Azoarcus tolulyticus: Difference between revisions
| (16 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
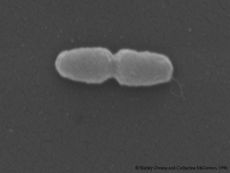
[[File:tolulyticus.jpg| | [[File:tolulyticus.jpg|230px|thumb|right|''Azoarcus tolulyticus'']] | ||
==Classification== | ==Classification== | ||
[[File:treeoflife.jpg| | [[File:treeoflife.jpg|400px|thumb|right|''Tree of Life'']] | ||
• Kingdom - Bacteria <Br>• Phylum - Proteobacteria<Br> • Class - Betaproteobacteria<Br> • Order - Rhodocyclales<Br> • Family - Rhodocyclaceae<Br> • Genus - Azoarcus<Br> | • Kingdom - Bacteria <Br>• Phylum - Proteobacteria<Br> • Class - Betaproteobacteria<Br> • Order - Rhodocyclales<Br> • Family - Rhodocyclaceae<Br> • Genus - Azoarcus<Br> | ||
| Line 16: | Line 16: | ||
==Description and Significance== | ==Description and Significance== | ||
[[File:toluene.png| | [[File:toluene.png|130px|thumb|right|Chemical structure of toluene]] | ||
First described in 1995 by researchers at Michigan State University, ''Azoarcus tolulyticus'', is a Gram negative, motile rod-shaped bacteria that can be 1.4-2.8um in length (size dependent on growth medium). It belongs to the nitrogen-fixing genus of ''Azoarcus''. ''A. tolulyticus'' is an anaerobic toluene degrader isolated | First described in 1995 by researchers at Michigan State University, ''Azoarcus tolulyticus'', is a Gram negative, motile rod-shaped bacteria that can be 1.4-2.8um in length (size dependent on growth medium). It belongs to the nitrogen-fixing genus of ''Azoarcus''. ''A. tolulyticus'' is an anaerobic toluene degrader isolated primarily from petroleum contaminated soils, marine and freshwaters. This microbe would be considered a non-halophilic mesophile as it grows almost exclusively at temperatures ranging from 25-37⁰C, NaCl concentrations below 1% and near neutral to slightly alkaline pH.<Br> | ||
In anaerobic environments where there is significant gasoline contamination, BTEX (benzene, toluene, ethylbenzene and xylene) compounds pose serious environmental and human health problems. | In anaerobic environments where there is significant gasoline contamination, BTEX (benzene, toluene, ethylbenzene and xylene) compounds pose serious environmental and human health problems. Along with irritation of the respiratory tract, eyes and skin by contact, BTEX compounds mainly target the nervous system of humans and animals with symptoms (dependent on concentration and exposure time) varying from vertigo up to severe neurotoxic effects and Cancer. ''Azoarcus tolulyticus'' is a facultative anaerobe which allows it to live best in low oxygen environments, like subsurface aquifers. Because of its innate ability to anaerobically degrade toluene which is enhanced through nitrogen stimulation to increase degradation, ''Azoarcus tolulyticus'' has the potential for use in the in situ bioremediation of gasoline or petroleum contaminated sites. | ||
==Genome Structure== | ==Genome Structure and Toluene Degrading Pathway== | ||
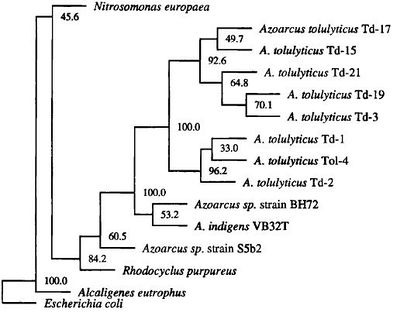
''Azoarcus tolulyticus'' strain Tol-4 was obtained from petroleum contaminated freshwater sediment, 24-25m deep in Northern Michigan. Its genome consists of 1458 base pairs with a G+C content of 56.1-56.6% Base pairs at positions 28 and 1491 correspond with that on the E. coli 16S rRNA gene. Helices located at positions 180 and 220 on the E. coli gene are signature sequences specific to the beta subdivision of the Proteobacteria. Automated fluorescence sequencing of the 16S rRNA revealed that different A. tolulyticus strains had high levels of similarity, of up to 99.9%, but not identical. PCR analysis has shown that multiple isolates have distinct repetitive extragenic palindromic PCR patterns.<Br><Br> | ''Azoarcus tolulyticus'' strain Tol-4 was obtained from petroleum contaminated freshwater sediment, 24-25m deep in Northern Michigan. Its genome consists of 1458 base pairs with a G+C content of 56.1-56.6% Base pairs at positions 28 and 1491 correspond with that on the E. coli 16S rRNA gene. Helices located at positions 180 and 220 on the E. coli gene are signature sequences specific to the beta subdivision of the Proteobacteria. Automated fluorescence sequencing of the 16S rRNA revealed that different A. tolulyticus strains had high levels of similarity, of up to 99.9%, but not identical. PCR analysis has shown that multiple isolates have distinct repetitive extragenic palindromic PCR patterns.<Br><Br> | ||
