Aquarium Niche: Difference between revisions
No edit summary |
|||
| (239 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | |||
==Introduction to Aquarium== | ==Introduction to Aquarium== | ||
[[Image: | [[Image:SW_aquarium.jpg|thumb|Aquarium|300px|right| Saltwater aquarium: Hosts a variety of unique aquatic species. Picture taken from National Oceanic and Atmospheric Administration (NOAA). [http://usasearch.gov/search?v%3aproject=firstgov-noaa-images&v%3afile=viv_1017%4028%3aUPJbp5&v%3aframe=viewimage&v%3astate=%28root%29%7croot&id=Ndoc8&rpaid=& (1)]]] | ||
An '''aquarium''' is an artificial ecosystem that is inhabitable by various aquatic species. The environment of the aquarium can differ depending on the residing species. For example, ''Xiphophorus helleri'' or '''Green Swordtail''' prefer to live in freshwater, while ''Paracanthurus hepatus'' or '''Surgeonfish''' prefer water with higher salt concentration [2]. Nothing quite adds to the room décor as adding an aquarium with beautifully colored fishes with lively aquatic plants. However, what keeps these colorful organisms alive and healthy are none other than the series of '''micro-organisms''', also known as '''microbes'''. | An '''aquarium''' (plural form is '''aquaria''' or aquariums) is an artificial ecosystem that is inhabitable by various aquatic species. The environment of the aquarium can differ depending on the residing species. For example, ''Xiphophorus helleri'' or '''Green Swordtail''' prefer to live in freshwater, while ''Paracanthurus hepatus'' or '''Surgeonfish''' prefer water with higher salt concentration [2]. Nothing quite adds to the room décor as adding an aquarium with beautifully colored fishes with lively aquatic plants. However, what keeps these colorful organisms alive and healthy are none other than the series of '''micro-organisms''', also known as '''microbes'''. However, aquariums are built as an “ideal” ecosystem; hence, filtering out most of the microbes that can be found in natural body of water. For example, ultraviolet sterilization is used in aquariums to destroy algae and nucleic material of many free-floating micropathogens [8]. Furthermore, most of the work done by natural microbes is conducted by machineries or by chemical substances sold in supermarkets. Therefore, it is in the best interest for '''aquarist''' (people who maintain aquariums) to restrict the number of microbes in the aquaria to avoid potentially harming the inhabitants. | ||
=== | What kind of microbes can survive such controlled environment? Do aquariums need microbes? What happens if bacteria are introduced into the aquariums? The focus of the topic is to discuss the microbes that can survive in the aquarium, and how they affect the niche. In addition, the topic covers the different type of aquariums and its conditions, and the non-microbes that inhabit the aquaria ecosystem. | ||
Aquarist must maintain specific pH, temperature levels, and lighting for different species in the aquarium. '''Salinity''', the concentration of salt in a given amount of water, becomes one of the characteristics to distinguish between the different types of aquariums. Salinity can be measured using a hydrometer, which compares the specific gravity of sample with pure water under the units of ppt (parts per thousand) or ppm (parts per million) [2]. In addition, the aquarium's hardness must be monitored. The levels of dissolved minerals, such as bicarbonates, in water | |||
===Maintaining Aquaria Niche=== | |||
Aquarist must maintain specific pH, temperature levels, and lighting for different species in the aquarium. '''Salinity''', the concentration of salt in a given amount of water, becomes one of the characteristics to distinguish between the different types of aquariums. Salinity can be measured using a hydrometer, which compares the specific gravity of sample with pure water under the units of ppt (parts per thousand) or ppm (parts per million) [2]. In addition, the aquarium's hardness must be monitored. The levels of dissolved minerals, such as bicarbonates, in water are described as the water's '''hardness''' [2]. | |||
==Types of Aquarium Niche== | ==Types of Aquarium Niche== | ||
Commercial aquariums come in variety of shapes and sizes, from tiny one gallon fish bowls to luxurious 100 gallon fish tanks, and normally can be set simply on a desk top or on top of a special aquarium stand. There are generally four types of aquariums a new aquarist can set up: | Commercial aquariums come in variety of shapes and sizes, from tiny one gallon fish bowls to luxurious 100 gallon fish tanks, and normally can be set simply on a desk top or on top of a special aquarium stand. There are generally four types of aquariums a new aquarist can set up: freshwater, brackish, and saltwater. Each type of aquarium requires specific care and maintenance, and different types of fish reside in each of the designed ecosystem. | ||
=== | ===Freshwater Aquarium=== | ||
In cold freshwater aquariums for fish such as goldfish | [[Image:Fresh.jpg|thumb|Aquarium|200px|left| Freshwater in Main where the Atlantic salmon can be spotted. Picture taken from NOAA. [http://www.nefsc.noaa.gov/salmon/finalcriticalhabitat.html (3)]]] | ||
'''Freshwater aquarium''' can be divided into tropical and cold freshwater. In '''tropical freshwater aquariums''', for fish such as ''Paracheirodon innesi'', ''Betta splendens'', and ''Belontia hasselti'', just to name a few, the temperature must be kept at 72-80°F (22-27°C) [8]. The pH must be kept between 6 and 8, the stability and the consistency of the pH also playing an important role in ensuring the health of the fish [8]. Since freshwater fish normally cannot tolerate any salinity, salinity must be kept under 3ppm (parts per million), preferrably at 0 [8]. In '''cold freshwater aquariums''', for fish such as ''Cyprinus carpio'' (koi) and ''Carassius auratus'' (goldfish), the temperature should be kept typically below 70°F (20°C). Thus, coldwater aquariums do not require heaters. The light should be kept at the standard level and the hardness of the water at 100-200mg/L CaCO3 [8]. | |||
===Brackish Water Aquarium=== | ===Brackish Water Aquarium=== | ||
[[Image: | [[Image:Brack.jpg|thumb|Aquarium|200px|right| Brackish water in Weeks Bay, Alabama. Picture taken from NOAA. [http://www.photolib.noaa.gov/htmls/nerr0200.htm (4)]]] | ||
'''Brackish water aquarium''' is an artificial ecosystem that simulates the natural environment of '''brackish water''', which has a salt concentration between freshwater and saltwater. Brackish water aquarium is kept at a temperature around 23-29°C, with a pH between 7 to 8, a hardness of 200 mg/liter of CaCO3, a specific gravity of 1.003-1.012, and a salinity between 7.6 and 14 ppt [2]. | '''Brackish water aquarium''' is an artificial ecosystem that simulates the natural environment of '''brackish water''', which has a salt concentration between freshwater and saltwater. Brackish water aquarium is kept at a temperature around 23-29°C, with a pH between 7 to 8, a hardness of 200 mg/liter of CaCO3, a specific gravity of 1.003-1.012, and a salinity between 7.6 and 14 ppt [2]. The lighting level should be high since most brackish water environments are exposed to the sun. Brackwish water can be found in Central American costal streams, East Africa mangrove swamps, and Southeast Asia estuaries [2]. A large variety of species inhabit the brackish water, such as ''Sailfin'', ''Monos'', and ''Cichlids'' [25]. | ||
===Saltwater Aquarium=== | |||
[[Image:Salt.jpg|thumb|Aquarium|200px|left| Saltwater Aquarium: Inhabiting fishes and coral reefs. Picture taken from NOAA. [http://www.magazine.noaa.gov/stories/mag25.htm (10)]]] | |||
'''Saltwater''' or '''Marine aquarium''' hosts a variety of colorful and exotic species, such as ''Premnas biaculeatus'' or Maroon Clownfish [11]. The temperature should be kept at 22-26°C (72-78°F), pH between 8.1 and 8.3, specific gravity of 1.020-1.022, and salinity between 27.2 and 33.7ppt [2]. Saltwater aquariums are difficult to maintain because salinity levels can fluctuate [11]. Most marine species live in the ocean where salinity levels are kept constant; thus, they cannot tolerate fluctuating levels of salt concentration in the waters [11]. Another division of marine aquarium is the '''Reef aquaria'''. This aquarium recreates the environment for the many fantastic reefs in the marine ecosystem. Temperature should be maintained at 23-25°C (72-78°F), pH of 8.1-8.3, specific gravity of 1.022-1.024, and salinity of 27.2-33.7ppt [2]. Inhabitants include ''Goniopora'' or Daisy Coral, and ''Gorgonia spp.'' or Sea Fan [2]. | |||
==Conditions under which the environment changes== | |||
There are various factors that come into play that could potentially harm the organisms that are living in the aquarium. | |||
First, an aquarist must make sure to dechlorinate the water before changing the water of the aquarium. This is because chlorine is present in tap water, which breaks down to hypochlorous acid (HOCl) and hypochlorite ion (OCl-) [11]. These two molecules kill beneficial microorganisms by first attacking the lipids in their peptidoglycan cell walls. They will also attack the enzymes and other structural proteins that are necessary for the microbes' health. | |||
