Tetanus in sub-Saharan Africa: Difference between revisions
No edit summary |
|||
| (49 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
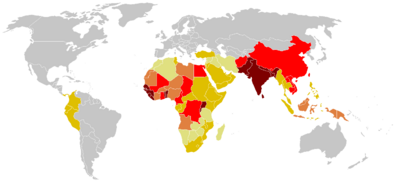
[[Image:Tetanos.png|thumb|400px|World Disease Incident (1990-2004). Strongly prevalent is colored in dark red, very few cases is in light yellow. (Courtesy of the World Health Organization, http://www.who.int)]] | [[Image:Tetanos.png|thumb|400px|World Disease Incident (1990-2004). Strongly prevalent is colored in dark red, very few cases is in light yellow. (Courtesy of the World Health Organization, http://www.who.int)]] | ||
==Introduction== | ==Introduction== | ||
Tetanus is a disease caused by the bacterium Clostridum tetani. The disease is caused by a neurotoxin released by the bacterium once inside the host, and causes deadly spastic paralysis. The neurotoxin is the second strongest toxin known to man, only | Tetanus is a disease caused by the bacterium Clostridum tetani. The disease is caused by a neurotoxin released by the bacterium once inside the host, and causes deadly spastic paralysis. The neurotoxin is the second strongest toxin known to man, the toxin which has a lethal dose of approximately 1ng/kg, is second only to the closely neurotoxin produced by the closely related Clostridum botulinum [4]. Records describing tetanus can be dated back to 5th century B.C. and are littered throughout medical military records from various periods of time [11,13]. The name is derived from the Greek word tetanos, from teinein, which means “to stretch,” [13]. The bacterium responsible for the disease, Clostridium tetani, was discovered in 1884 by Nicolaier, who injected animals with soil, and a pure culture was obtained in 1889 [11]. While tetanus used to be a rampant problem throughout the world in ages past, it has been controlled in recent decades through prevention in developed countries today. However, in developing regions of the world such as sub-Saharan Africa, it remains a critical issue in society: most maternal and neonatal deaths from this disease currently take place in this region [14]. With immunization from the tetanus toxoid, the disease is completely preventable. However, the WHO estimates up to 290,000 deaths each year, with 128,000 infant mortalities due to neonatal tetanus. In fact, the WHO reports it as the second leading cause of death from vaccine preventable disease [7]. | ||
==Description of Microbe== | ==Description of Microbe== | ||
| Line 10: | Line 11: | ||
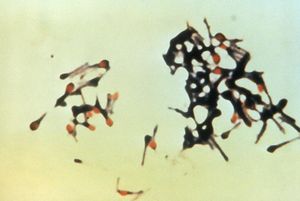
[[Image:Spore_forming_PHIL.jpg|thumb|alt=Alt|Clostridium tetani during sporulation. (Courtesy of the CDC Public Health Image Library ) http://phil.cdc.gov/]] | [[Image:Spore_forming_PHIL.jpg|thumb|alt=Alt|Clostridium tetani during sporulation. (Courtesy of the CDC Public Health Image Library ) http://phil.cdc.gov/]] | ||
===Structure and Function=== | ===Structure and Function=== | ||
Colstridum tetani are rod shaped bacilli, anaerobic fermenting, gram positive bacteria of the Clostridium genus. C. tetani are spore forming bacteria which form spores at their terminal ends, this gives them a tennis racquet like apprearance during sporulation [http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mmed&part=A1099]. C.tetani | |||
Colstridum tetani are rod shaped bacilli, anaerobic, strictly fermenting, gram positive bacteria of the [[Clostridium]] genus. C. tetani are spore-forming bacteria which form spores at their terminal ends, this gives them a tennis racquet-like apprearance during sporulation [http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mmed&part=A1099]. C.tetani live in low oxygen places such as soil and animal instestine [4]. C. tetani are the cause of the disease tetanus, and carry the toxin producing gene on a plasmid. The two species that have been found to be most susceptible to the tetanus neurotoxin are horses and humans. Tetanospasmin is the toxin produced by these toxigenic species, but non-toxic producing species have been found to exist. Most species of C. tetani are motile and form peritrichous flagella, and swarming is observed in some species. Other species are immotile and do not form flagella [2]. C. tetani is not the only species in the [[Clostridium]] genus that produces endotoxins which are medically important neurotoxins; a very similar organism, C. botulinum, produces the botulism toxin [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1765670&log$=activity]. | |||
C.tetani spores are incredibly resilient, and are able survive in the presence of oxygen, and very harsh conditions such are boiling water and disinfectants. Spores will germinate under low oxygen conditions, such as inside wounds on the human body. [3] | |||
===Genome=== | ===Genome=== | ||
The genome of toxigenic C.tetani E88 a contains a chromosome with 2,799,250-bp and a 28.6% G+C content. However the plasmid (pE88) containing the toxin gene (tetX) contains 74,082-bp and a much lower 24.5% G+C content. The plasmid also contains the regulator for the toxin gene. Both the regulator and the toxin gene show homology to | |||
The genome of toxigenic C.tetani E88 a contains a chromosome with 2,799,250-bp and a 28.6% G+C content. However, the plasmid (pE88) containing the toxin gene (tetX) contains 74,082-bp and a much lower 24.5% G+C content. The plasmid also contains the regulator for the toxin gene. Both the regulator and the toxin gene show homology to botulism neurotoxins and regulators. Unlike many other enteropathogens, the genome of C.Tetani has a very stable G+C content and not many transposable elements are found, which is evidence that it possesses genome stability and there has been a lack of lateral gene transfer. Some mobile elements are found on the plasmid however, including genes for transposase. The plasmid also contributes several other virulence factors, such a gene for collaginase, which may destroy host tissue integrity. The origin of the plasmid containing the toxin gene has yet to be revealed. It is not what environmental signals turn on tetX genes, or what regulates the activation of toxin transcription, however it is thought that alternative sigma factors may be responsible [http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=12552129]. | |||
===Bioenergetics=== | ===Bioenergetics=== | ||
During their | |||
