Salmonella typhimurium in the United States: Difference between revisions
No edit summary |
No edit summary |
||
| (28 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
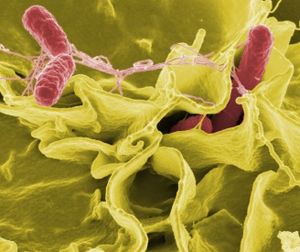
[[Image:Salmonella_Picture.JPG|thumb|alt=Puzzle globe logo|S. Typhimurium]] | {{Uncurated}} | ||
[[Image:Salmonella_Picture.JPG|thumb|alt=Puzzle globe logo|S. Typhimurium<sup>[21]</sup> ]] | |||
==Introduction== | ==Introduction== | ||
[[Salmonella]] is a type of bacteria that lives and grows within mammal intestinal tracts whenever the host eats raw contaminated foods that contains animal feces <sup>[1]</sup>. Salmonellosis is the medical diagnosis for an individual who has | ''[[Salmonella]]'' is a type of bacteria that lives and grows within mammal intestinal tracts whenever the host eats raw contaminated foods that contains animal feces <sup>[1]</sup>. Salmonellosis is the medical diagnosis for an individual who has been infected with the bacteria called ''Salmonella''. Those who are infected within 72 hours of this common foodborne disease commonly experience diarrhea, fever and abdominal pain, which usually last from 4 to 7 days <sup>[2]</sup>. Outbreaks of this microbial disease are common in restaurants, hospitals, and organizations housing individuals, which makes Salmonellosis so deadly yet easily overlooked <sup>[2]</sup>. The number of individuals in the general population infected with this disease consistently increases each year. | ||
==Description of | ==Description of ''Salmonella typhimurium''== | ||
===Description of the microbe=== | ===Description of the microbe=== | ||
[[Salmonella | ''[[Salmonella typhimurium]]'' is a Gram-negative microbe that cultures and grows within mammalian cells. The outer lipopolysaccharide (LPS) layer surrounds the thin peptioglycan layer, which makes it a Gram-negative cell. Normally, mammalian cells have enzymes that can help them protect from these microbes, studies shown that ''[[Salmonella typhi]]'' can alter their peptioglycan layer by releasing large amounts of LPS onto to the epithetial cells within the host's cytosol <sup>[3]</sup>. These unique cross-linkages between the host's cell and the microbe's N-acetly-muramic acid (NAM) alone allows ''S. typhimurium'' to alter its intracellular peptidoglycan structure and allows it to survive within mammalian cell. | ||
===Transmission of disease=== | ===Transmission of disease=== | ||
Pathogenesis of Salmonellosis begins as ''Salmonella'' enters the body as contaminated food is ingested. Virulence factors allow the microbe to invade cells, form a lipopolysaccharide coat, replicate intracellularly and possibly have the ability to disseminate toxin(s). After ingestion, colonization takes place in the ileum and colon as S. | Sources of transmission of ''Salmonella'' include unclean food in institutional kitchens and restaurants, feces of either sick or infected but apparently asymptomatic people and animals (especially exposed are caregivers and animals), polluted surface water and standing water, unhygenically thawed domestic birds and affiliation with reptiles (i.e. tortoise and snakes) is well documented.<sup>[4]</sup>''Salmonella'' has the ability to survive for durations lasting between weeks to months, depending on the habitat. Thus, ''Salmonella'' is often found in polluted water that has been contaminated with excretions of carrier animals. Important vectors of ''S. typhimirium'' include aquatic vertebrates, birds and reptiles. Also, poultry, cattle and sheep are susceptible to contamination by ''Salmonella''; therefore it can be found in food sources like meats and raw eggs. | ||
===Pathology of microbe=== | |||
Pathogenesis of Salmonellosis begins as ''Salmonella'' enters the body as contaminated food is ingested. Virulence factors allow the microbe to invade cells, form a lipopolysaccharide coat, replicate intracellularly and possibly have the ability to disseminate toxin(s). After ingestion, colonization takes place in the ileum and colon as ''S. typhimurium'' invades and proliferates within the endothelium and lymphoid follicles. After invasion, ''S. typhimurium'' replicates intracellularly and spreads to the mysentric lymph nodes and throughout the body until they are captured by the reticuloendothelial system.<sup>[5]</sup> Fortunately, most ''Salmonella'' strains are killed in extraintestinal sites while the most common ''Salmonella'' infection occurs in the intestines. While invading the intestines, ''S. typhimurium'' triggers an acute inflammatory response, which may cause ulcerations. Diarrhea is caused by the secretion of fluid and electrolytes by the large and small intestines during inflammation. The host defends spreading of ''S. typhimurium'' by activation of mucosal adenylate cyclase, causing increasing in cyclic AMP secretion. This event leads to the local production of prostaglandins or other components of inflammatory response <sup>[6]</sup>. | |||
===Prevention=== | ===Prevention=== | ||