Second, a great care must be taken when doing water changes and cleaning the tanks in order to not destroy the beneficial bacterial colonies that are present. It takes one month for ''[[Nitrosomonas]]'' and two months for ''[[Nitrobacter]]'' to develop into a mature colony in the aquarium [11]. Once these stable colonies are disturbed, the aquarium must cycle again before the environment is optimal for the health of the fish. | |||
Third, the location of the aquarium must also be chosen carefully not to allow drastic changes of the temperature of the water. The places that must be avoided include near the windows, vents, and radiators. | |||
==Microbes that Inhabit the Aquarium== | ==Microbes that Inhabit the Aquarium== | ||
Public aquariums can host large varieties of aquatic species in a closed and “ideal” ecosystem. However, there are numbers of microorganisms in nature that are not present in aquariums despite their profound importance to global marine ecology [5]. This is mainly due to the difficulty in creating a habitat for the microorganisms, and, at the same time, maintaining a suitable environment for the aquatic species [5]. In the aquarium, bacteria are mostly responsible for breakdown waste [6]. The most frequent process is the breakdown of protein into ammonia, then nitrite into nitrate [6]. This process of waste disposable is known as the '''Nitrogen Cycle'''. | Public aquariums can host large varieties of aquatic species in a closed and “ideal” ecosystem. However, there are numbers of microorganisms in nature that are not present in aquariums despite their profound importance to global marine ecology [5]. This is mainly due to the difficulty in creating a habitat for the microorganisms, and, at the same time, maintaining a suitable environment for the aquatic species [5]. In the aquarium, bacteria are mostly responsible for breakdown waste [6]. The most frequent process is the breakdown of protein into ammonia, then nitrite into nitrate [6]. This process of waste disposable is known as the '''Nitrogen Cycle''', which uses ''Nitrosomonas'' and ''Nitrobacter''. These two bacteria enter the aquaria on the surface of inhabitants, such as fishes, or can be bought in bottles, such as Turbo Start 700 (freshwater) and 900 (brackish and marine water) [26]. | ||
===Microbes Involved In Nitrogen Cycle=== | ===Microbes Involved In Nitrogen Cycle=== | ||
The waste excreted by the fish in the aquarium must always somehow be kept low at a non-toxic level. The cycle that exists in aquariums and natural aquatic environments alike is the nitrogen cycle. Since an aquarium is a fragile ecosystem on average containing only 10 gallons of water, it requires the aquarist’s interference in ensuring that the cycle is well established. In so doing, one must create this artificial ecosystem as close to the natural environment as possible. Only when all the players of the nitrogen cycle have successfully been established can more fish be added with a minimal loss. | The waste excreted by the fish in the aquarium must always somehow be kept low at a non-toxic level. The cycle that exists in aquariums and natural aquatic environments alike is the nitrogen cycle. Since an aquarium is a fragile ecosystem on average containing only 10 gallons of water, it requires the aquarist’s interference in ensuring that the cycle is well established. In so doing, one must create this artificial ecosystem as close to the natural environment as possible. Only when all the players of the nitrogen cycle have successfully been established can more fish be added with a minimal loss. | ||
''' | '''Nitrosomonas and Nitrobacter''' <br> | ||
''Nitrosomonas | [[Image:Nitrosomonas-Photo_by_Stan_Watson,_Woods_Hole_Oceanographic_Institute.jpg|thumb|Aquarium|200px|right| Picture of [[Nitrosomonas europaea]], a gram-negative bacteria that can oxidize ammonia [27]. Picture taken from microbewiki site.]] | ||
''[[Nitrosomonas europaea]]'' is a Gram-negative chemolithoautotroph that can derive all its energy needed for growth by the oxidation of ammonia. [14] The key enzyme used in order to oxidize the ammonia is ammonia monooxygenase, whose subunits are coded by ''amoA, amoB,'' and ''amoC'' genes. [17] They colonize in other environments such as soil, sewage, and walls of buildings. ''Nitrobacter'', an alphaproteobacterium, is a gram-negative facultative chemolithoautotroph. [12] Both of the nitrifying bacteria require specific environment for their maximum activity, all of which should be met by the environments the aquarist sets up for the fish in the aquarium. Because these organisms cannot form spores, the aquarium must be kept at the optimum condition at all times for the biological filtration of these nitrifying bacteria to function. They also form slimy biofilms in order to protect themselves from desiccation and other potential threats. | |||
The ideal pH for these beneficial bacteria is between 7.2 and 8.5, having a narrower range than tolerated by fish. | The ideal pH for these beneficial bacteria is between 7.2 and 8.5, having a narrower range than tolerated by fish. [13] Also, nitrifying bacteria are aerobic organisms and oxygen is critical for their nitrifying activity. As the oxygen level decreases beyond 1mg/L, dissolved oxygen becomes the limiting factor of the nitrification reactions. Thus, the oxygen level in the water must be at least 2mg/L in order for it to not have an adverse effect on the nitrifying activity. [13] At suboptimal levels of pH(4.5, 5.5, 6.5) and at suboptimal levels of oxygen (10 - 100 microM), nitrification was severely reduced. [13] However, when the nitrifying activities of intact biofilms (IB) and dispersed biofilms (DB) were compared, bacteria of IB were less affected by pH and oxygen level changes. [13] Thus, the nitrifying bacteria form biofilms in order to protect themselves from potentially detrimental environmental changes. | ||
Also, the nitrifying bacteria are most active between the temperatures of 68 - 86 °F; 50°F is the minimum temperature and 95°F is the maximum temperature for these nitrifying bacteria. [11] Furthermore, nitrifying bacteria are light sensitive, especially to UV light contained in the sun light. Room light can also have an adverse effect on the bacteria. [14] Thus, nitrifying bacteria tend to form colonies within the filteration system and beneath the gravel to avoid exposure to light. Finally, nitrifying bacteria can tolerate a wide range of salinity. Thus, a beneficial bacterial colonies established in a freshwater environment can then be used for aquariums in a saltwater environment. [11] This transition, though, must be carried out in a gradual manner for it to be effective. [11] Maximum change of 5ppm should not affect the activity of the nitrifying bacteria. [11] Thus ''[[Nitrosomonas]]'' and ''[[Nitrobacter]]'' are both seen in all of the four general types of aquariums mentioned in the previous section. | |||
===The '' | ===The ''Nitrosomona'' and ''Nitrobacter'' Interaction and in the Environment=== | ||
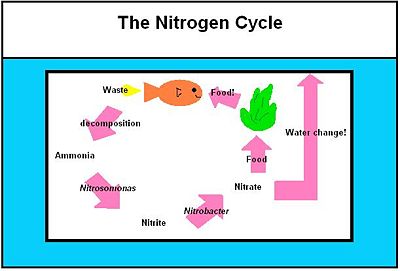
The first step in the nitrogen cycle is the excretion of waste by the fish. There are two types of the waste excreted by the fish: carbon dioxide and nitrogenous compounds. Carbon dioxide that is returned to the water via fish’s gills is then used as a primary carbon source by the photoautotrophs present in the tank. These organisms include algae and aquatic plants. Nitrogenous compounds that are excreted by the fish are usually in the form of ammonia, which is very toxic to the fish. This ammonia is then turned into ammonium ion in water via the following equation: | [[Image:Nitrogencycle.JPG|thumb|Aquarium|400px|right| The nitrogen cycle in the aquaria environment. Picture made by UCSD student in BIMM120, Marin N. ]] | ||
The first step in the nitrogen cycle is the excretion of waste by the fish. There are two types of the waste excreted by the fish: carbon dioxide and nitrogenous compounds. [28] Carbon dioxide that is returned to the water via fish’s gills is then used as a primary carbon source by the photoautotrophs present in the tank [11]. These organisms include algae and aquatic plants. Nitrogenous compounds that are excreted by the fish are usually in the form of ammonia, which is very toxic to the fish. This ammonia is then turned into ammonium ion in water via the following equation: | |||
NH3 + H2O ↔ NH4+ + OH- | NH3 + H2O ↔ NH4+ + OH- [28] | ||
This equation is driven to the right if the ammonium ion present is constantly being diminished by another organism. | This equation is driven to the right if the ammonium ion present is constantly being diminished by another organism. | ||
The second step in the nitrogen cycle is the conversion of the ammonium ions into nitrites. This step is carried out by a class of bacteria called nitrifying bacteria via a process called nitrification by the following oxidation reaction. The genus of bacteria involved in this step of the cycle is Nitrosomonas, specifically Nitrosomonas | The second step in the nitrogen cycle is the conversion of the ammonium ions into nitrites. This step is carried out by a class of bacteria called nitrifying bacteria via a process called nitrification by the following oxidation reaction [11]. The genus of bacteria involved in this step of the cycle is ''[[Nitrosomonas]]'', specifically ''[[Nitrosomonas europaea]]'' [11]. | ||
NH4+ + 3/2 O2 ↔ 2H+ + H2O + NO2- [28] | |||
In the third step, the nitrites are then converted into nitrates by another example of nitrifying bacteria, specifically of the genus ''[[Nitrobacter]]'' by the following oxidation reaction: | |||
