Rhabdoviridae: Difference between revisions
No edit summary |
No edit summary |
||
| (11 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
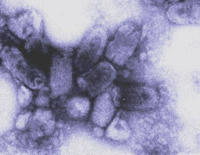
[[Image:Trimp6.gif|thumb|200px|right|A negatively stained rabies virus as seen under a transmission electron microscope. | {{Curated}} | ||
{{Viral Biorealm Family}} | |||
[[Image:Trimp6.gif|thumb|200px|right|A negatively stained rabies virus as seen under a transmission electron microscope. Image Courtesy [http://www.wadsworth.org/databank/rabies.htm Wadsworth Center, New York State Department of Health].]] | |||
==Baltimore Classification== | ==Baltimore Classification== | ||
| Line 5: | Line 8: | ||
===Higher order taxa=== | ===Higher order taxa=== | ||
Virus; Rhabdoviridae | Virus; ssRNA negative-strand viruses; Mononegavirales; Rhabdoviridae | ||
=== | ===Genera=== | ||
''Cytorhabdovirus'', ''Vesiculovirus'', ''Lyssavirus'', ''Ephemerovirus'', ''Novirhabdovirus'', ''Nucleorhabdovirus'' | |||
==Description and Significance== | ==Description and Significance== | ||
Rabies is a fatal infection of the central nervous system acquired through the bite of a rabid animal. If left untreated, this infection has a 100% fatality rate, and it has a global distribution that claims around 60,000 human lives each year, which is why rabies is one of the most dreaded and significant diseases. Infection is prevented in case of humans by the injection of rabies immunoglobulin followed by a series of injections with rabies vaccine. The cost of rabies control in humans exceeds millions of dollars annually. A large reservoir of rabies also exists in racoons, skunks, bats, foxes and other wild animals. | Rabies is a fatal infection of the central nervous system acquired through the bite of a rabid animal. If left untreated, this infection has a 100 % fatality rate, and it has a global distribution that claims around 60,000 human lives each year, which is why rabies is one of the most dreaded and significant diseases. Infection is prevented in case of humans by the injection of rabies immunoglobulin followed by a series of injections with rabies vaccine. The cost of rabies control in humans exceeds millions of dollars annually. A large reservoir of rabies also exists in racoons, skunks, bats, foxes and other wild animals. | ||
Rabies is a major health problem in developing countrues and has emerged again in the USA due to bat rabies virus variants. | |||
==Genome Structure== | ==Genome Structure== | ||
| Line 33: | Line 38: | ||
==Viral Ecology & Pathology== | ==Viral Ecology & Pathology== | ||
Normal hosts for the rabies virus are foxes, dogs, cats, bats, skunks, etc. while man is a dead-end infection for the virus. The pattern is endemic rather than epidemic and cattle rabies is a serious economic disease in these areas. Rabies virus enters the body by wound or abrasion of skin directly into bloodstream. Primary replication occurs locally in the muscle and connective tissue but there are no symptoms. The virus eventually infects peripheral nerves, then travels along neuronal axons to CNS, where it produces severe and fatal encephalitis. Only very few cases escape these severe consequences. Viraemia and haematogenous spread of the virus to CNS has not been shown yet. Ther incubation period varies from 3-8 weeks to a year, depending on the sire and size of inoculation. | Normal hosts for the rabies virus are foxes, dogs, cats, bats, skunks, etc. while man is a dead-end infection for the virus. The pattern is endemic rather than epidemic and cattle rabies is a serious economic disease in these areas. Rabies virus enters the body by wound or abrasion of skin directly into bloodstream. Humans become infected with the rabies virus either by a bite from a rabid animal or by mucosal exposure. Primary replication occurs locally in the muscle and connective tissue but there are no symptoms. The virus eventually infects peripheral nerves, then travels along neuronal axons to CNS, where it produces severe and fatal encephalitis. Only very few cases escape these severe consequences. Viraemia and haematogenous spread of the virus to CNS has not been shown yet. Ther incubation period varies from 3-8 weeks to a year, depending on the sire and size of inoculation. | ||
The presence of an arginine or a lysine residue at the position 333 of the glycoprotein is responsible for the pathogenicity of rabies virus. Avirulent rabies virus mutants do not contain these amino-acid residues at this position and two genotypes of lyssavirus, Mokola and Lagos bat, which do not carry either arginine or lysine at position 333 of the glycoprotein are not pathogenic in mice after intramuscular injection. | The presence of an arginine or a lysine residue at the position 333 of the glycoprotein is responsible for the pathogenicity of rabies virus. Avirulent rabies virus mutants do not contain these amino-acid residues at this position and two genotypes of lyssavirus, Mokola and Lagos bat, which do not carry either arginine or lysine at position 333 of the glycoprotein are not pathogenic in mice after intramuscular injection. | ||
==References | ==References== | ||
[http://www.wadsworth.org/databank/rabies.htm New York State Department of Health] | [http://www.wadsworth.org/databank/rabies.htm New York State Department of Health] | ||
| Line 43: | Line 48: | ||
[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=genome&cmd=Retrieve&dopt=Overview&list_uids=10362 National Center for Biotechnology Information] | [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=genome&cmd=Retrieve&dopt=Overview&list_uids=10362 National Center for Biotechnology Information] | ||
Zhen Fang Fu; "Rabies and rabies research: past, present and future"; <u>Vaccine</u>; 1997 Volume 15 Suppl | |||
Latest revision as of 00:33, 8 August 2010
A Viral Biorealm page on the family Rhabdoviridae
Baltimore Classification
Higher order taxa
Virus; ssRNA negative-strand viruses; Mononegavirales; Rhabdoviridae
Genera
Cytorhabdovirus, Vesiculovirus, Lyssavirus, Ephemerovirus, Novirhabdovirus, Nucleorhabdovirus
Description and Significance
Rabies is a fatal infection of the central nervous system acquired through the bite of a rabid animal. If left untreated, this infection has a 100 % fatality rate, and it has a global distribution that claims around 60,000 human lives each year, which is why rabies is one of the most dreaded and significant diseases. Infection is prevented in case of humans by the injection of rabies immunoglobulin followed by a series of injections with rabies vaccine. The cost of rabies control in humans exceeds millions of dollars annually. A large reservoir of rabies also exists in racoons, skunks, bats, foxes and other wild animals.