Its closest related genus is a selenate respiring species Thauera selenatis which maintains a 93% similarity. A. evansii is the mostly closely related ''Azoarcus'' species to ''A. tolulyticus'' although it does not it does not degrade toluene anaerobically.<Br><Br> | Its closest related genus is a selenate respiring species Thauera selenatis which maintains a 93% similarity. A. evansii is the mostly closely related ''Azoarcus'' species to ''A. tolulyticus'' although it does not it does not degrade toluene anaerobically.<Br><Br> | ||
[[File:tree.jpg| | [[File:tree.jpg|400px|thumb|center|Figure I. - Phylogenetic tree showing the relationships between strains of ''Azoarcus tolulyticus'' and related species. Tree above was obtained using DNAPAR and SEQBOOT with ''A. tolulyticus'' strains and related species. Percentages indicate estimates on the confidence of the above branches (Zhou 1995). | ||
]] | ]] | ||
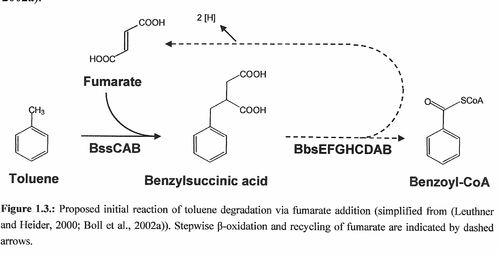
==Cell Structure | The key enzyme for anaerobic toluene and xylene degradation is the benzylsuccinate synthase (Bss). Its structure is that of the α2β2γ2 heterohexameric type and whose subunits are encoded by the bssCAB genes. This enzyme is a highly oxygen sensitive glycyl radical enzyme belonging to the family of pyruvate formiate lyases. It catalyses the addition of the methyl group of the aromatic ring to the double bond of a fumarate cosubstrate leading to (R)-benzylsuccinate. Within this initial reaction, the Bss is making toluene accessible for ring cleavage. FIGURE 1.3 Shows proteins encoded by an operon consisting of bbs genes catalyze beta-oxidation of benzylsuccinate leading to the central intermediate, benzoyl-CoA. <Br><Br> | ||
[[File:CoA.png|500px|thumb|center|]] | |||
Only a limited number of bssA sequences are available from pure cultures and include : | |||
''Azoarcus sp. T'', and ''Azoarcus sp. EbN1''. All of the reference cultures (some of which are not included here) belong to Proteobacteria. Furthermore, in most of these strains the bss genes are organized similarly within a bssDCABEFGH operon and upstream of the bbsA-H operon. The bssA gene is used as a specific functional marker for anaerobic BTEX degraders | |||
==Cell Structure and Metabolism== | |||
Eight facultative anaerobic isolates of ''A. tolulyticus'' have been identified each of which is capable of performing toluene-degrading denitrification. These bacteria show fatty acid profiles similar to other members of the genus ''Azoarcus'', however distinctions were found in that the newly discovered isolates contained an abundance of 12:0 fatty acid while lacking 3-OH 8:0 fatty acid content. All of the isolates are gram negative, cytochrome c oxidase and catalase positive, and all are capable of fixing nitrogen when grown on N2. Also each of these isolates are capable of producing N2 gas as products of cellular respiration under anaerobic conditions.<Br><Br> | Eight facultative anaerobic isolates of ''A. tolulyticus'' have been identified each of which is capable of performing toluene-degrading denitrification. These bacteria show fatty acid profiles similar to other members of the genus ''Azoarcus'', however distinctions were found in that the newly discovered isolates contained an abundance of 12:0 fatty acid while lacking 3-OH 8:0 fatty acid content. All of the isolates are gram negative, cytochrome c oxidase and catalase positive, and all are capable of fixing nitrogen when grown on N2. Also each of these isolates are capable of producing N2 gas as products of cellular respiration under anaerobic conditions.<Br><Br> | ||