The most dangerous factor of the ''Salmonella'' bacteria is that there is no visible or odorous difference between contaminated and non-contaminated foods. Also, studies have shown that foods such as eggs, chickens, pork, fish, unpasteurized dairy products, raw fruits and vegetables are most likely to carry the ''Salmonella'' bacteria. As a result, ''Salmonella'' prevention can be mainly achieved by cooking foods at the right temperature to insure that all bacteria are killed. As each meat or poultry product varies, the temperatures for each product is as follows: beef, veal, lamb steaks, roasts, and chops should be cooked to 145 degrees F; all cuts of pork cooked to 160 degrees F; ground beef, veal and lamb to 160 degrees F; all poultry to 165 degrees F; fish should reach 145 degrees F, and sauces, soups, and gravy should be brought to a boil when reheating; and lastly, leftovers should be heated to at least 165 degrees F<sup>[ | The most dangerous factor of the ''Salmonella'' bacteria is that there is no visible or odorous difference between contaminated and non-contaminated foods. Also, studies have shown that foods such as eggs, chickens, pork, fish, unpasteurized dairy products, raw fruits and vegetables are most likely to carry the ''Salmonella'' bacteria. As a result, ''Salmonella'' prevention can be mainly achieved by cooking foods at the right temperature to insure that all bacteria are killed. As each meat or poultry product varies, the temperatures for each product is as follows: beef, veal, lamb steaks, roasts, and chops should be cooked to 145 degrees F; all cuts of pork cooked to 160 degrees F; ground beef, veal and lamb to 160 degrees F; all poultry to 165 degrees F; fish should reach 145 degrees F, and sauces, soups, and gravy should be brought to a boil when reheating; and lastly, leftovers should be heated to at least 165 degrees F<sup>[7]</sup>. | ||
The most elementary and basic instruction of them all, but the most essential, is washing your hands. As animals can carry fecal matter in their feathers or fur, washing hands after animal contact and avoiding putting them near your face while around animals can prevent or limit ''Salmonella'' exposure. Another potential source is found when the food preparer fails to clean kitchen counters or cutting boards properly after using them to cut or prepare raw chicken, eggs, or other meat products containing the bacteria, allowing the bacteria to be transferred to other foods. While most contaminated foods come from animal food sources, including beef, poultry, milk and eggs, other foods including vegetables can also be contaminated. So, as a precaution wash raw fruits and vegetables thoroughly and keep them separate from all meat products when storing them. Further prevention can be taken by consuming only pasteurized dairy products and being aware of potential new sources of contamination and avoiding them until cleared by the FDA (i.e. the recent tomato and peanut butter scare)<sup>[ | |||
The most elementary and basic instruction of them all, but the most essential, is washing your hands. As animals can carry fecal matter in their feathers or fur, washing hands after animal contact and avoiding putting them near your face while around animals can prevent or limit ''Salmonella'' exposure. Another potential source is found when the food preparer fails to clean kitchen counters or cutting boards properly after using them to cut or prepare raw chicken, eggs, or other meat products containing the bacteria, allowing the bacteria to be transferred to other foods. While most contaminated foods come from animal food sources, including beef, poultry, milk and eggs, other foods including vegetables can also be contaminated. So, as a precaution wash raw fruits and vegetables thoroughly and keep them separate from all meat products when storing them. Further prevention can be taken by consuming only pasteurized dairy products and being aware of potential new sources of contamination and avoiding them until cleared by the FDA (i.e. the recent tomato and peanut butter scare)<sup>[8]</sup>. | |||
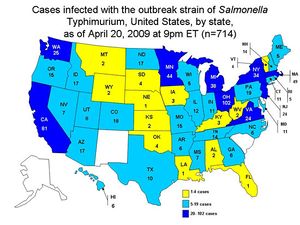
[[Image:Salmonella_Picture2.JPG|thumb|alt=Puzzle globe logo|Treated cases of ''S. Typhimurium'' in the United States <sup>[15]</sup> ]] | |||
==Why is this disease a problem in the United States== | ==Why is this disease a problem in the United States== | ||
Salmonellosis is an important medical problem in the United States as ''Salmonella | Salmonellosis is an important medical problem in the United States as ''Salmonella typhimurium'' commonly causes food-borne disease, with an estimated 1.4 million illnesses and 500 deaths annually <sup>[9]</sup>. ''Salmonella typhimurium'' organisms are common inhabitants of the gastrointestinal tracts of all animals, including cattle<sup>[9]</sup>. Thus, humans can be readily exposed to this organism thorough beef and dairy products. Another route of human exposure to ''Salmonella typhimurium'' is through unpasteurized orange juice. From 1995 through 2004, nineteen juice-associated outbreaks reported to the Centers of Disease Control and Prevention (CDC); seven (37%) were associated with orange juice <sup>[10]</sup>. This report is significant because orange juice is commonly consumed in the United States: 33% of a US population sample reported drinking orange juice in a give week in 2004 <sup>[10]</sup>. Of the 95 patients with ''S. typhimurium'' infection, 73 (77%) drank orange juice during the week before illness <sup>[9]</sup>. This report warned that ''Salmonella'' could contaminate fruits and vegetables, including juices, in addition to beef and dairy products. Although the route of contamination is unknown, noncompliance with the juice Hazard Analysis and Critical Control Point regulation likely contributed to the outbreak since a particular company's orange juice was labeled as “all natural fresh-squeezed” without an indication that the final product was not pasteurized or was not treated with any method to reduce pathogens <sup>[10]</sup>. Since contaminated orange juice was sold in the U.S. under various brand names and was consumed by the glass in restaurants, tracking contamination became an even more complicated problem <sup>[10]</sup>. | ||
