on the actual methods in which the bacteria, <i>N. elongata</i>, causes disease in the human host, possibly also due to the fact that its genome has not yet been sequenced. | |||
==Current Research== | ==Current Research== | ||
The majority of research done on <i>N. elongata</i> has been related to its pathogenicity, particularly case reports on the diagnosis of the associated <i>N. elongata</i> diseases of patients suffering from infections of the organism. Other research done on <i>N. elongata</i> involves the use of the organism as a comparison and identification model against bacterial organisms and strains of unknown genus and species. | |||
8.1 <i>Neisseria</i> species with Bacillary Morphology | |||
The genus <i>Neisseria</i> consists of gram-negative cocci or diplococci species, with the exception of <i>N. elongata</i> as the only known species isolated from humans with a bacillary morphology. However, current research on eight strains of another bacillary <i>Neisseria</i> species from human infections were isolated and characterized as <i>Neisseria bacilliformis</i> based on their significant phylogenic and phenotypic differences from the other <i>Neisseria</i> species. 16S rRNA gene sequencing was used as a gene comparison method between the new isolated strains with those of <i>N. elongata</i>, resulting in a match of less than 96%, which provided the strains as a distinct group within the <i>Neisseria</i> genus. Cellular fatty acid analysis of the eight strains yielded results similar to those of other <i>Neisseria</i> species, yet biochemical comparisons of the eight strains against known <i>Neisseria</i> species further supported the classification of the strains as a distinct species, <i>N. bacilliformis</i>, through tests for catalase reaction, nitrate reduction, and oxidase production. Through 16S rRNA gene sequencing, cellular fatty acid profiling, and biochemical tests, distinctions were able to be confidently made between <i>N. bacilliformis</i> and <i>N. elongata</i> (6). | |||
8.2 16S Ribosomal DNA Sequencing of <i>Neisseria elongata </i> subsp. <i> glycolytica</i> | |||
The use of 16S ribosomal DNA sequencing is a reliable technique to identify and confirming the identity of unknown bacterial strains. In this 2003 case report, this identification technique was used to diagnose septicemia from blood cultures isolated from a patient that was undergoing antimitotic chemotherapy. Phenotypic methods used to identify the strain based on just the biochemical properties of <i>N. elongata</i> provided uncertain results, thus 16S ribosomal DNA sequencing was used and identified the strain to be <i>N. elongata</i> subsp. <i>glycolytica</i> with a 99.5% homology between the two strains. The diagnosis of septicemia in this case report was the second case of invasive disease reported that was caused by <i>N. elongata</i> subsp. <i>glycolytica</i>, where the first report involved the diagnosis of endocarditis caused by the organism in 1995 (5). | |||
8.3 <i>Neisseria elongata</i> subsp. <i>elongata,</i> as a cause of human endocarditis | |||
<i>N. elongata</i> subsp. <i>elongata</i> was previously considered nonpathogenic up until 1996, when the first case of human endocarditis caused by the organism was reported. In this 2001 case report, the second case of human endocarditis was reported in a patient diagnosed with a <i>N. elongata</i> subsp. <i>elongata</i> infection. The organism was identified through biochemical tests that agreed with the known biochemical features of <i>N. elongata</i> subsp. <i>elongata</i>, particularly oxidase positive, and catalase, urea, and acid negative. Being the second reported case of this particular <i>N. elongata</i> subspecies, there is still a lack of data regarding the clinical course and severity of endocarditis caused by <i>N. elongata</i> subsp. <i>elongata</i>. Because there were no complications in the patient's infections, whereas the patient in the first reported case had a mycotic aneurysm complication, this case report was able to conclude that <i>N. elongata</i> subsp. <i>elongata</i> is an agent of human endocarditis, where risk factors involved can be due to dental manipulations and/or a history of valvular heart disease (5). | |||
==References== | ==References== | ||
(1) NCBI: Neisseria elongata, Accessed August 26, 2007, <http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=495&lvl=3&lin=f&keep=1&srchmode=1&unlock> | |||
(2) Bovre K, Holten F. "<i>Neisseria elongata</i> sp. nov., a rod-shaped member of the genus <i>Neisseria</i>. Re-evaluation of cell shape as a criterion in classification," "J Gen Microbiol." 1970. Volume 60. p. 67-75. | |||
