Acidithiobacillus ferrooxidans: Difference between revisions
No edit summary |
|||
| (32 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
==Classification== | ==Classification== | ||
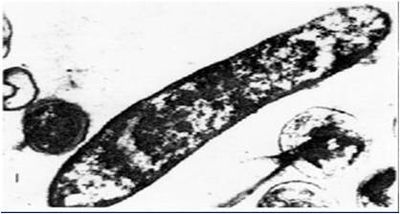
Bacteria | [[File:A. ferrooxidans image2.jpg|thumb|400px|right|''Acidithiobacillus ferrooxidans'' magnification 30,000 times. [Henry Lutz Ehrlich, Geomicrobiology, 2nd edition, (New York: Marcel Dekker, 1990)]]] | ||
'''Domain''': Bacteria | |||
'''Phylum''': Proteobacteria | |||
'''Class''': Gammaproteobacteria | |||
'''Order''': Acidithiobacillales | |||
'''Family''': Acidithiobacillaceae | |||
'''Genus''': ''Acidithiobacillus'' | |||
===Species=== | ===Species=== | ||
| Line 9: | Line 23: | ||
==Description and Significance== | ==Description and Significance== | ||
''A. | ''A. ferrooxidans'' is a Gram negative rod shaped bacterium that is commonly found in deep caves or acid mine drainage, such as coal waste (10, 11, 12). These acidophilic bacteria thrive in optimal pH level of 1.5 – 2.5 where they convert insoluble metals to their soluble state. Even low concentrations (ppm) of these metallic ions would be extremely toxic to other bacteria (7, 8). In addition, these bacteria have been utilized in industrial bioleaching efforts to extract otherwise unobtainable metals (9). | ||
==Genome Structure== | ==Genome Structure== | ||
| Line 15: | Line 29: | ||
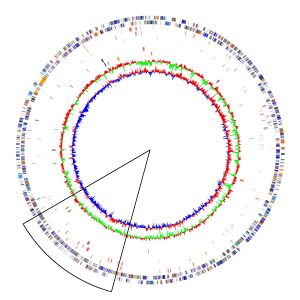
Two strains of ''Acidthiobacillus ferrooxidans'' have been successfully and completely sequenced. The strains sequenced are ATCC 53993 and ATCC 23270 which consists of genomic sizes of 2.88kb and 2.98kb respectively. ''Acidithiobacillus ferrooxidans'' contains a single circular chromosome consisting of around 3,000 genes. The greater majority of species sampled from multiple sites around the world were shown to contain plasmids, however ATCC 23270 did not contain a plasmid (15). ATCC 23270 contains a rich region of recombination which includes phage, transposases, and psuedogenes shown in the black triangle located in Figure 1. | Two strains of ''Acidthiobacillus ferrooxidans'' have been successfully and completely sequenced. The strains sequenced are ATCC 53993 and ATCC 23270 which consists of genomic sizes of 2.88kb and 2.98kb respectively. ''Acidithiobacillus ferrooxidans'' contains a single circular chromosome consisting of around 3,000 genes. The greater majority of species sampled from multiple sites around the world were shown to contain plasmids, however ATCC 23270 did not contain a plasmid (15). ATCC 23270 contains a rich region of recombination which includes phage, transposases, and psuedogenes shown in the black triangle located in Figure 1. | ||
[[File:Genome spiral.jpg|thumb|300px|left|''Acidithiobacillus ferrooxidans''. [http://www.biomedcentral.com/1471-2164/9/597/figure/F2 Jorge Valdés et al., "Figure 1: Circular representation of the A. ferrooxidans ATCC 23270 genome sequence.(15)"]]] | [[File:Genome spiral.jpg|thumb|300px|left|''Acidithiobacillus ferrooxidans''. [http://www.biomedcentral.com/1471-2164/9/597/figure/F2 Jorge Valdés et al., "Figure 1: Circular representation of the ''A. ferrooxidans'' ATCC 23270 genome sequence.(15)"]]] | ||
==Cell Structure and Metabolism== | ==Cell Structure and Metabolism== | ||
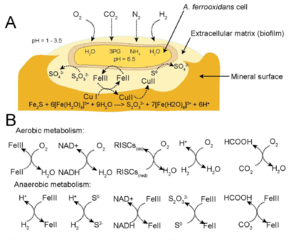
''A. ferrooxidans'' is a | ''A. ferrooxidans'' is a chemolithoautotrophic bacterium which can use many different electron donors to support growth. In addition, a primary habitat consists of acidic conditions producing a strong reducing environment. The cell is able to maintain homeostasis with a neutral pH of 6.5 within the cytoplasm preventing damage and a reducing environment in periplasm. This allows an increase in redox potential of O2/H2O to 1.12 V at pH 3.2 yielding higher energy when coupled with an electron donor (13, 14). | ||
In aerobic conditions, electron donors may include ferrous ions or sulfur compounds which are oxidized into ferric iron and sulfuric acid, respectively, yielding high energy (3, 4, 5). However, during anaerobic conditions ferric ions can replace oxygen as the electron acceptor with multiple substrates donating an electron (Figure 2B, 6). This pathway yields less energy than aerobic conditions, but energy can still be produced for growth. | In aerobic conditions, electron donors may include ferrous ions or sulfur compounds which are oxidized into ferric iron and sulfuric acid, respectively, yielding high energy (3, 4, 5). However, during anaerobic conditions ferric ions can replace oxygen as the electron acceptor with multiple substrates donating an electron (Figure 2B, 6). This pathway yields less energy than aerobic conditions, but energy can still be produced for growth. | ||