In terms of resistance, ''Salmonella | |||
In terms of resistance, ''Salmonella typhimurium'' definitive type 104 with chromosomally encoded resistance to five or more antimicrobial drugs (R-type ACSSuT +) has been increasingly prevalent in the United States since 1990 <sup>[11]</sup>. A geospatial analysis of ''Salmonella typhimurium'' infection show that patients infected with R-type ACSSuT + resided in postal zip code polygons of above average cattle farm density (P < 0.03) and were more likely to report direct contact with livestock (P < 0.01) than patients infected with other R-types <sup>[11]</sup>. Of the four quadrants of the country, the northwestern quadrant, including Washington State, reported the highest frequency of R-type ACSSuT + isolates. Risk factor for acquiring DT104 infection includes exposure to livestock and eating runny or undercooked eggs <sup>[11]</sup>. | |||
Although infection with ''Salmonella typhimurium'' usually causes mild self-limited illness, death may occur, especially in immune-compromised hosts <sup>[12]</sup>. Economical burden due to medical expenses and lost productivity makes salmonellosis a major issue in the United States. | |||
==What is being done to address this problem== | ==What is being done to address this problem== | ||
At present, the U.S. Center for Disease Control tracks and analyzes outbreaks of Salmonellosis and other foodborne illnesses in order to identify measures of control <sup>[ | At present, the U.S. Center for Disease Control tracks and analyzes outbreaks of Salmonellosis and other foodborne illnesses in order to identify measures of control <sup>[13]</sup>. State, local, and territorial health departments use the electronic Foodborne Outbreak Reporting System to submit reports of outbreaks to the CDC’s Foodborne Disease Outbreak Surveillence System <sup>[13]</sup>. When an outbreak occurs, the CDC tries to identify the source of the disease and coordinates with the U.S. Food and Drug Administration to recall all potentially contaminated products. The CDC also runs PulseNet USA, a molecular surveillance network to track foodborne infections in the U.S., which has been used to detect, investigate, and control foodborne outbreaks throughout all 50 states <sup>[14]</sup>. This program uses pulsed-field gel electrophoresis DNA subtyping to distinguish between different strains of foodborne illnesses, including Salmonellosis, and the database allows direct access for all participating laboratories <sup>[15]</sup>. Since 1997, the United States has permitted the irradiation of meat to help prevent foodborne diseases, and has recently allowed the irradiation of spinach and lettuce <sup>[15]</sup>. Irradiation of susceptible food products could greatly reduce the risk of bacterial contamination without altering the food itself <sup>[16]</sup>. These safety measures can help reduce the spread of ''Salmonella typhimurium'' and other foodborne diseases as well as help track and control outbreaks. | ||
As a result of recent outbreaks of ''Salmonella'', the U.S. Congress is working on legislation for higher safety standards for food production companies and more support for FDA inspections. They are also trying to separate the FDA into two agencies, having one strictly for food safety and the other for drugs safety <sup>[ | |||
As a result of recent outbreaks of ''Salmonella'', the U.S. Congress is working on legislation for higher safety standards for food production companies and more support for FDA inspections. They are also trying to separate the FDA into two agencies, having one strictly for food safety and the other for drugs safety <sup>[7]</sup>. | |||
==What else could be done to address this problem== | ==What else could be done to address this problem== | ||
The future of the prevention of ''Salmonella'' infections involves developing new methods of irradiation as well as the advancement of vaccination against the microbe. | The future of the prevention of ''Salmonella'' infections involves developing new methods of irradiation as well as the advancement of vaccination against the microbe. | ||
First, there is strong evidence that food irradiation can kill microbes. When ''S. | |||
Secondly, some recent research shows that vaccination might help to protect against ''S. | First, there is strong evidence that food irradiation can kill microbes. When ''S. typhimurium''present in the food are irradiated, the energy from the ray is transferred to water and other molecules within the microbe; the energy creates transient reactive chemicals that damage the DNA of the microbe causing defects in its genetic coding <sup>[18]</sup>. The microbe is unable to develop and replicate itself, and thus will die unless it can repair the damage. A variety of foods in the US is already approved by FDA and USDA for irradiation, and has been treated to kill ''S. typhimurium''. Examples of fruits and vegetables, such as iceberg lettuce and spinach, are being irradiated with gamma rays to kill bacteria by disrupting their DNA. For high-fat foods that cannot use gamma ray irradiation, such as nuts and meat, x-rays are used instead for disinfection <sup>[18]</sup>. Sensitivity to irradiation depends on the size of the DNA of the target microbe. When target organisms have large DNA, it is easier to kill the bacteria even with small doses of irradiation; however when an organism has smaller DNA, it takes requires more irradiation (thus the reason why irradiation is ineffective against viruses) | ||