(3) Haddow L. J., Mulgrew C., Ansari A., Miell J., Jackson G., Malnick H. and Gogal Rav G. “<i>Neisseria elongata</i> endocarditis: case report and literature review,” “European Society of Clinical Microbiology and Infectious Diseases.” 2003. Volume 9. p. 426-430. | |||
(4) Anderson B. M., Weyant R. S., Steigerwalt A. G., Moss C. W., Hollis D. G., Weaver R. E., Ashford D., and Brenner D. J. “Characterization of <i>Neisseria elongata</i> subsp. <i>glycolytica</i> Isolates Obtained from Human Wound Specimens and Blood Cultures,” “Journal of Clinical Microbiology.” 1994. Volume 33. p. 76-78. | |||
(5) Apisarnthanarak A., Dunagan C., and Dunne M. Jr. “<i>Neisseria elongata subsp. elongata,</i> as a cause of human endocarditis,” “Diagnostic Microbiology and Infectious Disease.” 2001. Volume 39. p. 265-266. | |||
(6) Han X. Y., Hong T., and Falsen E. “<i>Neisseria bacilliformis</i> sp. nov. Isolated from Human Infections,” “Journal of Clinical Microbiology.” 2005. Volume 44. p. 474-479. | |||
(7) Grant P. E., Brenner D. J., Steigerwalt A. G., Hollis D. G., and Weaver R. E. “<i>N. elongata</i> subsp. <i>nitroreducens</i> subsp. nov., Formerly CDC Group M-6, a Gram-Negative Bacterium Associated with Endocarditis,” “Journal of Clinical Microbiology.” 1990. p. 2591-2596. | |||
(8) Wong J. D., and Janda M. “Association of an Important <i>Neisseria</i> Species, <i>Neisseria elongata</i> subsp. <i>nitroreducens</i>, with Bacteremia, Endocarditis, and Osteomyelitis,” “Journal of Clinical Microbiology.” 1991. Volume 30. p. 719-720. | |||
(9) Dietrich G., Kurz S., Hubner C., Aepinus C., Theiss S., Guckenberger M., Panzner R., Weber J., and Frosch M. "Transcriptome analysis of <i>Neisseria meningitidis</i> during infection," "Journal of Bacteriology." 2003. Volume 185. p. 155-164. | |||
Edited by Cindy Wu, student of [mailto:ralarsen@ucsd.edu Rachel Larsen] | |||
Edited by | Edited by KLB | ||
Latest revision as of 03:25, 20 August 2010
A Microbial Biorealm page on the genus Neisseria elongata
Classification (1)
Higher order taxa
Bacteria; Proteobacteria; Betaproteobacteria; Neisseriales; Neisseriaceae; Neisseria
Species
|
NCBI: Taxonomy |
Neisseria elongata
Subspecies
N. elongata subsp. elongata, N. elongata subsp. glycolytica, N. elongata subsp. nitroreducens
Description and significance
Neisseria elongata, formerly known as Centers for Disease Control (CDC) group M6, was described by Bovre and Holten in 1970 as a gram-negative, rod-shaped bacterium of the family Neisseriaceae, where it is found in the oral bacterial flora of the human pharynx and throat or in the blood of those infected (2). N. elongata consists of three subspecies, N. elongata subsp. elongata, N. elongata subsp. glycolytica, and N. elongata subsp. nitroreducens, in which are separated based on their biochemical differences (4). Although these subspecies of N. elongata were previously believed to be nonpathogenic to humans, recent case studies from patients suffering from endocarditis, have indicated that all three N. elongata subspecies are associated with human disease, particularly endocarditis and osteomyelitis (3).
Although the N. elongata genome has not yet been sequenced, the importance of sequencing its genome will provide information on the three N. elongata subspecies that could possibly assist in distinguishing their pathogenic roles in endocarditis and osteomyelitis. Before the pathogenic roles of all three N. elongata subspecies were discovered, where N. elongata subsp. nitroreducens was the first subspecies to be discovered as pathogenic, N. elongata subsp. elongata and N. elongata subsp. glycolytica were considered just to be transient colonizers of the human upper respiratory tract and urogentical tract (4). Thus, the sequencing of their genomes could possibly provide further insight into the differences and similarities involved in the factors influencing the metabolism and virulent features of the three N. elongata subspecies.
Genome structure
The genome of N. elongata has yet to be sequenced along with the type and number of chromosomes found in the organism. However, comparisons between other bacterial organisms and N. elongata have been made through DNA hybridization studies, where the results of the comparisons between the DNA sequences indicate how closely related or unrelated the two species are to one another. Many case studies have used this method of DNA hybridization between N. elongata and isolated strains from infected patients to identify and determine whether or not the isolated strain belongs to one of the N. elongata subspecies (6). All three N. elongata subspecies have G+C contents ranging from 55-58%, where their G+C contents can also be used in comparison against other possible N. elongata strains isolated from infected patients (4). Currently, the single most reliable technique used to identify N. elongata strains is through the use of 16S rRNA gene sequencing (5).