''A. ferrooxidans'' can fix atmospheric carbon dioxide (CO2) as a carbon source essential for biomass (1). Nitrogen is a common limiting nutrient scarce in the environment; however, A. ferrooxidans can fix atmospheric nitrogen into ammonia (NH3) essential for nucleotides and amino acids (2). | ''A. ferrooxidans'' can fix atmospheric carbon dioxide (CO2) as a carbon source essential for biomass (1). Nitrogen is a common limiting nutrient scarce in the environment; however, ''A. ferrooxidans'' can fix atmospheric nitrogen into ammonia (NH3) essential for nucleotides and amino acids (2). | ||
[[File:A. ferrooxidans metabolic pathway.png|thumb|300px|right|''Acidithiobacillus ferrooxidans''. Figure 2: A: Example of bio-leaching. B: Aerobic and anaerobic pathways to yield energy for the bacterium (15)."]]] | |||
==Ecology and Industrial Applications== | ==Ecology and Industrial Applications== | ||
Interestingly, many isolations of ''A. ferrooxidans'' using purely inorganic media | Interestingly, many isolations of ''A. ferrooxidans'' using purely inorganic media were thought to be pure cultures, however were actually contaminated with members of the genus ''Acidiphilium'' (16). This prompted scientists to study a possible symbiosis between the two organisms. ''Acidiphilium'' was believed to aide ''A. ferrooxidans'' growth in unfavorable conditions by metabolizing organic materials. It is well documented that ''A. ferrooxidans'' is notably intolerant of low molecular weight organic acids (17). Cultivation of ''A. ferrooxidans'' is unfavorable on acidic ferrous iron agar because growth becomes inhibited by agar hydrolyzed products like: pyruvic acid, citric acid, oxaloacetic acid and glucose (18). Members of the genus ''Acidiphilium'' are oligotrophs, preferring low concentrations of organic compounds in growth media. Common heterotrophic media (eutrophic) is rich in glucose and other organic compounds that make it difficult for ''Acidiphilium cryptum'' to grow at all. This could explain the reason that these two microbes are commonly found together in co-culture. The ''Acidiphilia'' scavenge for organic compounds excreted by ''A. ferrooxidans'' such as: pyruvate, glutamate, aspartate, serine, and glycine (18). Additionally, some members of the genus ''Acidiphilium'' are capable of iron reduction, which is the opposite metabolism performed by ''A. ferrooxidans''. Growth is made much more favorable by the removal of organic compounds that harm ''A. ferrooxidans'' as well as the oxidation of ferrious iron. Lastly, a common oxidation product of pyrite (FeS<sub>2</sub>), thiosulfate, is very toxic to ''Acidiphilium''. ''A. ferrooxidans'' can oxidize thiosulfate and prevent any damage that might occur to ''Acidiphilium''. In addition, thiosulfate is very unstable and breaks down under acidic conditions, another by-product of ''A. ferrooxidans'' growth. | ||
Bioleaching, or biohydrometallurgy, are methods performed by industries to utilize ''A. ferrooxidans'' ability to oxidize metals from ore. Essentially, ''A. ferrooxidans'' oxidation metabolism is beneficial since it can attack insoluble sulfide containing minerals (i.e. copper, lead, zinc and nickel) and covert them to soluble metal sulfates (19). Currently, bioleaching involves a microbial community, not just one organism, to extract desirable metals from unrefined ores. | |||
Traditional concentration and smelting techniques produce hazardous byproducts, whereas ''A. ferrooxidans'' are a clean alternative. Coal can contain undesirable sulfur compounds created by natural weathering processes. This undesirable sulfur can be solubilized by ''A. ferrooxidans'' and removed by washing techniques; this process is known as desulfurization (20). Furthermore, these techniques have shown significant promise in a few other areas. | |||
Recycling of computers and electronics produces copious amounts of dust-like materials that are the result of shredding and separation. These dusty residues are known to contain valuable concentrations of metals that would otherwise be incinerated or discarded. In one experiment, over 90% of the available Cu, Zn, Ni and Al was leached out of this computer circuit board dust (21). | |||