Secondly, some recent research shows that vaccination might help to protect against ''S. typhimurium'' infection. Researchers have discovered that ''Salmonella'' depends on glucose for its carbon source. As a result, researchers followed up by focusing research on the glycolysis pathway. “It [glycolysis] occurs in most organisms including bacteria that occupy host cells. Disrupting the bacteria’s ability to use glucose could be used to create vaccine strains for other pathogenic bacteria” <sup>[19]</sup>. Scientists found that the ''Salmonella'' mutants that couldn’t import glucose into their cells were incompetent and unable to use glucose as the food source. As a result, cellular functions such as replication were unsuccessful, yet the harmless strains still stimulated an immune response by the host. Vaccine that inhibits the import of glucose into the microbe may be used as vaccine vectors to prevent ''Salmonella'' poisoning. | |||
==What models of prevention of Salmonellosis is available outside the United States?== | ==What models of prevention of Salmonellosis is available outside the United States?== | ||
In Mexico, an Integrated Food Chain Surveillance system (IFCS) was set up from 2002 to 2005 to measure the prevalence of different strains of ''Salmonella'' <sup>[ | In Mexico, an Integrated Food Chain Surveillance system (IFCS) was set up from 2002 to 2005 to measure the prevalence of different strains of ''Salmonella'' <sup>[20]</sup>. The implemented system split Mexico’s 32 states into 5 regions where the IFCS monitored samples from ill and asympomatic persons and retail pork, chicken and beef. This surveillance was designed to reflect the consumption patterns of each meat product. The significant findings from the study were three-fold including: high rates of meat contamination, high rates of ceftriaxone-resistant ''S. typhimurium'' in chicken, ill humans and swine, and emerging ciprofloxacin resistance in ''S. typhimurium''. However, The IFCS is limited by technology because it is unable to measure the health impact of contaminated food consumption, carry out cost-benefit analyses of health care costs, nor is it able to detect ''Salmonella'' outbreaks. Eventually, systems like these serve as a model for the reduction of ''Salmonella'' contamination throughout the food chain while preventing and controlling antibiotic-resistant ''Salmonella'' in food and retail meats. | ||
==Conclusion== | ==Conclusion== | ||
| Line 37: | Line 49: | ||
==References== | ==References== | ||
[1] “Salmonellosis.” National Institute of Allergy and Infectious Diseases. 19 Feb 2009. US Department of Health and Human Services. 24 Aug 2009 <http://www3.niaid.nih.gov/topics/salmonellosis/Transmission.htm> | <sup>[1]</sup>“Salmonellosis.” National Institute of Allergy and Infectious Diseases. 19 Feb 2009. US Department of Health and Human Services. 24 Aug 2009 <http://www3.niaid.nih.gov/topics/salmonellosis/Transmission.htm> | ||
<sup>[2]</sup>“Salmonellosis.” Division of Foodborne, Bacterial and Mycotic Diseases (DFBMD). 21 May 2008. Centers for Disease Control and Prevention. 24 Aug 2009 <http://www.cdc.gov/nczved/dfbmd/disease_listing/salmonellosis_gi.html#6> | |||
<sup>[3]</sup>Quintela, Jose Carlos, et al. “Peptidoglycan Structure of Salmonella Typhimurium Growing within Cultured Mammalian Cells.” Molecular Microbiology. 1997. Volume 23. p. 693-704. Wiley InterScience. 19 Aug 2009 <http://www3.interscience.wiley.com/cgi-bin/fulltext/119174833/PDFSTART> | |||
[ | <sup>[4]</sup>"Ongoing investigation into reptile associated salmonella infections". Health Protection Report 3 (14). 9 April 2009. http://www.hpa.org.uk/hpr/archives/2009/news1409.htm#reptiles. Retrieved 25 August 2009. | ||
[ | <sup>[5]</sup>Hendriksen, Susan, Karin Orsel, Jaap Wagenaar, Angelika Miko, and Engeline Duijkeren. "Animal-to-Human Transmission of Salmonella Typhimurium DT104A Variant." Emerging Infectious Diseases 10.12 (2004): 2225-227. Print. | ||
[ | <sup>[6]</sup>Ohl, Michael, and Samuel Miller. "Salmonella: A Model for Bacterial Pathogenesis." Annual Review of Medicine 52 (2001): 259-74. Print. | ||
[ | <sup>[7]</sup>Weber, Carol J. “Update on Salmonella Infection.” Society of Urologic Nurses and Associates Inc. Volume 29 Number 2. March 2009. | ||
[ | <sup>[8]</sup>Nordqvist, Christian. “What is Salmonella? What is Salmonella Infection?” Medicalnewstoday.com. 18 Aug. 2009. http://medicalnewstoday.com/articles/160942.php | ||
[ | <sup>[9]</sup>Kunze, David J., Loneragan, Guy H., Platt, Tammy M., Miller, Mark F., Besser, Thomas E., Koohmaraie, Mohammad, Stephens, Tyler, Brashears, Mindy M. Salmonella enterica Burden in Harvest-Ready Cattle Populations from the Southern High Plains of the United States. Appl. Environ. Microbiol. 2008 74: 345-351 | ||
[ | <sup>[10]</sup>Seema Jain, Sally A. Bidol, Jana L. Austin, Erica Berl, Franny Elson, Mysheika LeMaile‐Williams, Marshall Deasy III, Mària E. Moll, Vickie Rea, Jazmin D. Vojdani, Patricia A. Yu, Robert M. Hoekstra, Christopher R. Braden, and Michael F. Lynch. Multistate Outbreak of Salmonella Typhimurium and Saintpaul Infectons Associated with Unpasteurized Orange Juice – United States, 2005. Clinical Infectious Diseases 2009 48:8, 1065-1071 | ||