Cell structure and metabolism
N. elongata is a nonmotile, aerobic, catalase-negative, asaccharolytic, oxidase-positive, urea-negative, gram-negative coccobacilli with an optimal growth temperature at 35 degrees Celsius (5). Before the isolation and characterization of Neisseria bacilliformis, the second and more recent bacilliary Neisseria species that was isolated from human infections, N. elongata was the only bacillary Neisseria species derived from humans. The rod-like shape of N. elongata makes it unique from the other Neisseria species, which are either cocci or diplococci (6). Thus, it is critical to avoid classifying N. elongata strains based on the diplococcal morphology typically associated with the majority of the Neisseria species.
The classification of N. elongata into three different subspecies, N. elongata subsp. elongata, N. elongata subsp. glycolytica, and N. elongata subsp. nitroreducens, are based on the biochemical differences between each subspecies. N. elongata subsp. nitroreducens are different from N. elongata subsp. elongata and N. elongata subsp. glycolytica in its ability to reduce nitrate (7). N. elongata subsp. glycolytica differs from the other subspecies based on its feature of testing positive for catalase, an enzyme involved in the decomposition of toxic hydrogen peroxide to water and oxygen (5). N. elongata subsp. elongata differs from the other two subspecies due to its inability to produce acid from D-glucose (4).
Being strictly aerobic, N. elongata requires oxygen to oxidize substrates in order to obtain energy. The ability of N. elongata subsp. glycolytica and N. elongata subsp. nitroreducens to produce acid from D-glucose is another method of energy acquisition, as an accumulation of small amounts of acidic intermediates are formed when glucose is broken down (4).
Ecology and Pathology
N. elongata is found in the bacterial flora of the human pharynx, respiratory secretions, and blood (3, 8). Cases of N. elongata infections have been reported in the USA, Europe, Canada, Australia, and in the UK (3); the first diagnosis of N. elongata subsp. nitroreducens associated endocarditis was reported in New Zealand in 1978 (7). There has been no extensive research performed on the interactions of N. elongata with other organisms that live in its environment.
Through reports of case studies on patients from whom the N. elongata subspecies were isolated and characterized, it is indicated that N. elongata are capable of producing systemic diseases, such as endocarditis, septicemia, and osteomyelitis (8). N. elongata has only been found in humans, where risk factors for developing N. elongata infections are believed to include dental manipulations and/or a previous history of valvular heart disease, such as endocarditis, valve damage, or rheumatic fever (5). Studies have shown that N. elgonata is fully sensitive to the antibiotics amoxicillin, gentamicin, cefuroxime, and ciprofloxacin, yet having reduced sensitivity to penicillin and trimethoprim. However, endocarditis caused by N. elongata infections typically require early surgical invention as treatment with antibiotics alone may not be sufficient (3).
Although N. elongata infection cases were documented beginning in 1978 (7), the cases of N. elongata infections are uncommon in comparison to other species in the same Neisseria genus, particularly N. gonorrhoeae and N. menigitidis, which have caused between an estimated 500,000 to 1 million cases of septicemia and meningitis each year (9). Thus, there is a lack of extensive research that has been performed on the actual methods in which the bacteria, N. elongata, causes disease in the human host, possibly also due to the fact that its genome has not yet been sequenced.
Current Research
The majority of research done on N. elongata has been related to its pathogenicity, particularly case reports on the diagnosis of the associated N. elongata diseases of patients suffering from infections of the organism. Other research done on N. elongata involves the use of the organism as a comparison and identification model against bacterial organisms and strains of unknown genus and species.
8.1 Neisseria species with Bacillary Morphology
The genus Neisseria consists of gram-negative cocci or diplococci species, with the exception of N. elongata as the only known species isolated from humans with a bacillary morphology. However, current research on eight strains of another bacillary Neisseria species from human infections were isolated and characterized as Neisseria bacilliformis based on their significant phylogenic and phenotypic differences from the other Neisseria species. 16S rRNA gene sequencing was used as a gene comparison method between the new isolated strains with those of N. elongata, resulting in a match of less than 96%, which provided the strains as a distinct group within the Neisseria genus. Cellular fatty acid analysis of the eight strains yielded results similar to those of other Neisseria species, yet biochemical comparisons of the eight strains against known Neisseria species further supported the classification of the strains as a distinct species, N. bacilliformis, through tests for catalase reaction, nitrate reduction, and oxidase production. Through 16S rRNA gene sequencing, cellular fatty acid profiling, and biochemical tests, distinctions were able to be confidently made between N. bacilliformis and N. elongata (6).