During their vegetative state, C.tetani are anaerobic and can not survive in the presence of oxygen, and use fermentation as their energy source. C.tetani are peptolytic, and prefer to use ammino acids and protein substances for fermentations. However, they have been shown to be able to grow on glucose, but not on polypeptides. C.Tetani excretes many exopeptidase to break down the proteins in the host tissue and then imports the peptides into the cytosol, using a sodium ion symporter.Unlike some bacteria which use a proton motive force, C.tetani uses ATP as a product of fermentation to establish an ion motive force, by pumping out sodium ions via a V-type ATPase. Sodium-dependent systems are common among some thermophiles and other pathogenic bacteria. The influx of sodium is coupled to drive several transportation processes, some of which include multi-drug resistant exporters [4]. | |||
==Description of Disease== | ==Description of Disease== | ||
===Pathophysiology=== | ===Pathophysiology=== | ||
Two toxins are actually produced by Clostridium tetani, one responsible for the symptoms called tetanospasmin, and another with no known pathogenic properties called tetanolysin [12]. Tetanospasmin acts by cleaving proteins that are involved in vesicle fusion, thereby inhibiting neurotransmitter release. It acts at many sites located in the central nervous system, including the spinal cord and the brain, as well as sites in the autonomic nervous system, namely the sympathetic nervous system. Tetanospasmin is cleaved after translation into a light and heavy chain [12, 10]. It is the carboxyl terminal of the heavy chain that binds to gangliosides on peripheral nerves, allowing it to enter the system. Both binding and protein transport are attributed to the heavy chain. The toxin is passed to the central nervous system from the peripheral nervous system by way of trans-synaptic transmission and retrograde axonal transport, finally entering presynaptic cells [10]. In the presynaptic cells, the toxin’s light polypeptide chain cleaves an essential intergral membrane protein, called synaptobrevin, that is needed for the fusion of synaptic vesicles to the cell [10]. This action thus prevents the release of important inhibitory neurotransmitters such as GABA, to be released into the synaptic cleft. The final result of this inhibition is the unregulated release of excitatory signals, causing continual muscle contraction as well as painful muscle spasms that may ultimately lead to respiratory arrest and subsequently, death [12, 10]. | |||
The severity of the effect of the toxin greatly depends on the incubation time, which may range from 3 to 21 days, where around 8 days is most common. Typically, the shorter the incubation time, the more severe the disease and there is a higher chance of death [11]. Incubation time and severity are also directly related to both the amount and the location of the site of the bacteria; a shorter incubation time means that the site is closer to the central nervous system, explaining the more severe response to the toxin [11, 12]. | |||
===Symptoms=== | |||
The first symptoms of the disease are the cramping and/or twitching of muscles around the site of the wound in which the bacteria were introduced. This is usually followed by stiffness and uncontrollable muscle spasms around the jaw and the neck, eventually spreading throughout the body to the rest of the skeletal muscles. The spasms can last for weeks, and recovery may take several months, depending on the severity of the disease [11]. | |||
There are four types of tetanus; generalized, localized, cephalic, and neonatal [12]. Generalized tetanus is the most common, starting from the jaw and spreading throughout the body. Localized tetanus is very rare and as the name suggests, is confined to the area of the infected wound. It may, however, develop into generalized tetanus. Cephalic tetanus is also extremely rare, characterized by wounds on the face, and causes cranial nerve palsies [10]. Neonatal tetanus is very common in sub-Saharan Africa due to the improper conditions for delivery, and often times fatal. It commonly is transmitted when the umbilical cord is cut with an unsanitary instrument [11]. | |||
===Transmission of disease=== | ===Transmission of disease=== | ||
Tetanus is a disease caused by the bacterium Clostridium tetani, which releases an exotoxin inside the host [12]. Exposure to the bacteria usually happens by way of a wound; contact with soil containing dirt, feces, and other similar components raises the risk. Once a wound has allowed the bacteria to enter, they live in the necrotic tissue and produce the toxin, which is then carried throughout the body by the bloodstream and lymphatic system. Due to the fact that the organism is an anaerobe, the damaged tissue is required for its survival inside the host. The disease cannot be transmitted person to person [11]. | |||
===Clinical Treatment and Vaccinations=== | ===Clinical Treatment and Vaccinations=== | ||
Tetanus treatment begins with cleaning the wound, surgically if necessary, and | Tetanus treatment begins with cleaning the wound and removing, surgically if necessary, the source of the toxin which would be the infected and dead tissue To decrease the amount of bacteria,metronidazole treatment is encouraged but it actually has no effect on the actual toxin. In addition, the use of antibiotics, such as penicillin, clindamycin, erythromycin and metronidazle is used to battle tetanus longevity. To relax the muscles, often times bedrest is strongly encouraged coupled with muscle relaxants and sedatives. Drugs are given to control and attempt to prevent muscle spasms. It may even by advised to paralyze patients and use a mechanical ventilator if the muscle spasms are too severe. [9] | ||
Tetanus toxoid, a chemically modified toxin from tetanus that is no longer toxic but is still antigenic and is used as a vaccine, | Tetanus toxoid, a chemically modified toxin from tetanus that is no longer toxic but is still antigenic and is used as a vaccine, is a T cell dependent antigen that induces long-lasting immunity against tetanus. The immune response to tetanus toxoid vaccination shows that protection is dependent on the amount of IgG antibodies, the subclass distribution which is mainly dominated by IgG1, and the overall strength of binding between the antibody and the antigen of the produced antibodies. The binding strength between the antibody and the antigen is used to measure the efficiency of the antibodies to neutralize the antigen because it reflects the collective functional affinities of the antibodies that are formed during a humoral immune response. Tetanus toxoid and improved wound management, including the use of tetanus prophylaxis has shown to decline the rate of tetanus throughout the world. Prophylaxis is the medical procedure that aims to prevent a disease rather than to cure it. However, for areas like sub-Sahara Africa, the necessary resources may not be available. [10] | ||