[ | <sup>[11]</sup>T. E. Besser, M. Goldoft, L. C. Pritchett, R. Khakhri, D. D. Hancock, D. H. Rice, J. M. Gay, W. Johnson and C. C. Gay (2000). Multiresistant Salmonella Typhimurium DT104 infections of humans and domestic animals in the Pacific Northwest of the United States. Epidemiology and Infection, 124, pp 193-200. | ||
[ | <sup>[12]</sup>A Review of Human Salmonellosis: III. Magnitude of Salmonella Infection in the United States. Richard B. Chalker and Martin J. Blaser. Reviews of Infectious Diseases, Vol. 10, No. 1 (Jan. - Feb., 1988), pp. 111-124. The University of Chicago Press. http://www.jstor.org/stable/4454281 | ||
[ | <sup>[13]</sup>Surveillance for foodborne disease outbreaks - United States, 2006. MMWR.Morbidity and Mortality Weekly Report, 2009, 58, 22, 609-15, Centers for Disease Control and Prevention, Atlanta, GA. | ||
[ | <sup>[14]</sup>Swaminathan, Ribot, Hyyti-Trees, Rolando, Hunter, Kincaid, Hise, Gerner-Smidt. PulseNet USA: a five-year update. Foodborne pathogens and disease, 2006, 3, 1, 9-19, Mary Ann Liebert, Inc., Larchmont, NY. | ||
[ | <sup>[15]</sup>Investigation Update: Outbreak of Salmonella Typhimurium Infections, 2008-2009. April 29, 2009. Center for Disease Control and Prevention. http://www.cdc.gov/salmonella/typhimurium/update.html | ||
[ | <sup>[16]</sup>P. Gerner-Smidt, K. Hise, J. Kincaid, S. Hunter, S. Rolando, E. Hyytiä-Trees, E.M. Ribot, B. Swaminathan. Foodborne Pathogens and Disease. Spring 2006, 3(1): 9-19. doi:10.1089/fpd.2006.3.9. | ||
[ | <sup>[17]</sup>Chute, Nancy. "Can Irradiating Food Zap Salmonella Outbreaks? - US News and World Report." Health News Articles - US News Health. Web. 25 Aug. 2009. <http://health.usnews.com/articles/health/2009/02/26/can-irradiating-food-zap-salmonella-outbreaks.html?PageNr=1>. | ||
[ | <sup>[18]</sup>Stones, Mike. "Vaccination could stop salmonella food poisoning." Meat Processing - Meat Industry - Poultry, Meat Processors, Packaging, Production. Web. 25 Aug. 2009. <http://www.meatprocess.com/Safety-Legislation/Vaccination-could-stop-salmonella-food-poisoning>. | ||
[ | <sup>[19]</sup>Food Irradiation. Department of Health and Human Services. Centers for Disease Control and Prevention, 11 Oct. 2005. Web. 25 Aug. 2009. <http://www.cdc.gov/ncidod/dbmd/diseaseinfo/foodirradiation.htm>. | ||
[ | <sup>[20]</sup>Zaidi, Mussaret, Juan Calva, Maria Estrada-Garcia, Veronica Leon, Gabriela Vazquez, Gloria Figueroa, Estela Lopez, Jesus Contreras, Jason Abbott, Shaohua Zhao, Patrick McDermott, and Linda Tollefson. "Integrated Food Chain Surveillance System for Salmonella spp. in Mexico." Emerging Infectious Diseases 14.3 (2008): 429-35. Print. | ||
[ | <sup>[21]</sup>Fox, Maggie. "Bugs Go Feral in Space." News in Science. Web. | ||
Edited by Virginie Dang , Jaclyn Irwin , Ha Kim , So Kim , Anthony Ngo , Hyung-jo Yoon, students of [mailto:ralarsen@ucsd.edu Rachel Larsen] | Edited by Virginie Dang , Jaclyn Irwin , Ha Kim , So Kim , Anthony Ngo , Hyung-jo Yoon, students of [mailto:ralarsen@ucsd.edu Rachel Larsen] | ||
Latest revision as of 15:53, 16 September 2010
Introduction
Salmonella is a type of bacteria that lives and grows within mammal intestinal tracts whenever the host eats raw contaminated foods that contains animal feces [1]. Salmonellosis is the medical diagnosis for an individual who has been infected with the bacteria called Salmonella. Those who are infected within 72 hours of this common foodborne disease commonly experience diarrhea, fever and abdominal pain, which usually last from 4 to 7 days [2]. Outbreaks of this microbial disease are common in restaurants, hospitals, and organizations housing individuals, which makes Salmonellosis so deadly yet easily overlooked [2]. The number of individuals in the general population infected with this disease consistently increases each year.
Description of Salmonella typhimurium
Description of the microbe
Salmonella typhimurium is a Gram-negative microbe that cultures and grows within mammalian cells. The outer lipopolysaccharide (LPS) layer surrounds the thin peptioglycan layer, which makes it a Gram-negative cell. Normally, mammalian cells have enzymes that can help them protect from these microbes, studies shown that Salmonella typhi can alter their peptioglycan layer by releasing large amounts of LPS onto to the epithetial cells within the host's cytosol [3]. These unique cross-linkages between the host's cell and the microbe's N-acetly-muramic acid (NAM) alone allows S. typhimurium to alter its intracellular peptidoglycan structure and allows it to survive within mammalian cell.
Transmission of disease
Sources of transmission of Salmonella include unclean food in institutional kitchens and restaurants, feces of either sick or infected but apparently asymptomatic people and animals (especially exposed are caregivers and animals), polluted surface water and standing water, unhygenically thawed domestic birds and affiliation with reptiles (i.e. tortoise and snakes) is well documented.[4]Salmonella has the ability to survive for durations lasting between weeks to months, depending on the habitat. Thus, Salmonella is often found in polluted water that has been contaminated with excretions of carrier animals. Important vectors of S. typhimirium include aquatic vertebrates, birds and reptiles. Also, poultry, cattle and sheep are susceptible to contamination by Salmonella; therefore it can be found in food sources like meats and raw eggs.