NO2- + 1/2O2 ↔ NO3- [28] | |||
Nitrates, then, are in part used as fertilizers by aquatic plants and algae. [2] The rest can sufficiently be removed by the water changes carried out by the aquarist since nitrates are relatively non-toxic to the fish. | |||
==Disease Causing Microbes== | |||
Aquaria do not contain a wide number of microorganisms due to their highly controlled maintenance. Most of the microbes, however, can be introduced into the aquaria through newly acquired fishes. These fishes can then spread the bacteria to other species in the aquarium. | |||
===Bacterial Diseases=== | |||
Pathogenic bacteria can cause disease if the fish are susceptible. Bacterial diseases could affect the fish in multiple ways, anywhere from fin to tail, causing conditions such as body ulceration to septicemia. [2] It has been shown that up to 70 percent of the fish purchased in stores have these pathogenic bacteria already present in their blood, but show no symptoms unless triggered by weakened immune system or a less than optimal condition of the aquaria. [2] | |||
[[Image:Aeromonas1.jpg|thumb|Aquarium|200px|left| Picture of ''[[Aeromonas]]'', a type of bacteria that can cause hemorrhagic septicemia. Taken from Microbewiki site [29].]] | |||
Infection by ''[[Aeromonas]]'' is the most common form of bacterial infection seen in fish in aquariums. [22] Mortality rate is less than 10 percent and the condition can last for as long as 3 weeks. [22] Various forms of stress such as poor water quality, overcrowding, or rough handling can be the trigger to make the fish susceptible. [22] This species of bacteria can also cause hemorrhagic septicemia, especially in freshwater aquariums. In hemorrhagic septicemia, these pathogenic microbes enter the blood stream of the fish, resulting in septic shock, which can cause multiple organ failures, ultimately leading to death. [22] | |||
The symptoms become apparent by the visible ulcers present on the fish's body and exophthalmia, a condition also commonly known as pop-eye. [22] | |||
Infection by ''[[Vibrio]]'' species are also common in aquariums. These gram negative microbes ferment lactose and are more common in brackish and salt water aquariums. They cause wound infections, gastroenteritis, and hemorrhagic septicemia. [22] | |||
Fish tuberculosis is yet another common illness caused by bacteria in aquariums. Symptoms include ulcerated swellings under the skin. [2] This disease spreads quickly in the aquariums by cannibalism. [2] A care must be taken when handling a tank contaminated with fish tuberculosis since this could potentially infect humans as well. [2] | |||
=== | In order to cope with these various microbes, you can add various disinfectants such as benzalkonium chloride or chloramine T directly to the water. [2] You can also administer veterinary antibiotics specific to the nature of the bacteria infecting your tank. But when doing so, you must take into the fact that beneficial bacteria are also affected by these antibiotics. | ||
===Parasitic Diseases=== | |||
Many of the very common health problems, mostly affecting the skin and gills, are caused by parasites. The most common parasitic disease that affects aquarium fish is called ich. This condition is caused by ''[[Ichthyophthirius multifiliis]]'', and they can readily be observed as tiny white spots on the skin or gills.[2] When the parasite living on the fish reaches its full size, it bursts out leaves a hole behind; this, when multiple parasites affect the body of the fish, could ultimately lead to loss of body fluids. [2] In tropical aquariums this process taks only about 16 hours, but unless they are triggered by stress the fish's overall health is not affected. [2] | |||
Another example of a parasitic disease is called velvet disease. [2] This disease is caused by the organism ''Oodinium'', which belongs to a group of parasites called dinoflagellates. [2] They are normally free swimming in water, but they may encounter fish and attach themselves onto the skin and the gills of the host. [2] ''[[Oodinium]]'' also causes velvet disease in marine species. The symptoms of this disease include damage to the gills. [2] | |||
==Influence by Adjacent Communities== | |||
The aquarium is a protected ecosystem that is stabilized by the aquarists themselves; however, aquarists cannot protect the aquariums from all neighboring communities, especially the atomosphere. | |||
The air surrounding us contains more than 1800 types of bacteria, and these microbes inevitably have an effect on the aquariums. For example, some gram positive bacteria present in the atmosphere may enter the aquarium and colonize the water, causing water cloudiness.[11] These bacteria enters the aquarium when the wastes are not cycled by sufficient amount of nitrifying bacteria, resulting in the accumulation of the waste material in water. These bacteria can be eradicated by the use of an antibiotic, erythromycin specifically for gram positive bacteria. [11] | |||
Many non-microbes that are found in aquaria have significant influences in the aquaria ecosystem. | |||
==Non-microbes in Aquarium== | ==Non-microbes in Aquarium== | ||
===Aquarium Plants=== | ===Aquarium Plants=== | ||
'''Aquarium plants''' are important for the ecosystem of the aquarium, since most aquatic species live side by side with vegetations. Plants can provide shelter, food, breeding sites, nest-building materials, and territory markers [2]. | '''Aquarium plants''' are important for the ecosystem of the aquarium, since most aquatic species live side by side with vegetations. Plants can provide shelter, food, breeding sites, nest-building materials, and territory markers [2]. | ||
| Line 81: | Line 104: | ||
Plants are also important in maintaining the health of fishes. Plants remove not just certain mineral salts from the water, but also significant quantities of organic carbon compounds and even phenols [8]. They can reduce the number of certain bacteria that lead to health issues [8]. Some plants, such as ''Lemna spp.'' or duckweed, can produce antibiotics, while others can keep some damaging molecules, such as nitrates and phosphates, under control [8]. | Plants are also important in maintaining the health of fishes. Plants remove not just certain mineral salts from the water, but also significant quantities of organic carbon compounds and even phenols [8]. They can reduce the number of certain bacteria that lead to health issues [8]. Some plants, such as ''Lemna spp.'' or duckweed, can produce antibiotics, while others can keep some damaging molecules, such as nitrates and phosphates, under control [8]. | ||
Variety of different plants can be found in saltwater and freshwater aquariums. For example, ''Echinodorus sppp.'' or Amazon Swordplant and ''Anubias congensis'' or Congo Anubias can grow in freshwater. On the other hand, ''Penicillus sp.'' or Shaving Brush Plant are seen in saltwater [2]. Planting in Brackish water aquarium is difficult because there is limited number of brackish water tolerant plants being distributed. This includes ''Microsorium pteropus'', ''Crinum calamistratum'', ''Bacopa monnieri'', and ''Cryptocoryne ciliata'' [25]. | |||
===Algae=== | ===Algae=== | ||
[[Image:Algae.jpg|thumb|Aquarium| | [[Image:Green_algae.jpg|thumb|Aquarium|180px|left| Green algae are seen in all the aquarium environments. Picture taken by David Burdick. NOAA distributed. [http://www8.nos.noaa.gov/coris_glossary/index.aspx (9)]]] | ||
'''Algae''' are non-flowering, photosynthesizing plants that grow in most body of water. Algae are seen as problematic in aquariums because they restrict aquaria plant function by competing with them for nutrients and space [8]. However, algae provide food to a number of aquatic species, and can be used to test the growing conditions of the aquarium (allows aquarist to decide whether the aquarium is ready to inhabit plants and fishes), and control the nitrate levels in water [8]. There are various types of algae that can grow in aquariums: blue-green, green, red, and brown algae. | |||
[[Image:Algae.jpg|thumb|Aquarium|200px|right| Patches of red and green algae on rock surface. Picture taken from NOAA Ocean Explorer. [http://oceanexplorer.noaa.gov/explorations/04fire/logs/april08/media/bacteria_algae.html (7)]]] | |||
'''Blue-green algae''' are cyanobacteria that have the characteristics of both bacteria and algae. They can form rapidly and cover plants, leading to plant death [8]. Blue-green algae are seen in new aquariums that contain “raw” water, thinly planted, excessively illuminated with lights, and contain water pollutants [8]. '''Green algae''' can be free-floating single-celled or attached filamentous/encrusting types [8]. They can be found in aquariums with high illumination. Free-floating will cause green-water problems, while filamentous type produce tuft-like growths on plants [8]. However, they can act as food source for some fish species, such as ''Gyrinocheilus aymonieri'' or Sucking Loach [8]. Despite the name, '''red algae''' (also known as fir or brush algae) are greenish-brown, and forms tufts on plants and decorations [8]. Red algae form under aquarium with lower illumination because they are naturally found in deeper water with less light [9]. '''Brown algae''' form brown encrustations on aquarium panes, decorations, and plant leaves [8]. They are mostly seen in aquaria with poor plant growth [8]. Similar to red algae, brown algae grow in low illumination. | |||