Passive immunization with human tetanus immunoglobulin curtails the longevity and possibly assuages the severity of tetanus. The antiserum is derived from a plasma from a healthy human tetanus immune donor and has a half-life of approximately 27 days. There is also equine tetanus immunoglobulin available and is much less expensive to produce. However, it has a half-life of only two days and has an increased chance of extreme, life-threatening, allergic reactions. Despite these dangers, equine immunogobulin is the standard throughout the world because of its cheap production cost. [10] | |||
===Prevention=== | ===Prevention=== | ||
Tetanus is preventable by continued immunization. Immunization is expected to last ten years. In the United States, immunizations begin at birth with the DTaP series of shots that is a three-in-1 vaccine that targets diphtheria, pertussis, and tetanus. A Td vaccine is also a booster used to continue immunity from the ages of 11 to 65. If an individual suffers an injury such as a punctured-wound, a tetanus booster immunization should be administered immediately if more than ten years have passed since the last immunization. Although maintained immunization is the key way to prevent tetanus, developing areas such as the sub-Sahara | Tetanus is preventable by continued immunization. Immunization is expected to last ten years. In the United States, immunizations begin at birth with the DTaP series of booster shots that is a three-in-1 vaccine that targets diphtheria, pertussis, and tetanus. A Td vaccine is also a booster used to continue immunity from the ages of 11 to 65. If an individual suffers an injury such as a punctured-wound, a tetanus booster immunization should be administered immediately if more than ten years have passed since the last immunization. However, tetanus antibodies can take up to two weeks to form. Thus in extremely fatal cases of tetanus, the possibility of a successful recovery is slim. Although maintained immunization is the key way to prevent tetanus, developing areas such as the sub-Sahara do not have sufficient resources for continued prevention, which is why there is an increased infection rate in those areas. [24, 25] | ||
==Why is this disease prevalent in sub-Saharan Africa?== | ==Why is this disease prevalent in sub-Saharan Africa?== | ||
[[Image:PHIL_CDC.jpg|thumb|alt=Alt|An infant suffering from Neonatal Tetanus.(Courtesy of the CDC Public Health Image Library) http://phil.cdc.gov/]] | [[Image:PHIL_CDC.jpg|thumb|alt=Alt|An infant suffering from Neonatal Tetanus.(Courtesy of the CDC Public Health Image Library) http://phil.cdc.gov/]] | ||
Since maternal and neonatal tetanus cases are prevalent in poor, developing communities, many newborns die due to poor access to good healthcare. Furthermore, people in major developing countries, such as people in | Since maternal and neonatal tetanus cases are prevalent in poor, developing communities, many newborns die due to poor access to good healthcare. Furthermore, people in major developing countries, such as people in sub-Saharan Africa, may not receive the most promising vaccination coverage or immunization. Most women of childbearing age in the developing country are not even provided with immunization with tetanus toxoid, which immunizes women of childbearing age in order to enhance transfer of tetanus antibodies into their systems and protecting their newborns from catching neonatal tetanus [5]. | ||
Tetanus is most common in hot, damp climates with soil rich in organic matter for spores to grow. This explains why Sub-Saharan Africa is such a prominent country for neonatal tetanus. | Tetanus is most common in hot, damp climates with soil rich in organic matter for spores to grow. This explains why Sub-Saharan Africa is such a prominent country for neonatal tetanus. Tetanus spores, unfortunately, cannot be removed from the environment, causing tetanus to be spread through spores that were exposed to the intestines and feces of various animals. Spores can also be launched into the body through puncture wounds that have already been contaminated by the soil, animal waste, or any nature of debris [6]. | ||
Neonatal tetanus, the most common tetanus in the developing world, is the result of contamination of the umbilical stump by the usage of non-sterile instruments or animal dung to cut the cord after delivery. Many newborn deliveries are performed at homes by untrained birth attendants. Most people in | Neonatal tetanus, the most common tetanus in the developing world, is the result of contamination of the umbilical stump by the usage of non-sterile instruments or animal dung to cut the cord after delivery. Many newborn deliveries are performed at homes by untrained birth attendants. Most people in sub-Saharan African do not have a profound understanding of dangerous causes of neonatal tetanus on their children [5]. In fact, neonatal tetanus is a huge problem in this region because of the lack of maternal and paternal education, the unavailability of effective contraception, improper hygienic deliveries and cord care. Large numbers of sub-Saharan Africa physicians leave their homeland to practice medicine or upon completion of their medical school practices in search of expanding their work experience in higher-income countries. This leaves many Africans in distress of receiving proper healthcare systems. In sub-Saharan Africa, life expectancy is only 50 years with about less than 32% of the deliveries are attended by trained personnel. People in this region suffer from poor health systems, which greatly hinder the country from taking a huge step towards improving their infrastructures of healthcare and eliminating diseases [5,6]. | ||
==What is being done to address this problem== | ==What is being done to address this problem== | ||
[[Image:VACCINATION_PHIL.jpg|thumb|alt=Alt| | [[Image:VACCINATION_PHIL.jpg|thumb|alt=Alt|.(Courtesy of the CDC Public Health Image Library) http://phil.cdc.gov/]] | ||