Rabies is a major health problem in developing countrues and has emerged again in the USA due to bat rabies virus variants.
Genome Structure
The entire rabies genome has now been cloned, sequenced and expressed. There is a conserved polyadenylation signal at the end of each gene and a short intergenic region between each of the 5 genes.
Virion Structure of a Rabies virus
The particles have a unique bullet-shaped appearance, and are all rather similar. They are enveloped with prominent spikes on the surface, G protein- Haemagglutinates RCS, but are not variable in surface. Lined by the matrix protein, the envelope contains the nucleocapsid wound helically inside the core. Two non-structural proteins, L and NS, are associated with the nucleocapsid and act in concert as the viral polymerase. The particle is relatively labile, as with most enveloped viruses.
Reproductive Cycle of a Rabies virus in a Host Cell
When the rabis virus enters the host, the G protein spikes bind to recptors on the surface of host cells and the viruses enter the cell by endocytosis and fusion with the membrane of the vesicle, mediated by the G protein.
The receptor molecules for rabies virus are not known but are believes to be phospholipids rather than specific proteins. Both the L and NS proteins are necessary for transcription-- neither can function alone-- and replication occurs in the cytoplasm. Five monocistronic mRNAs are produced, capped at the 5' end and polyadenylated at the 3' end and each containing the leader sequence from the 3' end of the vRNA at the 5' end message. These mRNAs are made by sequential transcription of the ORFs in the virus genome and it has been shown that the intergenic sequence is responsible for both termination and re-initiation of transcription by the polymerase between each gene, thus producing separate transcripts.
The step is not well understood but it is known that progeny vRNA is made from a (+)sense intermediate. As in transcription, the genome is replicated by the L + P polymerase, but additional host cell factors are also required. These host cell factors are not yet known. These events all occur in a portion of the cytoplasm which acts as a virus 'factory' and appears as a charecteristic cytoplasmic inclusion body- perinuclear Negri bodies. The virions are assembled around the tightly coiled nucleoprotein core, and bud from cytoplasmic membranes and the outer membrane of the cell, acquiring the M + G proteins as they do so.
Viral Ecology & Pathology
Normal hosts for the rabies virus are foxes, dogs, cats, bats, skunks, etc. while man is a dead-end infection for the virus. The pattern is endemic rather than epidemic and cattle rabies is a serious economic disease in these areas. Rabies virus enters the body by wound or abrasion of skin directly into bloodstream. Humans become infected with the rabies virus either by a bite from a rabid animal or by mucosal exposure. Primary replication occurs locally in the muscle and connective tissue but there are no symptoms. The virus eventually infects peripheral nerves, then travels along neuronal axons to CNS, where it produces severe and fatal encephalitis. Only very few cases escape these severe consequences. Viraemia and haematogenous spread of the virus to CNS has not been shown yet. Ther incubation period varies from 3-8 weeks to a year, depending on the sire and size of inoculation.
The presence of an arginine or a lysine residue at the position 333 of the glycoprotein is responsible for the pathogenicity of rabies virus. Avirulent rabies virus mutants do not contain these amino-acid residues at this position and two genotypes of lyssavirus, Mokola and Lagos bat, which do not carry either arginine or lysine at position 333 of the glycoprotein are not pathogenic in mice after intramuscular injection.
References
New York State Department of Health
National Center for Biotechnology Information
Zhen Fang Fu; "Rabies and rabies research: past, present and future"; Vaccine; 1997 Volume 15 Suppl