[[Image:Coral.jpg|thumb|Aquarium|200px|right| Coral reef in the marine water inhabited by various aquatic creatures. [http://oceanservice.noaa.gov/education/kits/corals/coral07_importance.html (21)]]] | |||
===Coral Reefs=== | |||
'''Coral reefs''' are mostly found in marine water because freshwater are nutrient rich can lead to algae growth on the reefs, leading to nutrient restriction [18]. Some coral reefs can survive in brackish water; however, only in areas with higher salinity concentration [19]. Coral reefs have been known to inhabit 4,000 species of fish, and its research has potential for cures for many diseases, including cancer and arthritis [21]. | |||
==Current Research== | |||
1. '''"Low Temperature Decreases the Phylogenetic Diversity of Ammonia-Oxidizing Archaea and Bacteria in Aquarium Biofiltration Systems" '''[15] | |||
Currently, the researches have indicated that the nitrification reactions in aquarium biofiltration are carried out mainly by organisms of ''Nitrosomonas'', a bacteria. Recently, though, it was discovered that some organisms not in the domain ''[[Bacteria]]'', specifically ''[[Archaea]]'', is also resopnsible for the nitrification reactions. These ''[[Archea]]'' all have the genes for three subunits of ammonia monooxygenase (''amoA, amoB, amoC''), which is the key enzyme for the nitrification process. In this experiment, the researchers isolated organisms present in the water and sand of three aquariums of various temperatures, namely 19.9°C, 5.5°C, and 19.0°C, in order to detect the presence of these organisms as well as compare the temperature sensitivity of these organisms. Then, DNA was extracted in order to run PCR (using primers Arch-''amoAF'' and Arch-''amoAR'') to amplify the genes coding for the subunits of ammonia monooxygenase. The isolated DNA was also sequenced and BLASTed in order to determine the phylogeny of the ''[[Archaea]]'' isolated. As a result, they found that the phylogenic diversity of ammonia-oxidizying archaea(AOA) is much greater than those of ammonia-oxidizying bacteria in aquaria; however, the diversity of these ''[[Archaea]]'' and ''[[Bacteria]]'' were both minimized in cold temperature, indicating that the temperature is an important factor influencing the population of these beneficial bacteria. These results will be helpful in determining novel ways in which we can hasten the "seeding" process of the aquariums in order to create a safe ecosystem. | |||
''' | 2. '''"Distribution of mycobacteria in clinically healthy ornamental fish and their aquarium environment"''' [16] | ||
''' | Diseases caused by mycobacteria is the most common diseases affecting fish worldwide; however, little studies have been done on the effect of mycobacterial diseases in the fish living in aquariums. Mycobacterial infections have already been seen to cause emaciation, and also have multiple effects on their skin and internal organs. Thus, this study focuses on mycobacterial diseases that occur in decorative aquariums. Ziehl-Neelsen staining was used in order to identify acid-fast bacteria in the aquariums. As a result, 41 out of 58 aquariams indicated presence of mycobacteria species, namely ''[[M. fortuitum]], [[M. flavescens]], [[M. chelonae]], [[M. gordonae]], [[M. triviale]], [[M. diernhoferi]], [[M. celatum]], [[M. kansasii]]'' and ''[[M. intracellulare]]''. This result is important since mycobacterial species from aquarium environments may cause infection for both aquarium fish and fish handlers. This risk of human infection is increased by factors that affect the immunity of the human. | ||
3. '''"An improved nitrifying enrichment to remove ammonium and nitrite from freshwater aquaria systems"''' [23] | |||
The purpose of the research was to determine whether nitrifying cells called ammonia binding inoculum liquid (ABIL) can be used to lower the total ammonium-nitrogen (TAN) in the aquarium. Ammonia and nitrite are toxic substances, and need to be removed to maintain the health of aquaria ecosystem. The nitrifying activity of ABIL was measured in two conditions. 1) With 25-250mg TAN per liter, and 2) In freshwater aquarium with 10mg TAN per liter and dissolved oxygen concentration in the water above 6mg O2 per liter [23]. For the first condition, the nitrifying rate of ABIL was determined to be 0.33±0.12 g TAN g−1 VSS −1 day−1 (mean±standard deviation, n=3) [23]. For the second condition, results revealed that ABIL at dose of 5mg volatile suspended solids (VSS) per liter led to the total removal of ammonium (NH4+) and nitrite species from 10mg TAN per liter to below the detection level within four days [23]. Further research reveals that after 12 months of storage in freshwater aquarium with 10mg TAN per liter, nitrite-oxidation became slower, and nitrite began to accumulate. An additional research was conducted, in which the second condition was also inhabited with a nitrifying organism, polyurethane (PU) sponges, but no significant changes in nitrification were recorded. Although ABIL are effective in removing toxic substances, perlonged exposure in the aquaria can lower its function, and eventually lead to negative impact in aquaria ecosystem. This result will allow aquarist to expand their methods to keep the ammonia and nitrate level lower in aquariums; thus, improving the aquaria ecosystem for the inhabitants. | |||
4. '''"Elevated salinity selects for a less diverse ammonia-oxidizing population in aquarium biofilters"''' [24] | |||
The aim of the research was to determine whether ammonia-oxidizing bacteria (AOB) present in inoculums were able to grow and survive in freshwater and seawater biofilters for 2 months. They also tested whether the changes in salinity concentration can affect the activity and the population of betaproteobacterial, a type of AOB. DNA was extracted from biofilters sludge and soil, and analyzed using 1.2% agarose gel. The 465 bp fragment of the 16S rRNA gene from betaproteobacterial AOB was amplified, and ran in denaturing gradient gel electrophoresis. Furthermore, the specific 16S rRNA gene fragments of the first PCR round for AOB was cloned. Denaturing gradient gel electrophoresis of the ammonia-oxidizing bacterial community of the inoculum and the freshwater and the artificial seawater aquaria under the function of time showed that initially only one ammonia-oxidizer related to ''[[Nitrosomonas marina]]'' was detectable in both fresh and saltwater [24]. The AOB community in the seawater biofilters continued to be dominated by this single ammonia-oxidizer. In contrast, in the freshwater aquaria, the composition of the ammonia-oxidizer community became more diverse after one month, with 4–7 new bands appearing in the denaturing gradient gel fingerprint [24]. Since the inoculum is cultivated at an average salinity of 11g per liter, the research argued that the salinity level determines the diversity of the ammonia-oxidizer community in the inoculum, and in the seawater aquaria [24]. Furthermore, AOB can survive in freshwater and seawater biofilters after 2 months, but the diversity of AOB in freshwater increases, while in seawater, it remains the same. This experiment reveals a significant impact on understanding what kind of microbes can inhabit the aquaria, and how the environment of the aquarium can significantly affect those microbes. | |||
==Conclusion of Aquarium Niche== | |||
Aquarium hosts a large number of aquatic species that live in a variety of water conditions: saltwater, freshwater, and brackish water. It is important for aquarist to maintain the aquaria ecosystem because many species and important microbes cannot tolerate drastic or fluctuating changes to their environment. It is important to filter micro-organisms from aquariums, since a number of them can be detrimental to the health of the inhabitants. Microbes that are present in aquariums are ''[[Nitrosomonas]]'' and ''[[Nitrobacter]]'', which are important in Nitrogen cycle, the process of removing ammonia and nitrite from the aquariums. Microbes are most commonly introduced to the aquarium by external forces, such as acquiring new fishes with microbes present on their skins. Some of these microbes can lead to the spread of bacterial and parasitic diseases. Other niches found in the aquarium become important in the aquaria ecosystem. Plants and algae have important role in maintaining a suitable environment for the aquatic species. The research on aquarium micro-organism exists, and continues to answer questions that can help aquarist maintain a controlled ecosystem in the aquarium. | |||
==References== | ==References== | ||
#"aquarium_600.jpg". | #''NOAA Ocean Explorer''. "aquarium_600.jpg". 2006. Washington D.C., Retrieved: 26 August 2008. <http://oceanexplorer.noaa.gov/explorations/04fire/logs/april05/media/aquarium.html>. | ||
#Scott, Peter W. ''The Complete Aquarium''. New York: Alfred A. Knopf Inc., 1991. | #Scott, Peter W. ''The Complete Aquarium''. New York: Alfred A. Knopf Inc., 1991. | ||
# | #''NOAA Fisheries Service Northeast Salmon Team''. "Criticalhabitat_clip_image002_0000.jpg". 2008. Washington D.C., Retrieved: 20 August 2008. <http://www.nefsc.noaa.gov/salmon/finalcriticalhabitat.html>. | ||
#" | #''NOAA Photo Library''. "nerr0200.jpg". 2007. Washington D.C., Retrieved: 25 August 2008. <http://www.photolib.noaa.gov/htmls/nerr0200.htm>. | ||