In 1989 the World Health Assembly set a goal to eliminate maternal and neonatal tetanus in Africa by 1995. However, many of the African countries did not obtain this goal. The World Health Organization (WHO) and the United Nation Children’s Fund (UNICEF) set a new goal to eliminate neonatal tetanus globally by 2005; this goal was also missed [18]. In 2005, in response to these setbacks and challenges in global immunization, WHO and UNICEF created the Global Immunization Vision and Strategy (GIVS) - the first global ten-year plan to battle vaccine-preventable diseases through immunization [19]. GIVS primary goal is to decrease the deaths from vaccine-preventable diseases by two-thirds by 2015 compared to 2000. The four aims of GIVS are to immunize more people against more diseases, to introduce people to a variety of new vaccines and technologies, to combine other health interventions with the immunizations, and lastly to administer vaccination programmes within the framework of global interdependence [19]. With the help of GIVS, routine immunization coverage, stationary through the 1990s, has recently begun to rise, specifically in Sub-Saharan Africa. | |||
Pampers has paired with UNICEF to help reach its goal of decreasing neonatal and maternal tetanus over the next couple of years. To help, Pampers has donated the cost of one tetanus vaccine to the US Fund for UNICEF for every bag of specially-labeled diapers and wipes bought in the US and Canada between 2/10/09 and 5/1/09 [20]. In its first year, supporters helped Pampers donate enough money for more than forty-five million vaccines against tetanus in developing countries [20]. | |||
In September of 2000, the United Nations adopted the Millennium Development Goals (MDGs) as part of the United Nations Development Programme [21]. The MDGs include eight goals to be carried out by 2015 that focus on decreasing developmental challenges by eradicating hunger and poverty, improving education and gender equality, reducing child mortality, bettering maternal health, fighting prevalent diseases and developing global partnerships for development [21]. The MDGs work through global partnerships in which rich countries help poorer countries through aid, fair trade, and funding. While the poorer countries agree to govern better and give their people with better healthcare and education [21]. | |||
The Integrated Management of Childhood Illnesses (IMCI) is a similar program developed by the WHO and UNICEF that centers on the well-being of children by utilizing preventative and remedial measures. These measures are executed by families, communities, and health care facilities to decrease morbidity and mortality from common illnesses and by the promotion of growth and development in children under the age of five [22]. In health care facilities, IMCI emphasizes correct determination of illness and appropriate treatment; in home settings, it emphasizes proper administration of prescribed care and improved preventative care [22]. IMCI looks at all factors that put children at risk for illness which is extremely important in developing countries where children are often suffering from multiple illnesses. | |||
Save the Children is another group that helps children in almost 40 developing countries around the world live better lives by partnering with local NGOs and donors, like UNICEF, improve health care. In Africa, it first started in Swaziland in the 1960s and has spread to thirteen other African countries [23]. Save the Children aims to make sure that children in developing countries are able to learn, grow, and live healthy lives. | |||
==What else could be done to address this problem== | ==What else could be done to address this problem== | ||
In sub-Saharan Africa and in the world, tetanus vaccination has risen consistently over the past 20 years, yet there is still a considerable amount left to be done. In 2008, it was reported that 5 out of every 1000 children born in Africa would die from tetanus [15]. The actual numbers have been put much higher: the WHO estimates that 14 percent of infants that die before one month of age, die from tetanus [15]. WHO released a report in 1999 saying an estimated 124,072 cases of tetanus occurred in the Africa region, but only 3,883 cases were reported [16]. | |||
It is difficult to tell the exact magnitude of tetanus on the population. The lack of infrastructure in Africa leads to such statistics in more ways than one. If infrastructure were up to par, infants would be brought to a proper facility and if they were to die, their deaths would be recorded. But the second and more serious fault is that the lack of infrastructure leads to birth conditions that cause the initial infection. Unsanitary conditions caused by improper facilities leads to infection. | |||
Education Campaigns have been fostered in certain regions, training on proper cleaning of wounds, disinfecting tools, proper birthing procedures and conditions. These programs have some success but they are not widespread enough for relevant statistics to appear, let alone actual make a contribution to such a large problem. | |||
Education, proper facilities, and vaccination are the goals. These are the conditions that will put a hold on the "silent killer." A comprehensive training of clinic staff, hospital staff and a widespread chain of facilities are needed to standardize the level of care to acceptable heights. | |||
It is rather difficult to build the entire sub-Saharan Africa region to such a standard, and this is generally seen as a more long term goal. But something that has been occurring relatively quickly is vaccination. At $.07 USD per dose, it is a wonder that so many are still without vaccination [17]. | |||
There are literally hundreds of things people can do to help this situation - donate to UNICEF, buy Pampers, volunteer, raise money, raise awareness to name a few. The question should be less what can be done, and more why haven’t I done anything? So for what more can be done: more people can get involved. | |||
==Summary== | |||
Clostridium tetani produces the second strongest toxin known to man. This toxin causes the muscle spasms and spastic muscle paralysis which can ultimately lead to death. An estimated 290,000 people worldwide die from this disease with sub-Saharan Africa as a leading contributor to this statistic. Currently, Africa's infrastructure is below par and thus lacks proper facilities to handle/prevent the disease. | |||
Raising Africa's infrastructure to acceptable conditions may be the Challenge of our century. In an age where prosperity and progress reach new heights, Africa remains torn by war, famine, poverty and disease. A lot of things are uncertain and possibly out of our capablities, but tetanus is a killer that can be stopped. It is hard to justify such a devastating disease prospering anywhere on Earth with an effective and cheap vaccine available. The reason tetanus is still a threat to mass amounts of people in Sub-Saharan Africa can be attributed to the lack of infrastructure and our lack of exposure or possible indifference to the disease. There is a lot that can be done to prevent future generations from knowing this fear. Hopefully this topic will be out of date soon. Feel free to contribute anyway you can. | |||