''A. tolulyticus'' are short motile rods, although having lengths ranging from 1.4 to 2.1um when grown on toluene, can increase in size to 2.1 to 2.8um (or in long chains) when grown on M-R2A agar. Morphological characteristics when grown on M-R2A agar produces uniform, translucent colonies, yellowish in color and approximately 2-3mm in diameter containing a dark center. The cells lack tryptophanase, B-galactosidsase, urease, and arginine dihydrolase activity. As facultative anaerobes A. tolulyticus is able to use either nitrate or oxygen as terminal electron acceptors during cellular respiration. In aerobic environments oxygen serves as the terminal electron acceptor and is usually the limiting factor due to its low solubility in water along with diffusion constraints in subsurface environments. In anaerobic environments nitrate is used as the terminal electron acceptor. Nitrate is an adequate alternative due to its high solubility in water and high mobility in soil making the rates of degradation comparable to those of aerobic metabolism processes. Evidence has been shown that anaerobic degradation of toluene by ''A. tolulyticus'' has also been observed under Fe(III)-reducing, methanogenic, and sulfidogenic conditions. | ''A. tolulyticus'' are short motile rods, although having lengths ranging from 1.4 to 2.1um when grown on toluene, can increase in size to 2.1 to 2.8um (or in long chains) when grown on M-R2A agar. Morphological characteristics when grown on M-R2A agar produces uniform, translucent colonies, yellowish in color and approximately 2-3mm in diameter containing a dark center. The cells lack tryptophanase, B-galactosidsase, urease, and arginine dihydrolase activity. As facultative anaerobes A. tolulyticus is able to use either nitrate or oxygen as terminal electron acceptors during cellular respiration. In aerobic environments oxygen serves as the terminal electron acceptor and is usually the limiting factor due to its low solubility in water along with diffusion constraints in subsurface environments. In anaerobic environments nitrate is used as the terminal electron acceptor. Nitrate is an adequate alternative due to its high solubility in water and high mobility in soil making the rates of degradation comparable to those of aerobic metabolism processes. Evidence has been shown that anaerobic degradation of toluene by ''A. tolulyticus'' has also been observed under Fe(III)-reducing, methanogenic, and sulfidogenic conditions. | ||
==Ecology and | ==Ecology and Ecological Relevance== | ||
''Azoarcus tolulyticus'' which is included in the genus of the free-living nitrogen fixing bacteria are found in a variety of habitats including both contaminated and non-contaminated soils, aquifiers, and sediments. Table I. shows the diversity of habitats in which isolates have been obtained which range from Michigan to California. Of particular interest are the underground layers of water-bearing permeable rock and soil, known as aquifers. Groundwater coupled with aquifer sediments produce an anoxic underground habitat for these microbes to thrive.<Br><Br> | |||
[[File:Ecology of Azoarcus tolulyticus.png|800px|thumb|center|]]<br> | |||
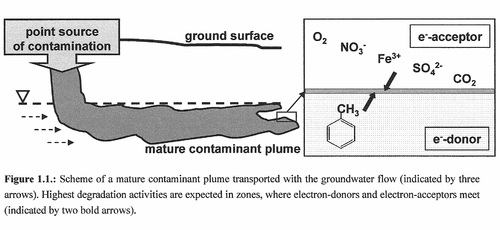
''Azoarcus tolulyticus'' | The importance of ''Azoarcus tolulyticus'' can be attributed to its bioremediation potential in anoxic toluene contaminated sites. Because toluene compounds have relatively high solubility rates and toxicity levels they pose a particular threat to drinking water resources and can impact the health and safety of the public. When a BTEX contamination occurs, the spilled substances are transported along with the groundwater flow and are difficult to remediate effectively. The contaminants in the water are sustainably reduced only due to in situ biological degradation by the metabolic activity of microbes such ''A. tolulyticus''. Aquifer systems and the processes occurring within them play a key role in maintaining water quality and to maintaining these indigenous microbes responsible for natural attenuation of pollutants. Microbes require both electron donors and electron acceptors for successful degradation of toluene and degradation activities are expected to be highest in biogeochemical gradient zones, where both compounds are at equilibrium. | ||
[[File: | |||
[[File:Aquifer.png|500px|thumb|center|]] | |||
<Br> | <Br> | ||
Due to the relatively low solubility of oxygen, the rate of oxygen being replenished to groundwater systems, allowing oxygen to be used as electron acceptor is rapidly depleted upon contamination by respiratory activities. ''A. tolulyticus'' populations are capable of using electron acceptors other than molecular oxygen. Their ability to use different anaerobic terminal electron-accepting processes are necessary for bioremediation in anaerobic conditions (aquifers). BTEX contaminants are often distributed into the ground in large volumes making them highly available as electron donors in non-limiting concentrations. The result is the availability of electron acceptors and their supply becomes a limiting factor for the respiratory activity of degrader capacities.<Br> | |||