#Consi, T.R. | #Consi, T.R. ''Micro-Safaris: The Display of Live Microorganisms in Public Aquariums''. Marine Technology Society Journal [0025-3324] yr:2001 vol:35 iss:1 pg:36 -47. | ||
#Wiegert, J. Freshwater and marine aquarium [0160-4317]. yr:2006 vol:29 iss:3 pg:112 -116 | #Wiegert, J. ''Freshwater and marine aquarium'' [0160-4317]. yr:2006 vol:29 iss:3 pg:112-116 | ||
#''NOAA Ocean Explorer''. "bacteria_algae_600.jpg". 2006. | #''NOAA Ocean Explorer''. "bacteria_algae_600.jpg". 2006. Washington D.C., Retrieved: 28 August 2008. <http://oceanexplorer.noaa.gov/explorations/04fire/logs/april08/media/bacteria_algae.html> | ||
#Dawes, John. ''Complete Encyclopedia of the Freshwater Aquarium''. Ontario, Canada: Firefly Books Ltd., 2001. | #Dawes, John. ''Complete Encyclopedia of the Freshwater Aquarium''. Ontario, Canada: Firefly Books Ltd., 2001. | ||
#Burdick, David. ''NOAA's Coral Reef Data''. "algae_186.jpg". 2006. Washington D.C., Retrieved: 28 August 2008. | |||
#''NOAA Magazine Online''. "aquarium.jpg". 2008. Washington D.C., Retrieved: 28 August 2008. <http://www.magazine.noaa.gov/stories/mag25.htm>. | |||
#Mills, Dick. ''The Marine Aquarium''. Virginia: Salamander Books Ltd., 1987. | |||
#Starkenburg SR, Larimer FW, Stein LY, Klotz MG, Chain PS, Sayavedra-Soto LA, Poret-Peterson AT, Gentry ME, Arp DJ, Ward B, Bottomley PJ. ''Complete genome sequence of Nitrobacter hamburgensis X14 and comparative genomic analysis of species within the genus Nitrobacter''. Appl Environ Microbiol. 2008 May;74(9):2852-63. Epub, 2008 March 7. | |||
#Mao Y, Bakken LR, Zhao L, Frostegård A. ''Functional robustness and gene pools of a wastewater nitrification reactor: comparison of dispersed and intact biofilms when stressed by low oxygen and low pH''. FEMS Microbiol Ecol. 2008 June 24. | |||
#Beyer S, Gilch S, Meyer O, Schmidt I. ''Transcription of Genes Coding for Metabolic Key Functions in Nitrosomonas europaea during Aerobic and Anaerobic Growth''. J Mol Microbiol Biotechnol. 2008 July 1. | |||
#Johnstone BH, Jones RD. ''Effects of Light and CO on the Survival of a Marine Ammonium-Oxidizing Bacterium during Energy Source Deprivation.'' Appli Environ Microbiol. 1988 Dec;54(12):2890-2893. | |||
#Hidetoshi Urakawa, Yoshiyuki Tajima, Yoshiyuki Numata, and Satoshi Tsuneda. ''Low Temperature Decreases the Phylogenetic Diversity of Ammonia-Oxidizing Archaea and Bacteria in Aquarium Biofiltration Systems.'' Applied and Environmental Microbiology. February 2008, p. 894-900, Vol. 74, No. 3. | |||
#V. Beran, L. Matlova, L. Dvorska, P. Svastova, and I. Pavlik. ''Distribution of mycobacteria in clinically healthy ornamental fish and their aquarium environment''. Journal of Fish Diseases [J. Fish Dis.]; vol. 29, no. 7. | |||
#''Corals reveal impact of land use''. ARC Center of Excellence: Coral Reef Studies. 31 may 2007. Retrieved: 28 August 2008. <http://www.coralcoe.org.au/news_stories/landimpacts.html>. | |||
#Monks, Neale. ''Brackish Aquarium''. Wet Web Media.Com. 2008. Retrieved: 28 August 2008. <http://www.wetwebmedia.com/ca/volume_4/V4I2/Brackish%20Systems/brackish.htm>. | |||
#Jennings, Greg. ''The New Encyclopedia of the Saltwater Aquarium''. Ontario, Canada: Firefly Books Ltd., 2007. | |||
#''Importance of Coral Reefs''. "coral07a_240.jpg". NOAA Ocean Service Education. 25 March 2008. Article retrieved: 28 August 2008. Picture retrived: 28 August 2008. <http://oceanservice.noaa.gov/education/kits/corals/coral07_importance.html>. | |||
#Strohmeyer, Carl. ''Treatment and Identification of Aeromonas and Vibrio in Aquariums and Ponds''. 2008. Retrieved: 28 August 2008. <http://EzineArticles.com/?expert=Carl_Strohmeyer>. | |||
#R. Grommen, I. Van Hauteghem, M. Van Wambeke, and W. Verstraete. ''An improved nitrifying enrichment to remove ammonium and nitrite from freshwater aquaria systems''. Aquaculture. Volume 211, Issues 1-4, , 23 August 2002, Pages 115-124. | |||
#Lenny Dauw, Roeland Grommen, and Willy Verstraete. ''Elevated salinity selects for a less diverse ammonia-oxidizing population in aquarium biofilters''. FEMS Microbiology Ecology. Volume 52, Issue 1, 1 March 2005, Pages 1-11 | |||
#Monks, Neale. Brackish Water Aquarium FAQ. 2007. <http://homepage.mac.com/nmonks/Projects/brackishfaq.html>. | |||
#''Cycling Your New Aquarium''. Well Aquariums by Living Pictures. 2004. Article retrived: 28 August 2008. <http://www.wallaquariums.com/cycling.htm>. | |||
#Watson, Stan. Picture of ''Nitrosomonas europaea''. US Department of Energy Office of Science. Picture retrived from Microbewiki: 28 August 2008. <http://microbewiki.kenyon.edu/index.php/Nitrosomonas_europaea>. | |||
#John Hochheimer, Fred Wheaton. "Biological Filters: Trickling and RBC Design." <http://nsgd.gso.uri.edu/vsgcp/vsgcpc98001/vsgcpc98001_part6.pdf>. | |||
#Picture of ''Aeromonas''. NOAA Fisheries Service. Picture retrived from Microbewiki: 29 August 2008. <http://microbewiki.kenyon.edu/index.php/Aeromonas>. | |||
''Edited by Yuichiro S. and Marin N. at University of California-San Diego, 29 August 2008.'' | |||
Latest revision as of 18:48, 22 April 2011
Introduction to Aquarium

An aquarium (plural form is aquaria or aquariums) is an artificial ecosystem that is inhabitable by various aquatic species. The environment of the aquarium can differ depending on the residing species. For example, Xiphophorus helleri or Green Swordtail prefer to live in freshwater, while Paracanthurus hepatus or Surgeonfish prefer water with higher salt concentration [2]. Nothing quite adds to the room décor as adding an aquarium with beautifully colored fishes with lively aquatic plants. However, what keeps these colorful organisms alive and healthy are none other than the series of micro-organisms, also known as microbes. However, aquariums are built as an “ideal” ecosystem; hence, filtering out most of the microbes that can be found in natural body of water. For example, ultraviolet sterilization is used in aquariums to destroy algae and nucleic material of many free-floating micropathogens [8]. Furthermore, most of the work done by natural microbes is conducted by machineries or by chemical substances sold in supermarkets. Therefore, it is in the best interest for aquarist (people who maintain aquariums) to restrict the number of microbes in the aquaria to avoid potentially harming the inhabitants.
What kind of microbes can survive such controlled environment? Do aquariums need microbes? What happens if bacteria are introduced into the aquariums? The focus of the topic is to discuss the microbes that can survive in the aquarium, and how they affect the niche. In addition, the topic covers the different type of aquariums and its conditions, and the non-microbes that inhabit the aquaria ecosystem.
Maintaining Aquaria Niche
Aquarist must maintain specific pH, temperature levels, and lighting for different species in the aquarium. Salinity, the concentration of salt in a given amount of water, becomes one of the characteristics to distinguish between the different types of aquariums. Salinity can be measured using a hydrometer, which compares the specific gravity of sample with pure water under the units of ppt (parts per thousand) or ppm (parts per million) [2]. In addition, the aquarium's hardness must be monitored. The levels of dissolved minerals, such as bicarbonates, in water are described as the water's hardness [2].
Types of Aquarium Niche
Commercial aquariums come in variety of shapes and sizes, from tiny one gallon fish bowls to luxurious 100 gallon fish tanks, and normally can be set simply on a desk top or on top of a special aquarium stand. There are generally four types of aquariums a new aquarist can set up: freshwater, brackish, and saltwater. Each type of aquarium requires specific care and maintenance, and different types of fish reside in each of the designed ecosystem.
Freshwater Aquarium

Freshwater aquarium can be divided into tropical and cold freshwater. In tropical freshwater aquariums, for fish such as Paracheirodon innesi, Betta splendens, and Belontia hasselti, just to name a few, the temperature must be kept at 72-80°F (22-27°C) [8]. The pH must be kept between 6 and 8, the stability and the consistency of the pH also playing an important role in ensuring the health of the fish [8]. Since freshwater fish normally cannot tolerate any salinity, salinity must be kept under 3ppm (parts per million), preferrably at 0 [8]. In cold freshwater aquariums, for fish such as Cyprinus carpio (koi) and Carassius auratus (goldfish), the temperature should be kept typically below 70°F (20°C). Thus, coldwater aquariums do not require heaters. The light should be kept at the standard level and the hardness of the water at 100-200mg/L CaCO3 [8].
Brackish Water Aquarium

Brackish water aquarium is an artificial ecosystem that simulates the natural environment of brackish water, which has a salt concentration between freshwater and saltwater. Brackish water aquarium is kept at a temperature around 23-29°C, with a pH between 7 to 8, a hardness of 200 mg/liter of CaCO3, a specific gravity of 1.003-1.012, and a salinity between 7.6 and 14 ppt [2]. The lighting level should be high since most brackish water environments are exposed to the sun. Brackwish water can be found in Central American costal streams, East Africa mangrove swamps, and Southeast Asia estuaries [2]. A large variety of species inhabit the brackish water, such as Sailfin, Monos, and Cichlids [25].