==References== | ==References== | ||
1.) [http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=1513&lvl=3&p=mapview&p=has_linkout&p=blast_url&p=genome_blast&lin=f&keep=1&srchmode=1&unlock NCBI taxonomy] | 1.) [http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=1513&lvl=3&p=mapview&p=has_linkout&p=blast_url&p=genome_blast&lin=f&keep=1&srchmode=1&unlock NCBI taxonomy] | ||
| Line 71: | Line 108: | ||
8.) [http://www.ncbi.nlm.nih.gov/pubmed/18539369 van Riet E, Retra K, Adegnika AA, Jol-van der Zijde CM, Uh HW, Lell B, Issifou S, Kremsner PG, Yazdanbakhsh M, van Tol MJ, Hartgers FC. "Cellular and humoral responses to tetanus vaccination in Gabonese Children." 1: Vaccine. 2008 Jul 4;26(29-30):3690-5. Epub 2008 May 15.] | 8.) [http://www.ncbi.nlm.nih.gov/pubmed/18539369 van Riet E, Retra K, Adegnika AA, Jol-van der Zijde CM, Uh HW, Lell B, Issifou S, Kremsner PG, Yazdanbakhsh M, van Tol MJ, Hartgers FC. "Cellular and humoral responses to tetanus vaccination in Gabonese Children." 1: Vaccine. 2008 Jul 4;26(29-30):3690-5. Epub 2008 May 15.] | ||
9.) [http://www.nlm.nih.gov/medlineplus/ency/article/000615.htm#Treatment Medline Plus] | 9.) [http://www.nlm.nih.gov/medlineplus/ency/article/000615.htm#Treatment Medline Plus Medline Plus. 2008.] | ||
[http://phil.cdc.gov/phil/home.asp Images courtesy of Center for Control and Disease Prevention. Public Health Image Library (PHIL).] | [http://phil.cdc.gov/phil/home.asp Images courtesy of Center for Control and Disease Prevention. Public Health Image Library (PHIL).] | ||
10.) [http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=10945801 Farrar JJ, Yen LM, Cook T, Fairweather N, Binh N, Parry J, Parry CM. "Tetanus". Journal of Neurology, Neurosurgery, and Psychiatry. 2000. Volume 69. pp. 292-301.] | |||
11.) [http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/tetanus-508.pdf “Tetanus.” CDC Pink Book. Retrieved 22 August 2009.] | |||
12.) [http://bja.oxfordjournals.org/cgi/content/full/87/3/477 T. M. Cook , R. T. Protheroe , and J. M. Handel. “Tetanus: a review of the literature.” British Journal of Anaesthesiology. Volume 87. pp. 477-487.] | |||
13.) [http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1073859&blobtype=pdf Pearce JMS. “Notes on tetanus (lockjaw).” J Neurol Neurosurg Psychiatry. 1996. Volume 60. p.332.] | |||
14.) [http://www.unicef.org/sowc09/docs/SOWC09-Panel-3.1-EN.pdf Unicef. Retrieved 25 August 2009.] | |||
15.) [http://www.unicef.org/wcaro/2009_3292.html Seydou Amadou Oumarou. "Niger: fighting against a silent killer called tetanus." 2009] | |||
16.) [http://www.afro.who.int/nnt/reports/elimination.pdf "MATERNAL AND NEONATAL TETANUS ELIMINATION." Retrieved 27 August 2009] | |||
17.) [http://www.enewsbuilder.net/focalpoint/e_article001294805.cfm?x=b11,0,w Christine Edier. "UNICEF and Proctor & Gamble Fight to Eliminate Maternal and Newborn Tetanus."] | |||
18.) [http://www.afro.who.int/nnt/planofaction.pdf "Maternal and Neonatal Tetanus Elimination in: Africa Region." WHO/ UNICEF. 2001.] | |||
19.) [http://www.who.int/immunization/givs/en/index.html "Global Immunization Vision and Strategy." WHO. 2009.] | |||
20.) [http://www.pampers.com/en_US/unicef/tab/partner "Pampers and UNICEF: Working Together for Healthy Babies." Procter & Gamble. 2009.] | |||
21.) [http://www.undp.org/mdg/basics.shtml "About the MDGs: Basics." UNDP. 2009.] | |||
22.) [http://www.afro.who.int/imci/index.html "Integrated Management of Childhood Illness (IMCI)." WHO/AFRO: Division of Family and Reproductive Health. 2009.] | |||
23.) [http://www.savethechildren.org/countries/africa/Africa-20Fact-20Sheet-204-09-20rev.pdf "Save the Children’s | |||
Presence in Africa. Fact Sheet: May 2009." Save the Children. 2009.] | |||
24.) [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TD4-476W4W1-7R&_user=4429&_coverDate=12%2F31%2F1992&_rdoc=1&_fmt=&_orig=search&_sort=d&_docanchor=&view=c&_acct=C000059602&_version=1&_urlVersion=0&_userid=4429&md5=9ba2572a95ebb910bb6f9043a70d4028 "Lack of early antitoxin response to tetanus booster" Public Health Laboratory Service, Communicable Disease Surveillance Centre, 61 Colindale Avenue, London NW9 5EQ, UK. 1992.] | |||
25.) [http://www.cdc.gov/mmwr/preview/mmwrhtml/00041645.htm Diphtheria, tetanus, and pertussis: recommendations for vaccine use and other preventive measures. Recommendations of the Immunization Practices Advisory committee (ACIP). 1991.] | |||
Edited by [mailto:fpeturss@ucsd.edu Freyr Petursson], [mailto:mglashee@ucsd.edu Megan Glasheen], Meghan Wu,[mailto:chrisfascenda@gmail.com Chris Fascenda], [mailto:dgcervan@ucsd.edu David Cervantes], Lisa Sugimori, students of [mailto:ralarsen@ucsd.edu Rachel Larsen] | |||
Latest revision as of 15:56, 16 September 2010
Introduction
Tetanus is a disease caused by the bacterium Clostridum tetani. The disease is caused by a neurotoxin released by the bacterium once inside the host, and causes deadly spastic paralysis. The neurotoxin is the second strongest toxin known to man, the toxin which has a lethal dose of approximately 1ng/kg, is second only to the closely neurotoxin produced by the closely related Clostridum botulinum [4]. Records describing tetanus can be dated back to 5th century B.C. and are littered throughout medical military records from various periods of time [11,13]. The name is derived from the Greek word tetanos, from teinein, which means “to stretch,” [13]. The bacterium responsible for the disease, Clostridium tetani, was discovered in 1884 by Nicolaier, who injected animals with soil, and a pure culture was obtained in 1889 [11]. While tetanus used to be a rampant problem throughout the world in ages past, it has been controlled in recent decades through prevention in developed countries today. However, in developing regions of the world such as sub-Saharan Africa, it remains a critical issue in society: most maternal and neonatal deaths from this disease currently take place in this region [14]. With immunization from the tetanus toxoid, the disease is completely preventable. However, the WHO estimates up to 290,000 deaths each year, with 128,000 infant mortalities due to neonatal tetanus. In fact, the WHO reports it as the second leading cause of death from vaccine preventable disease [7].