8.2 16S Ribosomal DNA Sequencing of Neisseria elongata subsp. glycolytica
The use of 16S ribosomal DNA sequencing is a reliable technique to identify and confirming the identity of unknown bacterial strains. In this 2003 case report, this identification technique was used to diagnose septicemia from blood cultures isolated from a patient that was undergoing antimitotic chemotherapy. Phenotypic methods used to identify the strain based on just the biochemical properties of N. elongata provided uncertain results, thus 16S ribosomal DNA sequencing was used and identified the strain to be N. elongata subsp. glycolytica with a 99.5% homology between the two strains. The diagnosis of septicemia in this case report was the second case of invasive disease reported that was caused by N. elongata subsp. glycolytica, where the first report involved the diagnosis of endocarditis caused by the organism in 1995 (5).
8.3 Neisseria elongata subsp. elongata, as a cause of human endocarditis
N. elongata subsp. elongata was previously considered nonpathogenic up until 1996, when the first case of human endocarditis caused by the organism was reported. In this 2001 case report, the second case of human endocarditis was reported in a patient diagnosed with a N. elongata subsp. elongata infection. The organism was identified through biochemical tests that agreed with the known biochemical features of N. elongata subsp. elongata, particularly oxidase positive, and catalase, urea, and acid negative. Being the second reported case of this particular N. elongata subspecies, there is still a lack of data regarding the clinical course and severity of endocarditis caused by N. elongata subsp. elongata. Because there were no complications in the patient's infections, whereas the patient in the first reported case had a mycotic aneurysm complication, this case report was able to conclude that N. elongata subsp. elongata is an agent of human endocarditis, where risk factors involved can be due to dental manipulations and/or a history of valvular heart disease (5).
References
(1) NCBI: Neisseria elongata, Accessed August 26, 2007, <http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=495&lvl=3&lin=f&keep=1&srchmode=1&unlock>
(2) Bovre K, Holten F. "Neisseria elongata sp. nov., a rod-shaped member of the genus Neisseria. Re-evaluation of cell shape as a criterion in classification," "J Gen Microbiol." 1970. Volume 60. p. 67-75.
(3) Haddow L. J., Mulgrew C., Ansari A., Miell J., Jackson G., Malnick H. and Gogal Rav G. “Neisseria elongata endocarditis: case report and literature review,” “European Society of Clinical Microbiology and Infectious Diseases.” 2003. Volume 9. p. 426-430.
(4) Anderson B. M., Weyant R. S., Steigerwalt A. G., Moss C. W., Hollis D. G., Weaver R. E., Ashford D., and Brenner D. J. “Characterization of Neisseria elongata subsp. glycolytica Isolates Obtained from Human Wound Specimens and Blood Cultures,” “Journal of Clinical Microbiology.” 1994. Volume 33. p. 76-78.
(5) Apisarnthanarak A., Dunagan C., and Dunne M. Jr. “Neisseria elongata subsp. elongata, as a cause of human endocarditis,” “Diagnostic Microbiology and Infectious Disease.” 2001. Volume 39. p. 265-266.
(6) Han X. Y., Hong T., and Falsen E. “Neisseria bacilliformis sp. nov. Isolated from Human Infections,” “Journal of Clinical Microbiology.” 2005. Volume 44. p. 474-479.
(7) Grant P. E., Brenner D. J., Steigerwalt A. G., Hollis D. G., and Weaver R. E. “N. elongata subsp. nitroreducens subsp. nov., Formerly CDC Group M-6, a Gram-Negative Bacterium Associated with Endocarditis,” “Journal of Clinical Microbiology.” 1990. p. 2591-2596.
(8) Wong J. D., and Janda M. “Association of an Important Neisseria Species, Neisseria elongata subsp. nitroreducens, with Bacteremia, Endocarditis, and Osteomyelitis,” “Journal of Clinical Microbiology.” 1991. Volume 30. p. 719-720.
(9) Dietrich G., Kurz S., Hubner C., Aepinus C., Theiss S., Guckenberger M., Panzner R., Weber J., and Frosch M. "Transcriptome analysis of Neisseria meningitidis during infection," "Journal of Bacteriology." 2003. Volume 185. p. 155-164.
Edited by Cindy Wu, student of Rachel Larsen
Edited by KLB