Pathology of microbe
Pathogenesis of Salmonellosis begins as Salmonella enters the body as contaminated food is ingested. Virulence factors allow the microbe to invade cells, form a lipopolysaccharide coat, replicate intracellularly and possibly have the ability to disseminate toxin(s). After ingestion, colonization takes place in the ileum and colon as S. typhimurium invades and proliferates within the endothelium and lymphoid follicles. After invasion, S. typhimurium replicates intracellularly and spreads to the mysentric lymph nodes and throughout the body until they are captured by the reticuloendothelial system.[5] Fortunately, most Salmonella strains are killed in extraintestinal sites while the most common Salmonella infection occurs in the intestines. While invading the intestines, S. typhimurium triggers an acute inflammatory response, which may cause ulcerations. Diarrhea is caused by the secretion of fluid and electrolytes by the large and small intestines during inflammation. The host defends spreading of S. typhimurium by activation of mucosal adenylate cyclase, causing increasing in cyclic AMP secretion. This event leads to the local production of prostaglandins or other components of inflammatory response [6].
Prevention
The most dangerous factor of the Salmonella bacteria is that there is no visible or odorous difference between contaminated and non-contaminated foods. Also, studies have shown that foods such as eggs, chickens, pork, fish, unpasteurized dairy products, raw fruits and vegetables are most likely to carry the Salmonella bacteria. As a result, Salmonella prevention can be mainly achieved by cooking foods at the right temperature to insure that all bacteria are killed. As each meat or poultry product varies, the temperatures for each product is as follows: beef, veal, lamb steaks, roasts, and chops should be cooked to 145 degrees F; all cuts of pork cooked to 160 degrees F; ground beef, veal and lamb to 160 degrees F; all poultry to 165 degrees F; fish should reach 145 degrees F, and sauces, soups, and gravy should be brought to a boil when reheating; and lastly, leftovers should be heated to at least 165 degrees F[7].
The most elementary and basic instruction of them all, but the most essential, is washing your hands. As animals can carry fecal matter in their feathers or fur, washing hands after animal contact and avoiding putting them near your face while around animals can prevent or limit Salmonella exposure. Another potential source is found when the food preparer fails to clean kitchen counters or cutting boards properly after using them to cut or prepare raw chicken, eggs, or other meat products containing the bacteria, allowing the bacteria to be transferred to other foods. While most contaminated foods come from animal food sources, including beef, poultry, milk and eggs, other foods including vegetables can also be contaminated. So, as a precaution wash raw fruits and vegetables thoroughly and keep them separate from all meat products when storing them. Further prevention can be taken by consuming only pasteurized dairy products and being aware of potential new sources of contamination and avoiding them until cleared by the FDA (i.e. the recent tomato and peanut butter scare)[8].
Why is this disease a problem in the United States
Salmonellosis is an important medical problem in the United States as Salmonella typhimurium commonly causes food-borne disease, with an estimated 1.4 million illnesses and 500 deaths annually [9]. Salmonella typhimurium organisms are common inhabitants of the gastrointestinal tracts of all animals, including cattle[9]. Thus, humans can be readily exposed to this organism thorough beef and dairy products. Another route of human exposure to Salmonella typhimurium is through unpasteurized orange juice. From 1995 through 2004, nineteen juice-associated outbreaks reported to the Centers of Disease Control and Prevention (CDC); seven (37%) were associated with orange juice [10]. This report is significant because orange juice is commonly consumed in the United States: 33% of a US population sample reported drinking orange juice in a give week in 2004 [10]. Of the 95 patients with S. typhimurium infection, 73 (77%) drank orange juice during the week before illness [9]. This report warned that Salmonella could contaminate fruits and vegetables, including juices, in addition to beef and dairy products. Although the route of contamination is unknown, noncompliance with the juice Hazard Analysis and Critical Control Point regulation likely contributed to the outbreak since a particular company's orange juice was labeled as “all natural fresh-squeezed” without an indication that the final product was not pasteurized or was not treated with any method to reduce pathogens [10]. Since contaminated orange juice was sold in the U.S. under various brand names and was consumed by the glass in restaurants, tracking contamination became an even more complicated problem [10].
In terms of resistance, Salmonella typhimurium definitive type 104 with chromosomally encoded resistance to five or more antimicrobial drugs (R-type ACSSuT +) has been increasingly prevalent in the United States since 1990 [11]. A geospatial analysis of Salmonella typhimurium infection show that patients infected with R-type ACSSuT + resided in postal zip code polygons of above average cattle farm density (P < 0.03) and were more likely to report direct contact with livestock (P < 0.01) than patients infected with other R-types [11]. Of the four quadrants of the country, the northwestern quadrant, including Washington State, reported the highest frequency of R-type ACSSuT + isolates. Risk factor for acquiring DT104 infection includes exposure to livestock and eating runny or undercooked eggs [11].
Although infection with Salmonella typhimurium usually causes mild self-limited illness, death may occur, especially in immune-compromised hosts [12]. Economical burden due to medical expenses and lost productivity makes salmonellosis a major issue in the United States.