Description of Microbe
Lineage( abbreviated ) Bacteria; Firmicutes; Clostridia; Clostridiales; Clostridiaceae; Clostridium [1]
Structure and Function
Colstridum tetani are rod shaped bacilli, anaerobic, strictly fermenting, gram positive bacteria of the Clostridium genus. C. tetani are spore-forming bacteria which form spores at their terminal ends, this gives them a tennis racquet-like apprearance during sporulation [2]. C.tetani live in low oxygen places such as soil and animal instestine [4]. C. tetani are the cause of the disease tetanus, and carry the toxin producing gene on a plasmid. The two species that have been found to be most susceptible to the tetanus neurotoxin are horses and humans. Tetanospasmin is the toxin produced by these toxigenic species, but non-toxic producing species have been found to exist. Most species of C. tetani are motile and form peritrichous flagella, and swarming is observed in some species. Other species are immotile and do not form flagella [2]. C. tetani is not the only species in the Clostridium genus that produces endotoxins which are medically important neurotoxins; a very similar organism, C. botulinum, produces the botulism toxin [3]. C.tetani spores are incredibly resilient, and are able survive in the presence of oxygen, and very harsh conditions such are boiling water and disinfectants. Spores will germinate under low oxygen conditions, such as inside wounds on the human body. [3]
Genome
The genome of toxigenic C.tetani E88 a contains a chromosome with 2,799,250-bp and a 28.6% G+C content. However, the plasmid (pE88) containing the toxin gene (tetX) contains 74,082-bp and a much lower 24.5% G+C content. The plasmid also contains the regulator for the toxin gene. Both the regulator and the toxin gene show homology to botulism neurotoxins and regulators. Unlike many other enteropathogens, the genome of C.Tetani has a very stable G+C content and not many transposable elements are found, which is evidence that it possesses genome stability and there has been a lack of lateral gene transfer. Some mobile elements are found on the plasmid however, including genes for transposase. The plasmid also contributes several other virulence factors, such a gene for collaginase, which may destroy host tissue integrity. The origin of the plasmid containing the toxin gene has yet to be revealed. It is not what environmental signals turn on tetX genes, or what regulates the activation of toxin transcription, however it is thought that alternative sigma factors may be responsible [4].
Bioenergetics
During their vegetative state, C.tetani are anaerobic and can not survive in the presence of oxygen, and use fermentation as their energy source. C.tetani are peptolytic, and prefer to use ammino acids and protein substances for fermentations. However, they have been shown to be able to grow on glucose, but not on polypeptides. C.Tetani excretes many exopeptidase to break down the proteins in the host tissue and then imports the peptides into the cytosol, using a sodium ion symporter.Unlike some bacteria which use a proton motive force, C.tetani uses ATP as a product of fermentation to establish an ion motive force, by pumping out sodium ions via a V-type ATPase. Sodium-dependent systems are common among some thermophiles and other pathogenic bacteria. The influx of sodium is coupled to drive several transportation processes, some of which include multi-drug resistant exporters [4].
Description of Disease
Pathophysiology
Two toxins are actually produced by Clostridium tetani, one responsible for the symptoms called tetanospasmin, and another with no known pathogenic properties called tetanolysin [12]. Tetanospasmin acts by cleaving proteins that are involved in vesicle fusion, thereby inhibiting neurotransmitter release. It acts at many sites located in the central nervous system, including the spinal cord and the brain, as well as sites in the autonomic nervous system, namely the sympathetic nervous system. Tetanospasmin is cleaved after translation into a light and heavy chain [12, 10]. It is the carboxyl terminal of the heavy chain that binds to gangliosides on peripheral nerves, allowing it to enter the system. Both binding and protein transport are attributed to the heavy chain. The toxin is passed to the central nervous system from the peripheral nervous system by way of trans-synaptic transmission and retrograde axonal transport, finally entering presynaptic cells [10]. In the presynaptic cells, the toxin’s light polypeptide chain cleaves an essential intergral membrane protein, called synaptobrevin, that is needed for the fusion of synaptic vesicles to the cell [10]. This action thus prevents the release of important inhibitory neurotransmitters such as GABA, to be released into the synaptic cleft. The final result of this inhibition is the unregulated release of excitatory signals, causing continual muscle contraction as well as painful muscle spasms that may ultimately lead to respiratory arrest and subsequently, death [12, 10].
The severity of the effect of the toxin greatly depends on the incubation time, which may range from 3 to 21 days, where around 8 days is most common. Typically, the shorter the incubation time, the more severe the disease and there is a higher chance of death [11]. Incubation time and severity are also directly related to both the amount and the location of the site of the bacteria; a shorter incubation time means that the site is closer to the central nervous system, explaining the more severe response to the toxin [11, 12].
Symptoms
The first symptoms of the disease are the cramping and/or twitching of muscles around the site of the wound in which the bacteria were introduced. This is usually followed by stiffness and uncontrollable muscle spasms around the jaw and the neck, eventually spreading throughout the body to the rest of the skeletal muscles. The spasms can last for weeks, and recovery may take several months, depending on the severity of the disease [11].
There are four types of tetanus; generalized, localized, cephalic, and neonatal [12]. Generalized tetanus is the most common, starting from the jaw and spreading throughout the body. Localized tetanus is very rare and as the name suggests, is confined to the area of the infected wound. It may, however, develop into generalized tetanus. Cephalic tetanus is also extremely rare, characterized by wounds on the face, and causes cranial nerve palsies [10]. Neonatal tetanus is very common in sub-Saharan Africa due to the improper conditions for delivery, and often times fatal. It commonly is transmitted when the umbilical cord is cut with an unsanitary instrument [11].
Transmission of disease
Tetanus is a disease caused by the bacterium Clostridium tetani, which releases an exotoxin inside the host [12]. Exposure to the bacteria usually happens by way of a wound; contact with soil containing dirt, feces, and other similar components raises the risk. Once a wound has allowed the bacteria to enter, they live in the necrotic tissue and produce the toxin, which is then carried throughout the body by the bloodstream and lymphatic system. Due to the fact that the organism is an anaerobe, the damaged tissue is required for its survival inside the host. The disease cannot be transmitted person to person [11].
