Nitrospira: Difference between revisions
No edit summary |
No edit summary |
||
| (10 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{ | {{Curated}} | ||
{{Biorealm Genus}} | |||
[[Image:nsnb2.jpg|thumb|400px|right|''Nitrospira.'' Courtesy of [http://www.ci.englewood.co.us/ Englewood.]]] | [[Image:nsnb2.jpg|thumb|400px|right|''Nitrospira.'' Courtesy of [http://www.ci.englewood.co.us/ Englewood.]]] | ||
| Line 14: | Line 13: | ||
''Nitrospira marina, N. moscoviensis, N. sp.'' | ''Nitrospira marina, N. moscoviensis, N. sp.'' | ||
{| | |||
| height="10" bgcolor="#FFDF95" | | |||
'''NCBI: [http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=1234 Taxonomy] Genome ''' | |||
|} | |||
==Description and Significance== | ==Description and Significance== | ||
| Line 24: | Line 28: | ||
==Cell Structure and Metabolism== | ==Cell Structure and Metabolism== | ||
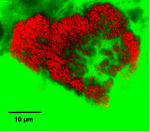
[[Image:nitrospira_aggregate.JPG|thumb|150px|left|Cell aggregate of Nitrospira-like bacteria detected by fluorescence in situ hybridization (FISH) in biofilm from a wastewater treatment plant. Source: Daims et al., Appl. Environ. Microbiol. 67(11):5273-5284]] | |||
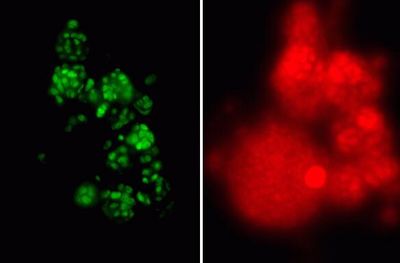
[[Image:Nitro_agg2.JPG|thumb|150px|right| Uptake of pyruvate, detected by FISH-microautoradiography, by ammonia-oxidizing bacteria (green) and Nitrospira-like bacteria (red) in biofilm. Black spots indicate uptake of radioactively labeled pyruvate. Source: Daims et al., Appl. Environ. Microbiol. 67(11):5273-5284]] | |||
''Nitrospira'' is a chemolithoautotrophic nitrite-oxidizing bacterium that is generally found in freshwater or saltwater. ''Nitrospira''-like bacteria take up inorganic carbon (like HCO<sub>3</sub><sup>-</sup> and CO<sub>2</sub>) as well as pyruvate under aerobic conditions. They can not use acetate, butyrate, or propionate, however. ''Nitrospira'' have doubling time of every 12 - 32 hours. | ''Nitrospira'' is a chemolithoautotrophic nitrite-oxidizing bacterium that is generally found in freshwater or saltwater. ''Nitrospira''-like bacteria take up inorganic carbon (like HCO<sub>3</sub><sup>-</sup> and CO<sub>2</sub>) as well as pyruvate under aerobic conditions. They can not use acetate, butyrate, or propionate, however. ''Nitrospira'' have doubling time of every 12 - 32 hours. | ||
==Ecology== | ==Ecology== | ||
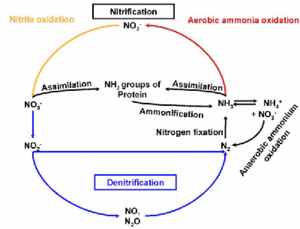
[[Image:nitrogen_cycle.gif|thumb|300px|right|The biogeochemical nitrogen cycle. Source: Dept. of Microbial Ecology, University of Vienna]] | |||
''Nitrospira'' can live in marine or nonmarine habitats. It has been isolated from ocean water, freshwater, aquarium water, deltaic sediment, deep-sea sediments, soils, and an iron pipe of a heating system (Daims ''et al.'' 2001). ''Nitrospira'' is part of a nitrification process which is important in the biogeochemical nitrogen cycle. Nitrification is the oxidation of ammonia to nitrite by autotrophic bacteria of the genus ''Nitrosomonas'' and oxidation of the nitrite to nitrate by bacteria in the genus ''Nitrobacter'' or ''Nitrospira''. This is important in marine environments because too much ammonia or nitrite can cause death in fish. However, ''Nitrospira'' and similar bacteria are slow-growing organisms, which means that a newly set-up aquarium without an established population of these bacteria can accumulate toxic concentrations of ammonia and nitrite. In an attempt to fix this problem, commercial companies have tried to market special preparations of ammonia-oxidizing and nitrite-oxidizing bacteria (the mixes included ''Nitrobacter'' instead of ''Nitrospira'') that could be put into a new aquarium to establish a healthy nitrogen cycle. However, these mixes were inexplicably ineffective so tests were done to analyze the bacterial content of aquaria water. While bacteria from the genus ''Nitrobacter'' are nitrite-oxidizing organisms and could theoretically fill the nitrite-oxidizing niche, the tests indicated relatively high numbers of ''Nitrospira'' and no ''Nitrobacter ''bacteria at all. Thus, ''Nitrospira'' is now considered the dominate nitrite-oxidizing bacterium in aquariums, (as well as in wastewater treatment systems and other reactors as shown by other similar studies) (Hovanec ''et al''.1998). Though water that is too rich in ammonia or has a pH that is too low will inhibit ''Nitrospira'''s nitrifying activity. | ''Nitrospira'' can live in marine or nonmarine habitats. It has been isolated from ocean water, freshwater, aquarium water, deltaic sediment, deep-sea sediments, soils, and an iron pipe of a heating system (Daims ''et al.'' 2001). ''Nitrospira'' is part of a nitrification process which is important in the biogeochemical nitrogen cycle. Nitrification is the oxidation of ammonia to nitrite by autotrophic bacteria of the genus ''Nitrosomonas'' and oxidation of the nitrite to nitrate by bacteria in the genus ''Nitrobacter'' or ''Nitrospira''. This