Saltwater Aquarium

Saltwater or Marine aquarium hosts a variety of colorful and exotic species, such as Premnas biaculeatus or Maroon Clownfish [11]. The temperature should be kept at 22-26°C (72-78°F), pH between 8.1 and 8.3, specific gravity of 1.020-1.022, and salinity between 27.2 and 33.7ppt [2]. Saltwater aquariums are difficult to maintain because salinity levels can fluctuate [11]. Most marine species live in the ocean where salinity levels are kept constant; thus, they cannot tolerate fluctuating levels of salt concentration in the waters [11]. Another division of marine aquarium is the Reef aquaria. This aquarium recreates the environment for the many fantastic reefs in the marine ecosystem. Temperature should be maintained at 23-25°C (72-78°F), pH of 8.1-8.3, specific gravity of 1.022-1.024, and salinity of 27.2-33.7ppt [2]. Inhabitants include Goniopora or Daisy Coral, and Gorgonia spp. or Sea Fan [2].
Conditions under which the environment changes
There are various factors that come into play that could potentially harm the organisms that are living in the aquarium.
First, an aquarist must make sure to dechlorinate the water before changing the water of the aquarium. This is because chlorine is present in tap water, which breaks down to hypochlorous acid (HOCl) and hypochlorite ion (OCl-) [11]. These two molecules kill beneficial microorganisms by first attacking the lipids in their peptidoglycan cell walls. They will also attack the enzymes and other structural proteins that are necessary for the microbes' health.
Second, a great care must be taken when doing water changes and cleaning the tanks in order to not destroy the beneficial bacterial colonies that are present. It takes one month for Nitrosomonas and two months for Nitrobacter to develop into a mature colony in the aquarium [11]. Once these stable colonies are disturbed, the aquarium must cycle again before the environment is optimal for the health of the fish.
Third, the location of the aquarium must also be chosen carefully not to allow drastic changes of the temperature of the water. The places that must be avoided include near the windows, vents, and radiators.
Microbes that Inhabit the Aquarium
Public aquariums can host large varieties of aquatic species in a closed and “ideal” ecosystem. However, there are numbers of microorganisms in nature that are not present in aquariums despite their profound importance to global marine ecology [5]. This is mainly due to the difficulty in creating a habitat for the microorganisms, and, at the same time, maintaining a suitable environment for the aquatic species [5]. In the aquarium, bacteria are mostly responsible for breakdown waste [6]. The most frequent process is the breakdown of protein into ammonia, then nitrite into nitrate [6]. This process of waste disposable is known as the Nitrogen Cycle, which uses Nitrosomonas and Nitrobacter. These two bacteria enter the aquaria on the surface of inhabitants, such as fishes, or can be bought in bottles, such as Turbo Start 700 (freshwater) and 900 (brackish and marine water) [26].
Microbes Involved In Nitrogen Cycle
The waste excreted by the fish in the aquarium must always somehow be kept low at a non-toxic level. The cycle that exists in aquariums and natural aquatic environments alike is the nitrogen cycle. Since an aquarium is a fragile ecosystem on average containing only 10 gallons of water, it requires the aquarist’s interference in ensuring that the cycle is well established. In so doing, one must create this artificial ecosystem as close to the natural environment as possible. Only when all the players of the nitrogen cycle have successfully been established can more fish be added with a minimal loss.
Nitrosomonas and Nitrobacter
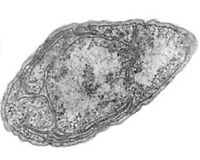
Nitrosomonas europaea is a Gram-negative chemolithoautotroph that can derive all its energy needed for growth by the oxidation of ammonia. [14] The key enzyme used in order to oxidize the ammonia is ammonia monooxygenase, whose subunits are coded by amoA, amoB, and amoC genes. [17] They colonize in other environments such as soil, sewage, and walls of buildings. Nitrobacter, an alphaproteobacterium, is a gram-negative facultative chemolithoautotroph. [12] Both of the nitrifying bacteria require specific environment for their maximum activity, all of which should be met by the environments the aquarist sets up for the fish in the aquarium. Because these organisms cannot form spores, the aquarium must be kept at the optimum condition at all times for the biological filtration of these nitrifying bacteria to function. They also form slimy biofilms in order to protect themselves from desiccation and other potential threats.
The ideal pH for these beneficial bacteria is between 7.2 and 8.5, having a narrower range than tolerated by fish. [13] Also, nitrifying bacteria are aerobic organisms and oxygen is critical for their nitrifying activity. As the oxygen level decreases beyond 1mg/L, dissolved oxygen becomes the limiting factor of the nitrification reactions. Thus, the oxygen level in the water must be at least 2mg/L in order for it to not have an adverse effect on the nitrifying activity. [13] At suboptimal levels of pH(4.5, 5.5, 6.5) and at suboptimal levels of oxygen (10 - 100 microM), nitrification was severely reduced. [13] However, when the nitrifying activities of intact biofilms (IB) and dispersed biofilms (DB) were compared, bacteria of IB were less affected by pH and oxygen level changes. [13] Thus, the nitrifying bacteria form biofilms in order to protect themselves from potentially detrimental environmental changes.
Also, the nitrifying bacteria are most active between the temperatures of 68 - 86 °F; 50°F is the minimum temperature and 95°F is the maximum temperature for these nitrifying bacteria. [11] Furthermore, nitrifying bacteria are light sensitive, especially to UV light contained in the sun light. Room light can also have an adverse effect on the bacteria. [14] Thus, nitrifying bacteria tend to form colonies within the filteration system and beneath the gravel to avoid exposure to light. Finally, nitrifying bacteria can tolerate a wide range of salinity. Thus, a beneficial bacterial colonies established in a freshwater environment can then be used for aquariums in a saltwater environment. [11] This transition, though, must be carried out in a gradual manner for it to be effective. [11] Maximum change of 5ppm should not affect the activity of the nitrifying bacteria. [11] Thus Nitrosomonas and Nitrobacter are both seen in all of the four general types of aquariums mentioned in the previous section.
The Nitrosomona and Nitrobacter Interaction and in the Environment
The first step in the nitrogen cycle is the excretion of waste by the fish. There are two types of the waste excreted by the fish: carbon dioxide and nitrogenous compounds. [28] Carbon dioxide that is returned to the water via fish’s gills is then used as a primary carbon source by the photoautotrophs present in the tank [11]. These organisms include algae and aquatic plants. Nitrogenous compounds that are excreted by the fish are usually in the form of ammonia, which is very toxic to the fish. This ammonia is then turned into ammonium ion in water via the following equation:
NH3 + H2O ↔ NH4+ + OH- [28]
This equation is driven to the right if the ammonium ion present is constantly being diminished by another organism.
The second step in the nitrogen cycle is the conversion of the ammonium ions into nitrites. This step is carried out by a class of bacteria called nitrifying bacteria via a process called nitrification by the following oxidation reaction [11]. The genus of bacteria involved in this step of the cycle is Nitrosomonas, specifically Nitrosomonas europaea [11].
NH4+ + 3/2 O2 ↔ 2H+ + H2O + NO2- [28]
In the third step, the nitrites are then converted into nitrates by another example of nitrifying bacteria, specifically of the genus Nitrobacter by the following oxidation reaction:
NO2- + 1/2O2 ↔ NO3- [28]
Nitrates, then, are in part used as fertilizers by aquatic plants and algae. [2] The rest can sufficiently be removed by the water changes carried out by the aquarist since nitrates are relatively non-toxic to the fish.
Disease Causing Microbes
Aquaria do not contain a wide number of microorganisms due to their highly controlled maintenance. Most of the microbes, however, can be introduced into the aquaria through newly acquired fishes. These fishes can then spread the bacteria to other species in the aquarium.
Bacterial Diseases
Pathogenic bacteria can cause disease if the fish are susceptible. Bacterial diseases could affect the fish in multiple ways, anywhere from fin to tail, causing conditions such as body ulceration to septicemia. [2] It has been shown that up to 70 percent of the fish purchased in stores have these pathogenic bacteria already present in their blood, but show no symptoms unless triggered by weakened immune system or a less than optimal condition of the aquaria. [2]
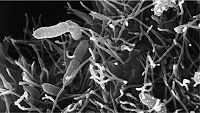
Infection by Aeromonas is the most common form of bacterial infection seen in fish in aquariums. [22] Mortality rate is less than 10 percent and the condition can last for as long as 3 weeks. [22] Various forms of stress such as poor water quality, overcrowding, or rough handling can be the trigger to make the fish susceptible. [22] This species of bacteria can also cause hemorrhagic septicemia, especially in freshwater aquariums. In hemorrhagic septicemia, these pathogenic microbes enter the blood stream of the fish, resulting in septic shock, which can cause multiple organ failures, ultimately leading to death. [22] The symptoms become apparent by the visible ulcers present on the fish's body and exophthalmia, a condition also commonly known as pop-eye. [22]
Infection by Vibrio species are also common in aquariums. These gram negative microbes ferment lactose and are more common in brackish and salt water aquariums. They cause wound infections, gastroenteritis, and hemorrhagic septicemia. [22]
Fish tuberculosis is yet another common illness caused by bacteria in aquariums. Symptoms include ulcerated swellings under the skin. [2] This disease spreads quickly in the aquariums by cannibalism. [2] A care must be taken when handling a tank contaminated with fish tuberculosis since this could potentially infect humans as well. [2]
In order to cope with these various microbes, you can add various disinfectants such as benzalkonium chloride or chloramine T directly to the water. [2] You can also administer veterinary antibiotics specific to the nature of the bacteria infecting your tank. But when doing so, you must take into the fact that beneficial bacteria are also affected by these antibiotics.