The prospect of in situ bioremediation treatments using ''A. tolulyticus'' potentially could be the best solution at achieving cost effective cleanup technology for treating contaminated sites. Although microbial degradation of these compounds is known to occur under aerobic conditions, contamination such as those in aquifiers and soil, housing anaerobic conditions make degradation by aerobic microbes not possible. The ability of ''A. tolulyticus'' to utilize nitrate (and other inorganic compounds) as terminal electron acceptors allows for degradation of toxic chemicals in these habitats. | |||
==References== | ==References== | ||
Latest revision as of 04:38, 27 April 2011
Classification
• Kingdom - Bacteria
• Phylum - Proteobacteria
• Class - Betaproteobacteria
• Order - Rhodocyclales
• Family - Rhodocyclaceae
• Genus - Azoarcus
Species
• Azoarcus tolulyticus
|
NCBI: Taxonomy |
Description and Significance
First described in 1995 by researchers at Michigan State University, Azoarcus tolulyticus, is a Gram negative, motile rod-shaped bacteria that can be 1.4-2.8um in length (size dependent on growth medium). It belongs to the nitrogen-fixing genus of Azoarcus. A. tolulyticus is an anaerobic toluene degrader isolated primarily from petroleum contaminated soils, marine and freshwaters. This microbe would be considered a non-halophilic mesophile as it grows almost exclusively at temperatures ranging from 25-37⁰C, NaCl concentrations below 1% and near neutral to slightly alkaline pH.
In anaerobic environments where there is significant gasoline contamination, BTEX (benzene, toluene, ethylbenzene and xylene) compounds pose serious environmental and human health problems. Along with irritation of the respiratory tract, eyes and skin by contact, BTEX compounds mainly target the nervous system of humans and animals with symptoms (dependent on concentration and exposure time) varying from vertigo up to severe neurotoxic effects and Cancer. Azoarcus tolulyticus is a facultative anaerobe which allows it to live best in low oxygen environments, like subsurface aquifers. Because of its innate ability to anaerobically degrade toluene which is enhanced through nitrogen stimulation to increase degradation, Azoarcus tolulyticus has the potential for use in the in situ bioremediation of gasoline or petroleum contaminated sites.
Genome Structure and Toluene Degrading Pathway
Azoarcus tolulyticus strain Tol-4 was obtained from petroleum contaminated freshwater sediment, 24-25m deep in Northern Michigan. Its genome consists of 1458 base pairs with a G+C content of 56.1-56.6% Base pairs at positions 28 and 1491 correspond with that on the E. coli 16S rRNA gene. Helices located at positions 180 and 220 on the E. coli gene are signature sequences specific to the beta subdivision of the Proteobacteria. Automated fluorescence sequencing of the 16S rRNA revealed that different A. tolulyticus strains had high levels of similarity, of up to 99.9%, but not identical. PCR analysis has shown that multiple isolates have distinct repetitive extragenic palindromic PCR patterns.
Its closest related genus is a selenate respiring species Thauera selenatis which maintains a 93% similarity. A. evansii is the mostly closely related Azoarcus species to A. tolulyticus although it does not it does not degrade toluene anaerobically.
The key enzyme for anaerobic toluene and xylene degradation is the benzylsuccinate synthase (Bss). Its structure is that of the α2β2γ2 heterohexameric type and whose subunits are encoded by the bssCAB genes. This enzyme is a highly oxygen sensitive glycyl radical enzyme belonging to the family of pyruvate formiate lyases. It catalyses the addition of the methyl group of the aromatic ring to the double bond of a fumarate cosubstrate leading to (R)-benzylsuccinate. Within this initial reaction, the Bss is making toluene accessible for ring cleavage. FIGURE 1.3 Shows proteins encoded by an operon consisting of bbs genes catalyze beta-oxidation of benzylsuccinate leading to the central intermediate, benzoyl-CoA.