What is being done to address this problem
At present, the U.S. Center for Disease Control tracks and analyzes outbreaks of Salmonellosis and other foodborne illnesses in order to identify measures of control [13]. State, local, and territorial health departments use the electronic Foodborne Outbreak Reporting System to submit reports of outbreaks to the CDC’s Foodborne Disease Outbreak Surveillence System [13]. When an outbreak occurs, the CDC tries to identify the source of the disease and coordinates with the U.S. Food and Drug Administration to recall all potentially contaminated products. The CDC also runs PulseNet USA, a molecular surveillance network to track foodborne infections in the U.S., which has been used to detect, investigate, and control foodborne outbreaks throughout all 50 states [14]. This program uses pulsed-field gel electrophoresis DNA subtyping to distinguish between different strains of foodborne illnesses, including Salmonellosis, and the database allows direct access for all participating laboratories [15]. Since 1997, the United States has permitted the irradiation of meat to help prevent foodborne diseases, and has recently allowed the irradiation of spinach and lettuce [15]. Irradiation of susceptible food products could greatly reduce the risk of bacterial contamination without altering the food itself [16]. These safety measures can help reduce the spread of Salmonella typhimurium and other foodborne diseases as well as help track and control outbreaks.
As a result of recent outbreaks of Salmonella, the U.S. Congress is working on legislation for higher safety standards for food production companies and more support for FDA inspections. They are also trying to separate the FDA into two agencies, having one strictly for food safety and the other for drugs safety [7].
What else could be done to address this problem
The future of the prevention of Salmonella infections involves developing new methods of irradiation as well as the advancement of vaccination against the microbe.
First, there is strong evidence that food irradiation can kill microbes. When S. typhimuriumpresent in the food are irradiated, the energy from the ray is transferred to water and other molecules within the microbe; the energy creates transient reactive chemicals that damage the DNA of the microbe causing defects in its genetic coding [18]. The microbe is unable to develop and replicate itself, and thus will die unless it can repair the damage. A variety of foods in the US is already approved by FDA and USDA for irradiation, and has been treated to kill S. typhimurium. Examples of fruits and vegetables, such as iceberg lettuce and spinach, are being irradiated with gamma rays to kill bacteria by disrupting their DNA. For high-fat foods that cannot use gamma ray irradiation, such as nuts and meat, x-rays are used instead for disinfection [18]. Sensitivity to irradiation depends on the size of the DNA of the target microbe. When target organisms have large DNA, it is easier to kill the bacteria even with small doses of irradiation; however when an organism has smaller DNA, it takes requires more irradiation (thus the reason why irradiation is ineffective against viruses)
Secondly, some recent research shows that vaccination might help to protect against S. typhimurium infection. Researchers have discovered that Salmonella depends on glucose for its carbon source. As a result, researchers followed up by focusing research on the glycolysis pathway. “It [glycolysis] occurs in most organisms including bacteria that occupy host cells. Disrupting the bacteria’s ability to use glucose could be used to create vaccine strains for other pathogenic bacteria” [19]. Scientists found that the Salmonella mutants that couldn’t import glucose into their cells were incompetent and unable to use glucose as the food source. As a result, cellular functions such as replication were unsuccessful, yet the harmless strains still stimulated an immune response by the host. Vaccine that inhibits the import of glucose into the microbe may be used as vaccine vectors to prevent Salmonella poisoning.
What models of prevention of Salmonellosis is available outside the United States?
In Mexico, an Integrated Food Chain Surveillance system (IFCS) was set up from 2002 to 2005 to measure the prevalence of different strains of Salmonella [20]. The implemented system split Mexico’s 32 states into 5 regions where the IFCS monitored samples from ill and asympomatic persons and retail pork, chicken and beef. This surveillance was designed to reflect the consumption patterns of each meat product. The significant findings from the study were three-fold including: high rates of meat contamination, high rates of ceftriaxone-resistant S. typhimurium in chicken, ill humans and swine, and emerging ciprofloxacin resistance in S. typhimurium. However, The IFCS is limited by technology because it is unable to measure the health impact of contaminated food consumption, carry out cost-benefit analyses of health care costs, nor is it able to detect Salmonella outbreaks. Eventually, systems like these serve as a model for the reduction of Salmonella contamination throughout the food chain while preventing and controlling antibiotic-resistant Salmonella in food and retail meats.
Conclusion
In conclusion, Salmonella contamination has become an increasing problem in the United States. Salmonella outbreaks have become more prevalent in the last few years, which means that the safety measures put in place by the CDC and the FDA to prevent foodborne illnesses need to be improved. New and stricter safety measures should be put in place in the food production industry, the main source of Salmonella outbreaks at the present time. The public also should be made aware of safety measures and food preparation techniques that can limit the chances of contracting salmonellosis.
References
[1]“Salmonellosis.” National Institute of Allergy and Infectious Diseases. 19 Feb 2009. US Department of Health and Human Services. 24 Aug 2009 <http://www3.niaid.nih.gov/topics/salmonellosis/Transmission.htm>
[2]“Salmonellosis.” Division of Foodborne, Bacterial and Mycotic Diseases (DFBMD). 21 May 2008. Centers for Disease Control and Prevention. 24 Aug 2009 <http://www.cdc.gov/nczved/dfbmd/disease_listing/salmonellosis_gi.html#6>
[3]Quintela, Jose Carlos, et al. “Peptidoglycan Structure of Salmonella Typhimurium Growing within Cultured Mammalian Cells.” Molecular Microbiology. 1997. Volume 23. p. 693-704. Wiley InterScience. 19 Aug 2009 <http://www3.interscience.wiley.com/cgi-bin/fulltext/119174833/PDFSTART>
[4]"Ongoing investigation into reptile associated salmonella infections". Health Protection Report 3 (14). 9 April 2009. http://www.hpa.org.uk/hpr/archives/2009/news1409.htm#reptiles. Retrieved 25 August 2009.