Parasitic Diseases
Many of the very common health problems, mostly affecting the skin and gills, are caused by parasites. The most common parasitic disease that affects aquarium fish is called ich. This condition is caused by Ichthyophthirius multifiliis, and they can readily be observed as tiny white spots on the skin or gills.[2] When the parasite living on the fish reaches its full size, it bursts out leaves a hole behind; this, when multiple parasites affect the body of the fish, could ultimately lead to loss of body fluids. [2] In tropical aquariums this process taks only about 16 hours, but unless they are triggered by stress the fish's overall health is not affected. [2]
Another example of a parasitic disease is called velvet disease. [2] This disease is caused by the organism Oodinium, which belongs to a group of parasites called dinoflagellates. [2] They are normally free swimming in water, but they may encounter fish and attach themselves onto the skin and the gills of the host. [2] Oodinium also causes velvet disease in marine species. The symptoms of this disease include damage to the gills. [2]
Influence by Adjacent Communities
The aquarium is a protected ecosystem that is stabilized by the aquarists themselves; however, aquarists cannot protect the aquariums from all neighboring communities, especially the atomosphere.
The air surrounding us contains more than 1800 types of bacteria, and these microbes inevitably have an effect on the aquariums. For example, some gram positive bacteria present in the atmosphere may enter the aquarium and colonize the water, causing water cloudiness.[11] These bacteria enters the aquarium when the wastes are not cycled by sufficient amount of nitrifying bacteria, resulting in the accumulation of the waste material in water. These bacteria can be eradicated by the use of an antibiotic, erythromycin specifically for gram positive bacteria. [11]
Many non-microbes that are found in aquaria have significant influences in the aquaria ecosystem.
Non-microbes in Aquarium
Aquarium Plants
Aquarium plants are important for the ecosystem of the aquarium, since most aquatic species live side by side with vegetations. Plants can provide shelter, food, breeding sites, nest-building materials, and territory markers [2].
Photosynthesis is one of the most important functions of aquarium plants: 6 CO2 + H2O + Light (Energy) --> Glucose + 6 O2. Simply put, aquarium plants consume carbon dioxide, and, with the presence of light, produce oxygen. The amount of light used in the aquarium can affect the plant’s photosynthesis. With sufficient illumination, the consumption of carbon dioxide and production of oxygen are high in aquarium plants [8]. The result is a drop in carbon dioxide level in water and rise in oxygen concentration [8]. When the aquarium lights are turned off (night time), respiration slows down. As a result, there is a drop in dissolved oxygen concentration, and increase in carbon dioxide in the water [8].
Plants are also important in maintaining the health of fishes. Plants remove not just certain mineral salts from the water, but also significant quantities of organic carbon compounds and even phenols [8]. They can reduce the number of certain bacteria that lead to health issues [8]. Some plants, such as Lemna spp. or duckweed, can produce antibiotics, while others can keep some damaging molecules, such as nitrates and phosphates, under control [8].
Variety of different plants can be found in saltwater and freshwater aquariums. For example, Echinodorus sppp. or Amazon Swordplant and Anubias congensis or Congo Anubias can grow in freshwater. On the other hand, Penicillus sp. or Shaving Brush Plant are seen in saltwater [2]. Planting in Brackish water aquarium is difficult because there is limited number of brackish water tolerant plants being distributed. This includes Microsorium pteropus, Crinum calamistratum, Bacopa monnieri, and Cryptocoryne ciliata [25].
Algae

Algae are non-flowering, photosynthesizing plants that grow in most body of water. Algae are seen as problematic in aquariums because they restrict aquaria plant function by competing with them for nutrients and space [8]. However, algae provide food to a number of aquatic species, and can be used to test the growing conditions of the aquarium (allows aquarist to decide whether the aquarium is ready to inhabit plants and fishes), and control the nitrate levels in water [8]. There are various types of algae that can grow in aquariums: blue-green, green, red, and brown algae.

Blue-green algae are cyanobacteria that have the characteristics of both bacteria and algae. They can form rapidly and cover plants, leading to plant death [8]. Blue-green algae are seen in new aquariums that contain “raw” water, thinly planted, excessively illuminated with lights, and contain water pollutants [8]. Green algae can be free-floating single-celled or attached filamentous/encrusting types [8]. They can be found in aquariums with high illumination. Free-floating will cause green-water problems, while filamentous type produce tuft-like growths on plants [8]. However, they can act as food source for some fish species, such as Gyrinocheilus aymonieri or Sucking Loach [8]. Despite the name, red algae (also known as fir or brush algae) are greenish-brown, and forms tufts on plants and decorations [8]. Red algae form under aquarium with lower illumination because they are naturally found in deeper water with less light [9]. Brown algae form brown encrustations on aquarium panes, decorations, and plant leaves [8]. They are mostly seen in aquaria with poor plant growth [8]. Similar to red algae, brown algae grow in low illumination.

Coral Reefs
Coral reefs are mostly found in marine water because freshwater are nutrient rich can lead to algae growth on the reefs, leading to nutrient restriction [18]. Some coral reefs can survive in brackish water; however, only in areas with higher salinity concentration [19]. Coral reefs have been known to inhabit 4,000 species of fish, and its research has potential for cures for many diseases, including cancer and arthritis [21].
Current Research
1. "Low Temperature Decreases the Phylogenetic Diversity of Ammonia-Oxidizing Archaea and Bacteria in Aquarium Biofiltration Systems" [15]
Currently, the researches have indicated that the nitrification reactions in aquarium biofiltration are carried out mainly by organisms of Nitrosomonas, a bacteria. Recently, though, it was discovered that some organisms not in the domain Bacteria, specifically Archaea, is also resopnsible for the nitrification reactions. These Archea all have the genes for three subunits of ammonia monooxygenase (amoA, amoB, amoC), which is the key enzyme for the nitrification process. In this experiment, the researchers isolated organisms present in the water and sand of three aquariums of various temperatures, namely 19.9°C, 5.5°C, and 19.0°C, in order to detect the presence of these organisms as well as compare the temperature sensitivity of these organisms. Then, DNA was extracted in order to run PCR (using primers Arch-amoAF and Arch-amoAR) to amplify the genes coding for the subunits of ammonia monooxygenase. The isolated DNA was also sequenced and BLASTed in order to determine the phylogeny of the Archaea isolated. As a result, they found that the phylogenic diversity of ammonia-oxidizying archaea(AOA) is much greater than those of ammonia-oxidizying bacteria in aquaria; however, the diversity of these Archaea and Bacteria were both minimized in cold temperature, indicating that the temperature is an important factor influencing the population of these beneficial bacteria. These results will be helpful in determining novel ways in which we can hasten the "seeding" process of the aquariums in order to create a safe ecosystem.
2. "Distribution of mycobacteria in clinically healthy ornamental fish and their aquarium environment" [16]
Diseases caused by mycobacteria is the most common diseases affecting fish worldwide; however, little studies have been done on the effect of mycobacterial diseases in the fish living in aquariums. Mycobacterial infections have already been seen to cause emaciation, and also have multiple effects on their skin and internal organs. Thus, this study focuses on mycobacterial diseases that occur in decorative aquariums. Ziehl-Neelsen staining was used in order to identify acid-fast bacteria in the aquariums. As a result, 41 out of 58 aquariams indicated presence of mycobacteria species, namely M. fortuitum, M. flavescens, M. chelonae, M. gordonae, M. triviale, M. diernhoferi, M. celatum, M. kansasii and M. intracellulare. This result is important since mycobacterial species from aquarium environments may cause infection for both aquarium fish and fish handlers. This risk of human infection is increased by factors that affect the immunity of the human.
3. "An improved nitrifying enrichment to remove ammonium and nitrite from freshwater aquaria systems" [23]
The purpose of the research was to determine whether nitrifying cells called ammonia binding inoculum liquid (ABIL) can be used to lower the total ammonium-nitrogen (TAN) in the aquarium. Ammonia and nitrite are toxic substances, and need to be removed to maintain the health of aquaria ecosystem. The nitrifying activity of ABIL was measured in two conditions. 1) With 25-250mg TAN per liter, and 2) In freshwater aquarium with 10mg TAN per liter and dissolved oxygen concentration in the water above 6mg O2 per liter [23]. For the first condition, the nitrifying rate of ABIL was determined to be 0.33±0.12 g TAN g−1 VSS −1 day−1 (mean±standard deviation, n=3) [23]. For the second condition, results revealed that ABIL at dose of 5mg volatile suspended solids (VSS) per liter led to the total removal of ammonium (NH4+) and nitrite species from 10mg TAN per liter to below the detection level within four days [23]. Further research reveals that after 12 months of storage in freshwater aquarium with 10mg TAN per liter, nitrite-oxidation became slower, and nitrite began to accumulate. An additional research was conducted, in which the second condition was also inhabited with a nitrifying organism, polyurethane (PU) sponges, but no significant changes in nitrification were recorded. Although ABIL are effective in removing toxic substances, perlonged exposure in the aquaria can lower its function, and eventually lead to negative impact in aquaria ecosystem. This result will allow aquarist to expand their methods to keep the ammonia and nitrate level lower in aquariums; thus, improving the aquaria ecosystem for the inhabitants.