Clinical Treatment and Vaccinations
Tetanus treatment begins with cleaning the wound and removing, surgically if necessary, the source of the toxin which would be the infected and dead tissue To decrease the amount of bacteria,metronidazole treatment is encouraged but it actually has no effect on the actual toxin. In addition, the use of antibiotics, such as penicillin, clindamycin, erythromycin and metronidazle is used to battle tetanus longevity. To relax the muscles, often times bedrest is strongly encouraged coupled with muscle relaxants and sedatives. Drugs are given to control and attempt to prevent muscle spasms. It may even by advised to paralyze patients and use a mechanical ventilator if the muscle spasms are too severe. [9]
Tetanus toxoid, a chemically modified toxin from tetanus that is no longer toxic but is still antigenic and is used as a vaccine, is a T cell dependent antigen that induces long-lasting immunity against tetanus. The immune response to tetanus toxoid vaccination shows that protection is dependent on the amount of IgG antibodies, the subclass distribution which is mainly dominated by IgG1, and the overall strength of binding between the antibody and the antigen of the produced antibodies. The binding strength between the antibody and the antigen is used to measure the efficiency of the antibodies to neutralize the antigen because it reflects the collective functional affinities of the antibodies that are formed during a humoral immune response. Tetanus toxoid and improved wound management, including the use of tetanus prophylaxis has shown to decline the rate of tetanus throughout the world. Prophylaxis is the medical procedure that aims to prevent a disease rather than to cure it. However, for areas like sub-Sahara Africa, the necessary resources may not be available. [10]
Passive immunization with human tetanus immunoglobulin curtails the longevity and possibly assuages the severity of tetanus. The antiserum is derived from a plasma from a healthy human tetanus immune donor and has a half-life of approximately 27 days. There is also equine tetanus immunoglobulin available and is much less expensive to produce. However, it has a half-life of only two days and has an increased chance of extreme, life-threatening, allergic reactions. Despite these dangers, equine immunogobulin is the standard throughout the world because of its cheap production cost. [10]
Prevention
Tetanus is preventable by continued immunization. Immunization is expected to last ten years. In the United States, immunizations begin at birth with the DTaP series of booster shots that is a three-in-1 vaccine that targets diphtheria, pertussis, and tetanus. A Td vaccine is also a booster used to continue immunity from the ages of 11 to 65. If an individual suffers an injury such as a punctured-wound, a tetanus booster immunization should be administered immediately if more than ten years have passed since the last immunization. However, tetanus antibodies can take up to two weeks to form. Thus in extremely fatal cases of tetanus, the possibility of a successful recovery is slim. Although maintained immunization is the key way to prevent tetanus, developing areas such as the sub-Sahara do not have sufficient resources for continued prevention, which is why there is an increased infection rate in those areas. [24, 25]
Why is this disease prevalent in sub-Saharan Africa?

Since maternal and neonatal tetanus cases are prevalent in poor, developing communities, many newborns die due to poor access to good healthcare. Furthermore, people in major developing countries, such as people in sub-Saharan Africa, may not receive the most promising vaccination coverage or immunization. Most women of childbearing age in the developing country are not even provided with immunization with tetanus toxoid, which immunizes women of childbearing age in order to enhance transfer of tetanus antibodies into their systems and protecting their newborns from catching neonatal tetanus [5].
Tetanus is most common in hot, damp climates with soil rich in organic matter for spores to grow. This explains why Sub-Saharan Africa is such a prominent country for neonatal tetanus. Tetanus spores, unfortunately, cannot be removed from the environment, causing tetanus to be spread through spores that were exposed to the intestines and feces of various animals. Spores can also be launched into the body through puncture wounds that have already been contaminated by the soil, animal waste, or any nature of debris [6].
Neonatal tetanus, the most common tetanus in the developing world, is the result of contamination of the umbilical stump by the usage of non-sterile instruments or animal dung to cut the cord after delivery. Many newborn deliveries are performed at homes by untrained birth attendants. Most people in sub-Saharan African do not have a profound understanding of dangerous causes of neonatal tetanus on their children [5]. In fact, neonatal tetanus is a huge problem in this region because of the lack of maternal and paternal education, the unavailability of effective contraception, improper hygienic deliveries and cord care. Large numbers of sub-Saharan Africa physicians leave their homeland to practice medicine or upon completion of their medical school practices in search of expanding their work experience in higher-income countries. This leaves many Africans in distress of receiving proper healthcare systems. In sub-Saharan Africa, life expectancy is only 50 years with about less than 32% of the deliveries are attended by trained personnel. People in this region suffer from poor health systems, which greatly hinder the country from taking a huge step towards improving their infrastructures of healthcare and eliminating diseases [5,6].
What is being done to address this problem
In 1989 the World Health Assembly set a goal to eliminate maternal and neonatal tetanus in Africa by 1995. However, many of the African countries did not obtain this goal. The World Health Organization (WHO) and the United Nation Children’s Fund (UNICEF) set a new goal to eliminate neonatal tetanus globally by 2005; this goal was also missed [18]. In 2005, in response to these setbacks and challenges in global immunization, WHO and UNICEF created the Global Immunization Vision and Strategy (GIVS) - the first global ten-year plan to battle vaccine-preventable diseases through immunization [19]. GIVS primary goal is to decrease the deaths from vaccine-preventable diseases by two-thirds by 2015 compared to 2000. The four aims of GIVS are to immunize more people against more diseases, to introduce people to a variety of new vaccines and technologies, to combine other health interventions with the immunizations, and lastly to administer vaccination programmes within the framework of global interdependence [19]. With the help of GIVS, routine immunization coverage, stationary through the 1990s, has recently begun to rise, specifically in Sub-Saharan Africa.
Pampers has paired with UNICEF to help reach its goal of decreasing neonatal and maternal tetanus over the next couple of years. To help, Pampers has donated the cost of one tetanus vaccine to the US Fund for UNICEF for every bag of specially-labeled diapers and wipes bought in the US and Canada between 2/10/09 and 5/1/09 [20]. In its first year, supporters helped Pampers donate enough money for more than forty-five million vaccines against tetanus in developing countries [20].