[5]Hendriksen, Susan, Karin Orsel, Jaap Wagenaar, Angelika Miko, and Engeline Duijkeren. "Animal-to-Human Transmission of Salmonella Typhimurium DT104A Variant." Emerging Infectious Diseases 10.12 (2004): 2225-227. Print.
[6]Ohl, Michael, and Samuel Miller. "Salmonella: A Model for Bacterial Pathogenesis." Annual Review of Medicine 52 (2001): 259-74. Print.
[7]Weber, Carol J. “Update on Salmonella Infection.” Society of Urologic Nurses and Associates Inc. Volume 29 Number 2. March 2009.
[8]Nordqvist, Christian. “What is Salmonella? What is Salmonella Infection?” Medicalnewstoday.com. 18 Aug. 2009. http://medicalnewstoday.com/articles/160942.php
[9]Kunze, David J., Loneragan, Guy H., Platt, Tammy M., Miller, Mark F., Besser, Thomas E., Koohmaraie, Mohammad, Stephens, Tyler, Brashears, Mindy M. Salmonella enterica Burden in Harvest-Ready Cattle Populations from the Southern High Plains of the United States. Appl. Environ. Microbiol. 2008 74: 345-351
[10]Seema Jain, Sally A. Bidol, Jana L. Austin, Erica Berl, Franny Elson, Mysheika LeMaile‐Williams, Marshall Deasy III, Mària E. Moll, Vickie Rea, Jazmin D. Vojdani, Patricia A. Yu, Robert M. Hoekstra, Christopher R. Braden, and Michael F. Lynch. Multistate Outbreak of Salmonella Typhimurium and Saintpaul Infectons Associated with Unpasteurized Orange Juice – United States, 2005. Clinical Infectious Diseases 2009 48:8, 1065-1071
[11]T. E. Besser, M. Goldoft, L. C. Pritchett, R. Khakhri, D. D. Hancock, D. H. Rice, J. M. Gay, W. Johnson and C. C. Gay (2000). Multiresistant Salmonella Typhimurium DT104 infections of humans and domestic animals in the Pacific Northwest of the United States. Epidemiology and Infection, 124, pp 193-200.
[12]A Review of Human Salmonellosis: III. Magnitude of Salmonella Infection in the United States. Richard B. Chalker and Martin J. Blaser. Reviews of Infectious Diseases, Vol. 10, No. 1 (Jan. - Feb., 1988), pp. 111-124. The University of Chicago Press. http://www.jstor.org/stable/4454281
[13]Surveillance for foodborne disease outbreaks - United States, 2006. MMWR.Morbidity and Mortality Weekly Report, 2009, 58, 22, 609-15, Centers for Disease Control and Prevention, Atlanta, GA.
[14]Swaminathan, Ribot, Hyyti-Trees, Rolando, Hunter, Kincaid, Hise, Gerner-Smidt. PulseNet USA: a five-year update. Foodborne pathogens and disease, 2006, 3, 1, 9-19, Mary Ann Liebert, Inc., Larchmont, NY.
[15]Investigation Update: Outbreak of Salmonella Typhimurium Infections, 2008-2009. April 29, 2009. Center for Disease Control and Prevention. http://www.cdc.gov/salmonella/typhimurium/update.html
[16]P. Gerner-Smidt, K. Hise, J. Kincaid, S. Hunter, S. Rolando, E. Hyytiä-Trees, E.M. Ribot, B. Swaminathan. Foodborne Pathogens and Disease. Spring 2006, 3(1): 9-19. doi:10.1089/fpd.2006.3.9.
[17]Chute, Nancy. "Can Irradiating Food Zap Salmonella Outbreaks? - US News and World Report." Health News Articles - US News Health. Web. 25 Aug. 2009. <http://health.usnews.com/articles/health/2009/02/26/can-irradiating-food-zap-salmonella-outbreaks.html?PageNr=1>.
[18]Stones, Mike. "Vaccination could stop salmonella food poisoning." Meat Processing - Meat Industry - Poultry, Meat Processors, Packaging, Production. Web. 25 Aug. 2009. <http://www.meatprocess.com/Safety-Legislation/Vaccination-could-stop-salmonella-food-poisoning>.
[19]Food Irradiation. Department of Health and Human Services. Centers for Disease Control and Prevention, 11 Oct. 2005. Web. 25 Aug. 2009. <http://www.cdc.gov/ncidod/dbmd/diseaseinfo/foodirradiation.htm>.
[20]Zaidi, Mussaret, Juan Calva, Maria Estrada-Garcia, Veronica Leon, Gabriela Vazquez, Gloria Figueroa, Estela Lopez, Jesus Contreras, Jason Abbott, Shaohua Zhao, Patrick McDermott, and Linda Tollefson. "Integrated Food Chain Surveillance System for Salmonella spp. in Mexico." Emerging Infectious Diseases 14.3 (2008): 429-35. Print.
[21]Fox, Maggie. "Bugs Go Feral in Space." News in Science. Web.
Edited by Virginie Dang , Jaclyn Irwin , Ha Kim , So Kim , Anthony Ngo , Hyung-jo Yoon, students of Rachel Larsen