is important in marine environments because too much ammonia or nitrite can cause death in fish. However, ''Nitrospira'' and similar bacteria are slow-growing organisms, which means that a newly set-up aquarium without an established population of these bacteria can accumulate toxic concentrations of ammonia and nitrite. In an attempt to fix this problem, commercial companies have tried to market special preparations of ammonia-oxidizing and nitrite-oxidizing bacteria (the mixes included ''Nitrobacter'' instead of ''Nitrospira'') that could be put into a new aquarium to establish a healthy nitrogen cycle. However, these mixes were inexplicably ineffective so tests were done to analyze the bacterial content of aquaria water. While bacteria from the genus ''Nitrobacter'' are nitrite-oxidizing organisms and could theoretically fill the nitrite-oxidizing niche, the tests indicated relatively high numbers of ''Nitrospira'' and no ''Nitrobacter ''bacteria at all. Thus, ''Nitrospira'' is now considered the dominate nitrite-oxidizing bacterium in aquariums, (as well as in wastewater treatment systems and other reactors as shown by other similar studies) (Hovanec ''et al''.1998). Though water that is too rich in ammonia or has a pH that is too low will inhibit ''Nitrospira'''s nitrifying activity. | ||
==References== | ==References== | ||
Latest revision as of 20:34, 6 August 2010
A Microbial Biorealm page on the genus Nitrospira
Classification
Higher order taxa:
Bacteria; Nitrospirae; Nitrospira (class); Nitrospirales; Nitrospiraceae; Nitrospira
Species:
Nitrospira marina, N. moscoviensis, N. sp.
|
NCBI: Taxonomy Genome |
Description and Significance
Nitrospira are nitrite-oxidizing bacteria that are important in marine habitats. In aquariums, for example, if the ammonia/nitrite/nitrate cycle is thrown off, the ecosystems suffers and fish can get sick or die. Therefore, nitrite-oxidizing bacteria as well as the other bacteria in this system are important for healthy marine ecosystems. In addition, Nitrospira-like bacteria are the main nitrite oxidizers in wastewater treatment plants and in laboratory scale reactors, not Nitrobacter sp., as was previously thought.
Genome Structure
Projects to sequence the genome of Nitrospira-like organisms are being undertaken by organizations such as the University of Vienna: Institute of Ecology and Conservation Biology, in order to analyze the nitrite-oxidizing mechanisms and the Nitrospira ability to compete with other nitrite-oxidizing bacteria. Also starting on the genome of Nitrospira marina Nb-295 is the The Gordon and Betty Moore Foundation Marine Microbiology Initiative, and J. Craig Venter Institute.
Cell Structure and Metabolism
Nitrospira is a chemolithoautotrophic nitrite-oxidizing bacterium that is generally found in freshwater or saltwater. Nitrospira-like bacteria take up inorganic carbon (like HCO3- and CO2) as well as pyruvate under aerobic conditions. They can not use acetate, butyrate, or propionate, however. Nitrospira have doubling time of every 12 - 32 hours.
Ecology
Nitrospira can live in marine or nonmarine habitats. It has been isolated from ocean water, freshwater, aquarium water, deltaic sediment, deep-sea sediments, soils, and an iron pipe of a heating system (Daims et al. 2001). Nitrospira is part of a nitrification process which is important in the biogeochemical nitrogen cycle. Nitrification is the oxidation of ammonia to nitrite by autotrophic bacteria of the genus Nitrosomonas and oxidation of the nitrite to nitrate by bacteria in the genus Nitrobacter or Nitrospira. This is important in marine environments because too much ammonia or nitrite can cause death in fish. However, Nitrospira and similar bacteria are slow-growing organisms, which means that a newly set-up aquarium without an established population of these bacteria can accumulate toxic concentrations of ammonia and nitrite. In an attempt to fix this problem, commercial companies have tried to market special preparations of ammonia-oxidizing and nitrite-oxidizing bacteria (the mixes included Nitrobacter instead of Nitrospira) that could be put into a new aquarium to establish a healthy nitrogen cycle. However, these mixes were inexplicably ineffective so tests were done to analyze the bacterial content of aquaria water. While bacteria from the genus Nitrobacter are nitrite-oxidizing organisms and could theoretically fill the nitrite-oxidizing niche, the tests indicated relatively high numbers of Nitrospira and no Nitrobacter bacteria at all. Thus, Nitrospira is now considered the dominate nitrite-oxidizing bacterium in aquariums, (as well as in wastewater treatment systems and other reactors as shown by other similar studies) (Hovanec et al.1998). Though water that is too rich in ammonia or has a pH that is too low will inhibit Nitrospira's nitrifying activity.
References
University of Vienna: Institute of Ecology and Conservation Biology.