In September of 2000, the United Nations adopted the Millennium Development Goals (MDGs) as part of the United Nations Development Programme [21]. The MDGs include eight goals to be carried out by 2015 that focus on decreasing developmental challenges by eradicating hunger and poverty, improving education and gender equality, reducing child mortality, bettering maternal health, fighting prevalent diseases and developing global partnerships for development [21]. The MDGs work through global partnerships in which rich countries help poorer countries through aid, fair trade, and funding. While the poorer countries agree to govern better and give their people with better healthcare and education [21].
The Integrated Management of Childhood Illnesses (IMCI) is a similar program developed by the WHO and UNICEF that centers on the well-being of children by utilizing preventative and remedial measures. These measures are executed by families, communities, and health care facilities to decrease morbidity and mortality from common illnesses and by the promotion of growth and development in children under the age of five [22]. In health care facilities, IMCI emphasizes correct determination of illness and appropriate treatment; in home settings, it emphasizes proper administration of prescribed care and improved preventative care [22]. IMCI looks at all factors that put children at risk for illness which is extremely important in developing countries where children are often suffering from multiple illnesses.
Save the Children is another group that helps children in almost 40 developing countries around the world live better lives by partnering with local NGOs and donors, like UNICEF, improve health care. In Africa, it first started in Swaziland in the 1960s and has spread to thirteen other African countries [23]. Save the Children aims to make sure that children in developing countries are able to learn, grow, and live healthy lives.
What else could be done to address this problem
In sub-Saharan Africa and in the world, tetanus vaccination has risen consistently over the past 20 years, yet there is still a considerable amount left to be done. In 2008, it was reported that 5 out of every 1000 children born in Africa would die from tetanus [15]. The actual numbers have been put much higher: the WHO estimates that 14 percent of infants that die before one month of age, die from tetanus [15]. WHO released a report in 1999 saying an estimated 124,072 cases of tetanus occurred in the Africa region, but only 3,883 cases were reported [16].
It is difficult to tell the exact magnitude of tetanus on the population. The lack of infrastructure in Africa leads to such statistics in more ways than one. If infrastructure were up to par, infants would be brought to a proper facility and if they were to die, their deaths would be recorded. But the second and more serious fault is that the lack of infrastructure leads to birth conditions that cause the initial infection. Unsanitary conditions caused by improper facilities leads to infection.
Education Campaigns have been fostered in certain regions, training on proper cleaning of wounds, disinfecting tools, proper birthing procedures and conditions. These programs have some success but they are not widespread enough for relevant statistics to appear, let alone actual make a contribution to such a large problem.
Education, proper facilities, and vaccination are the goals. These are the conditions that will put a hold on the "silent killer." A comprehensive training of clinic staff, hospital staff and a widespread chain of facilities are needed to standardize the level of care to acceptable heights. It is rather difficult to build the entire sub-Saharan Africa region to such a standard, and this is generally seen as a more long term goal. But something that has been occurring relatively quickly is vaccination. At $.07 USD per dose, it is a wonder that so many are still without vaccination [17].
There are literally hundreds of things people can do to help this situation - donate to UNICEF, buy Pampers, volunteer, raise money, raise awareness to name a few. The question should be less what can be done, and more why haven’t I done anything? So for what more can be done: more people can get involved.
Summary
Clostridium tetani produces the second strongest toxin known to man. This toxin causes the muscle spasms and spastic muscle paralysis which can ultimately lead to death. An estimated 290,000 people worldwide die from this disease with sub-Saharan Africa as a leading contributor to this statistic. Currently, Africa's infrastructure is below par and thus lacks proper facilities to handle/prevent the disease.
Raising Africa's infrastructure to acceptable conditions may be the Challenge of our century. In an age where prosperity and progress reach new heights, Africa remains torn by war, famine, poverty and disease. A lot of things are uncertain and possibly out of our capablities, but tetanus is a killer that can be stopped. It is hard to justify such a devastating disease prospering anywhere on Earth with an effective and cheap vaccine available. The reason tetanus is still a threat to mass amounts of people in Sub-Saharan Africa can be attributed to the lack of infrastructure and our lack of exposure or possible indifference to the disease. There is a lot that can be done to prevent future generations from knowing this fear. Hopefully this topic will be out of date soon. Feel free to contribute anyway you can.
References
1.) NCBI taxonomy
7.) World Health Organization. Vaccine-Preventable Diseases. "Tetanus". http://www.who.int. WHO 2009.
9.) Medline Plus Medline Plus. 2008.
Images courtesy of Center for Control and Disease Prevention. Public Health Image Library (PHIL).
11.) “Tetanus.” CDC Pink Book. Retrieved 22 August 2009.
13.) Pearce JMS. “Notes on tetanus (lockjaw).” J Neurol Neurosurg Psychiatry. 1996. Volume 60. p.332.
14.) Unicef. Retrieved 25 August 2009.
15.) Seydou Amadou Oumarou. "Niger: fighting against a silent killer called tetanus." 2009
16.) "MATERNAL AND NEONATAL TETANUS ELIMINATION." Retrieved 27 August 2009
17.) Christine Edier. "UNICEF and Proctor & Gamble Fight to Eliminate Maternal and Newborn Tetanus."
18.) "Maternal and Neonatal Tetanus Elimination in: Africa Region." WHO/ UNICEF. 2001.
19.) "Global Immunization Vision and Strategy." WHO. 2009.
20.) "Pampers and UNICEF: Working Together for Healthy Babies." Procter & Gamble. 2009.
21.) "About the MDGs: Basics." UNDP. 2009.
23.) [http://www.savethechildren.org/countries/africa/Africa-20Fact-20Sheet-204-09-20rev.pdf "Save the Children’s Presence in Africa. Fact Sheet: May 2009." Save the Children. 2009.]
Edited by Freyr Petursson, Megan Glasheen, Meghan Wu,Chris Fascenda, David Cervantes, Lisa Sugimori, students of Rachel Larsen