Only a limited number of bssA sequences are available from pure cultures and include : Azoarcus sp. T, and Azoarcus sp. EbN1. All of the reference cultures (some of which are not included here) belong to Proteobacteria. Furthermore, in most of these strains the bss genes are organized similarly within a bssDCABEFGH operon and upstream of the bbsA-H operon. The bssA gene is used as a specific functional marker for anaerobic BTEX degraders
Cell Structure and Metabolism
Eight facultative anaerobic isolates of A. tolulyticus have been identified each of which is capable of performing toluene-degrading denitrification. These bacteria show fatty acid profiles similar to other members of the genus Azoarcus, however distinctions were found in that the newly discovered isolates contained an abundance of 12:0 fatty acid while lacking 3-OH 8:0 fatty acid content. All of the isolates are gram negative, cytochrome c oxidase and catalase positive, and all are capable of fixing nitrogen when grown on N2. Also each of these isolates are capable of producing N2 gas as products of cellular respiration under anaerobic conditions.
A. tolulyticus are short motile rods, although having lengths ranging from 1.4 to 2.1um when grown on toluene, can increase in size to 2.1 to 2.8um (or in long chains) when grown on M-R2A agar. Morphological characteristics when grown on M-R2A agar produces uniform, translucent colonies, yellowish in color and approximately 2-3mm in diameter containing a dark center. The cells lack tryptophanase, B-galactosidsase, urease, and arginine dihydrolase activity. As facultative anaerobes A. tolulyticus is able to use either nitrate or oxygen as terminal electron acceptors during cellular respiration. In aerobic environments oxygen serves as the terminal electron acceptor and is usually the limiting factor due to its low solubility in water along with diffusion constraints in subsurface environments. In anaerobic environments nitrate is used as the terminal electron acceptor. Nitrate is an adequate alternative due to its high solubility in water and high mobility in soil making the rates of degradation comparable to those of aerobic metabolism processes. Evidence has been shown that anaerobic degradation of toluene by A. tolulyticus has also been observed under Fe(III)-reducing, methanogenic, and sulfidogenic conditions.
Ecology and Ecological Relevance
Azoarcus tolulyticus which is included in the genus of the free-living nitrogen fixing bacteria are found in a variety of habitats including both contaminated and non-contaminated soils, aquifiers, and sediments. Table I. shows the diversity of habitats in which isolates have been obtained which range from Michigan to California. Of particular interest are the underground layers of water-bearing permeable rock and soil, known as aquifers. Groundwater coupled with aquifer sediments produce an anoxic underground habitat for these microbes to thrive.
The importance of Azoarcus tolulyticus can be attributed to its bioremediation potential in anoxic toluene contaminated sites. Because toluene compounds have relatively high solubility rates and toxicity levels they pose a particular threat to drinking water resources and can impact the health and safety of the public. When a BTEX contamination occurs, the spilled substances are transported along with the groundwater flow and are difficult to remediate effectively. The contaminants in the water are sustainably reduced only due to in situ biological degradation by the metabolic activity of microbes such A. tolulyticus. Aquifer systems and the processes occurring within them play a key role in maintaining water quality and to maintaining these indigenous microbes responsible for natural attenuation of pollutants. Microbes require both electron donors and electron acceptors for successful degradation of toluene and degradation activities are expected to be highest in biogeochemical gradient zones, where both compounds are at equilibrium.
Due to the relatively low solubility of oxygen, the rate of oxygen being replenished to groundwater systems, allowing oxygen to be used as electron acceptor is rapidly depleted upon contamination by respiratory activities. A. tolulyticus populations are capable of using electron acceptors other than molecular oxygen. Their ability to use different anaerobic terminal electron-accepting processes are necessary for bioremediation in anaerobic conditions (aquifers). BTEX contaminants are often distributed into the ground in large volumes making them highly available as electron donors in non-limiting concentrations. The result is the availability of electron acceptors and their supply becomes a limiting factor for the respiratory activity of degrader capacities.
The prospect of in situ bioremediation treatments using A. tolulyticus potentially could be the best solution at achieving cost effective cleanup technology for treating contaminated sites. Although microbial degradation of these compounds is known to occur under aerobic conditions, contamination such as those in aquifiers and soil, housing anaerobic conditions make degradation by aerobic microbes not possible. The ability of A. tolulyticus to utilize nitrate (and other inorganic compounds) as terminal electron acceptors allows for degradation of toxic chemicals in these habitats.
References
Author
Page authored by Eric Tovar and Alyse Waldhorn, student of Prof. Jay Lennon at Michigan State University.
<-- Do not remove this line-->