4. "Elevated salinity selects for a less diverse ammonia-oxidizing population in aquarium biofilters" [24]
The aim of the research was to determine whether ammonia-oxidizing bacteria (AOB) present in inoculums were able to grow and survive in freshwater and seawater biofilters for 2 months. They also tested whether the changes in salinity concentration can affect the activity and the population of betaproteobacterial, a type of AOB. DNA was extracted from biofilters sludge and soil, and analyzed using 1.2% agarose gel. The 465 bp fragment of the 16S rRNA gene from betaproteobacterial AOB was amplified, and ran in denaturing gradient gel electrophoresis. Furthermore, the specific 16S rRNA gene fragments of the first PCR round for AOB was cloned. Denaturing gradient gel electrophoresis of the ammonia-oxidizing bacterial community of the inoculum and the freshwater and the artificial seawater aquaria under the function of time showed that initially only one ammonia-oxidizer related to Nitrosomonas marina was detectable in both fresh and saltwater [24]. The AOB community in the seawater biofilters continued to be dominated by this single ammonia-oxidizer. In contrast, in the freshwater aquaria, the composition of the ammonia-oxidizer community became more diverse after one month, with 4–7 new bands appearing in the denaturing gradient gel fingerprint [24]. Since the inoculum is cultivated at an average salinity of 11g per liter, the research argued that the salinity level determines the diversity of the ammonia-oxidizer community in the inoculum, and in the seawater aquaria [24]. Furthermore, AOB can survive in freshwater and seawater biofilters after 2 months, but the diversity of AOB in freshwater increases, while in seawater, it remains the same. This experiment reveals a significant impact on understanding what kind of microbes can inhabit the aquaria, and how the environment of the aquarium can significantly affect those microbes.
Conclusion of Aquarium Niche
Aquarium hosts a large number of aquatic species that live in a variety of water conditions: saltwater, freshwater, and brackish water. It is important for aquarist to maintain the aquaria ecosystem because many species and important microbes cannot tolerate drastic or fluctuating changes to their environment. It is important to filter micro-organisms from aquariums, since a number of them can be detrimental to the health of the inhabitants. Microbes that are present in aquariums are Nitrosomonas and Nitrobacter, which are important in Nitrogen cycle, the process of removing ammonia and nitrite from the aquariums. Microbes are most commonly introduced to the aquarium by external forces, such as acquiring new fishes with microbes present on their skins. Some of these microbes can lead to the spread of bacterial and parasitic diseases. Other niches found in the aquarium become important in the aquaria ecosystem. Plants and algae have important role in maintaining a suitable environment for the aquatic species. The research on aquarium micro-organism exists, and continues to answer questions that can help aquarist maintain a controlled ecosystem in the aquarium.
References
- NOAA Ocean Explorer. "aquarium_600.jpg". 2006. Washington D.C., Retrieved: 26 August 2008. <http://oceanexplorer.noaa.gov/explorations/04fire/logs/april05/media/aquarium.html>.
- Scott, Peter W. The Complete Aquarium. New York: Alfred A. Knopf Inc., 1991.
- NOAA Fisheries Service Northeast Salmon Team. "Criticalhabitat_clip_image002_0000.jpg". 2008. Washington D.C., Retrieved: 20 August 2008. <http://www.nefsc.noaa.gov/salmon/finalcriticalhabitat.html>.
- NOAA Photo Library. "nerr0200.jpg". 2007. Washington D.C., Retrieved: 25 August 2008. <http://www.photolib.noaa.gov/htmls/nerr0200.htm>.
- Consi, T.R. Micro-Safaris: The Display of Live Microorganisms in Public Aquariums. Marine Technology Society Journal [0025-3324] yr:2001 vol:35 iss:1 pg:36 -47.
- Wiegert, J. Freshwater and marine aquarium [0160-4317]. yr:2006 vol:29 iss:3 pg:112-116
- NOAA Ocean Explorer. "bacteria_algae_600.jpg". 2006. Washington D.C., Retrieved: 28 August 2008. <http://oceanexplorer.noaa.gov/explorations/04fire/logs/april08/media/bacteria_algae.html>
- Dawes, John. Complete Encyclopedia of the Freshwater Aquarium. Ontario, Canada: Firefly Books Ltd., 2001.
- Burdick, David. NOAA's Coral Reef Data. "algae_186.jpg". 2006. Washington D.C., Retrieved: 28 August 2008.
- NOAA Magazine Online. "aquarium.jpg". 2008. Washington D.C., Retrieved: 28 August 2008. <http://www.magazine.noaa.gov/stories/mag25.htm>.
- Mills, Dick. The Marine Aquarium. Virginia: Salamander Books Ltd., 1987.
- Starkenburg SR, Larimer FW, Stein LY, Klotz MG, Chain PS, Sayavedra-Soto LA, Poret-Peterson AT, Gentry ME, Arp DJ, Ward B, Bottomley PJ. Complete genome sequence of Nitrobacter hamburgensis X14 and comparative genomic analysis of species within the genus Nitrobacter. Appl Environ Microbiol. 2008 May;74(9):2852-63. Epub, 2008 March 7.
- Mao Y, Bakken LR, Zhao L, Frostegård A. Functional robustness and gene pools of a wastewater nitrification reactor: comparison of dispersed and intact biofilms when stressed by low oxygen and low pH. FEMS Microbiol Ecol. 2008 June 24.
- Beyer S, Gilch S, Meyer O, Schmidt I. Transcription of Genes Coding for Metabolic Key Functions in Nitrosomonas europaea during Aerobic and Anaerobic Growth. J Mol Microbiol Biotechnol. 2008 July 1.
- Johnstone BH, Jones RD. Effects of Light and CO on the Survival of a Marine Ammonium-Oxidizing Bacterium during Energy Source Deprivation. Appli Environ Microbiol. 1988 Dec;54(12):2890-2893.
- Hidetoshi Urakawa, Yoshiyuki Tajima, Yoshiyuki Numata, and Satoshi Tsuneda. Low Temperature Decreases the Phylogenetic Diversity of Ammonia-Oxidizing Archaea and Bacteria in Aquarium Biofiltration Systems. Applied and Environmental Microbiology. February 2008, p. 894-900, Vol. 74, No. 3.
- V. Beran, L. Matlova, L. Dvorska, P. Svastova, and I. Pavlik. Distribution of mycobacteria in clinically healthy ornamental fish and their aquarium environment. Journal of Fish Diseases [J. Fish Dis.]; vol. 29, no. 7.
- Corals reveal impact of land use. ARC Center of Excellence: Coral Reef Studies. 31 may 2007. Retrieved: 28 August 2008. <http://www.coralcoe.org.au/news_stories/landimpacts.html>.
- Monks, Neale. Brackish Aquarium. Wet Web Media.Com. 2008. Retrieved: 28 August 2008. <http://www.wetwebmedia.com/ca/volume_4/V4I2/Brackish%20Systems/brackish.htm>.
- Jennings, Greg. The New Encyclopedia of the Saltwater Aquarium. Ontario, Canada: Firefly Books Ltd., 2007.
- Importance of Coral Reefs. "coral07a_240.jpg". NOAA Ocean Service Education. 25 March 2008. Article retrieved: 28 August 2008. Picture retrived: 28 August 2008. <http://oceanservice.noaa.gov/education/kits/corals/coral07_importance.html>.
- Strohmeyer, Carl. Treatment and Identification of Aeromonas and Vibrio in Aquariums and Ponds. 2008. Retrieved: 28 August 2008. <http://EzineArticles.com/?expert=Carl_Strohmeyer>.
- R. Grommen, I. Van Hauteghem, M. Van Wambeke, and W. Verstraete. An improved nitrifying enrichment to remove ammonium and nitrite from freshwater aquaria systems. Aquaculture. Volume 211, Issues 1-4, , 23 August 2002, Pages 115-124.
- Lenny Dauw, Roeland Grommen, and Willy Verstraete. Elevated salinity selects for a less diverse ammonia-oxidizing population in aquarium biofilters. FEMS Microbiology Ecology. Volume 52, Issue 1, 1 March 2005, Pages 1-11
- Monks, Neale. Brackish Water Aquarium FAQ. 2007. <http://homepage.mac.com/nmonks/Projects/brackishfaq.html>.
- Cycling Your New Aquarium. Well Aquariums by Living Pictures. 2004. Article retrived: 28 August 2008. <http://www.wallaquariums.com/cycling.htm>.
- Watson, Stan. Picture of Nitrosomonas europaea. US Department of Energy Office of Science. Picture retrived from Microbewiki: 28 August 2008. <http://microbewiki.kenyon.edu/index.php/Nitrosomonas_europaea>.
- John Hochheimer, Fred Wheaton. "Biological Filters: Trickling and RBC Design." <http://nsgd.gso.uri.edu/vsgcp/vsgcpc98001/vsgcpc98001_part6.pdf>.
- Picture of Aeromonas. NOAA Fisheries Service. Picture retrived from Microbewiki: 29 August 2008. <http://microbewiki.kenyon.edu/index.php/Aeromonas>.
Edited by Yuichiro S. and Marin N. at University of California-San Diego, 29 August 2008.