==References== | ==References== | ||
| Line 89: | Line 80: | ||
15. Valdés, J., et. al. 2008. Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications, BMC Genomics 9:597 | 15. Valdés, J., et. al. 2008. Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications, BMC Genomics 9:597 | ||
16. Guay, R., and Silver, M. 1975. “Thiobacillus acidophilus sp. Nov.; isolation and some physiological characteristics”. Can. J. Microbiol. 21(3): 281-288 | |||
17. Cabrera, G., J.M. Gómez and D. Cantero. 2005. “Influence of heavy metals on growth and ferrous sulphate oxidation by Acidithiobacillus ferrooxidans in pure and mixed cultures”. Process Biochemistry. 40: 2683-2687 | |||
18. Gurung , Anirudra and Ranadhir Chakraborty. 2009. “The role of Acidithiobacillus ferrooxidans in alleviating the inhibitory effect of thiosulfate on the growth of acidophilic Acidiphilium species isolated from acid mine drainage samples from Garubathan, India”. Can. J. Microbiol. 55: 1040-1048. | |||
19. Tuovinen, Olli H. and Ilona J. Fry. 1993. “Bioleaching and mineral biotechnology”. Current Opinion in Biotechnology. 4: 344-355. | |||
20. Juszczak, Anna, Florian Domka, Mieczyslaw Kozlowski and Helena Wachowska. 1994. “Microbial desulfurization of coal with thiobacillus ferrooxidans bacteria”. Fuel. Vol. 74 No. 5 pp. 725-728. | |||
21. Brandl, H., R. Bosshard and M. Wegmann. 2000. “Computer-munching microbes: metal leaching from electronic scrap by bacteria and fungi”. Hydrometallurgy. 59: 319-326. | |||
==Authors== | |||
Page authored by Scott Henderson and Matthew Hranchook, students of [http://www.kbs.msu.edu/faculty/lennon/ Prof. Jay Lennon] at Michigan State University. | |||
Latest revision as of 15:23, 8 July 2011
Classification
Domain: Bacteria
Phylum: Proteobacteria
Class: Gammaproteobacteria
Order: Acidithiobacillales
Family: Acidithiobacillaceae
Genus: Acidithiobacillus
Species
Acidithiobacillus ferrooxidans PubMed Genome of ATCC 23270 Genome of ATCC 53993
Description and Significance
A. ferrooxidans is a Gram negative rod shaped bacterium that is commonly found in deep caves or acid mine drainage, such as coal waste (10, 11, 12). These acidophilic bacteria thrive in optimal pH level of 1.5 – 2.5 where they convert insoluble metals to their soluble state. Even low concentrations (ppm) of these metallic ions would be extremely toxic to other bacteria (7, 8). In addition, these bacteria have been utilized in industrial bioleaching efforts to extract otherwise unobtainable metals (9).
Genome Structure
Two strains of Acidthiobacillus ferrooxidans have been successfully and completely sequenced. The strains sequenced are ATCC 53993 and ATCC 23270 which consists of genomic sizes of 2.88kb and 2.98kb respectively. Acidithiobacillus ferrooxidans contains a single circular chromosome consisting of around 3,000 genes. The greater majority of species sampled from multiple sites around the world were shown to contain plasmids, however ATCC 23270 did not contain a plasmid (15). ATCC 23270 contains a rich region of recombination which includes phage, transposases, and psuedogenes shown in the black triangle located in Figure 1.
Cell Structure and Metabolism
A. ferrooxidans is a chemolithoautotrophic bacterium which can use many different electron donors to support growth. In addition, a primary habitat consists of acidic conditions producing a strong reducing environment. The cell is able to maintain homeostasis with a neutral pH of 6.5 within the cytoplasm preventing damage and a reducing environment in periplasm. This allows an increase in redox potential of O2/H2O to 1.12 V at pH 3.2 yielding higher energy when coupled with an electron donor (13, 14).
In aerobic conditions, electron donors may include ferrous ions or sulfur compounds which are oxidized into ferric iron and sulfuric acid, respectively, yielding high energy (3, 4, 5). However, during anaerobic conditions ferric ions can replace oxygen as the electron acceptor with multiple substrates donating an electron (Figure 2B, 6). This pathway yields less energy than aerobic conditions, but energy can still be produced for growth.
A. ferrooxidans can fix atmospheric carbon dioxide (CO2) as a carbon source essential for biomass (1). Nitrogen is a common limiting nutrient scarce in the environment; however, A. ferrooxidans can fix atmospheric nitrogen into ammonia (NH3) essential for nucleotides and amino acids (2).
]
Ecology and Industrial Applications
Interestingly, many isolations of A. ferrooxidans using purely inorganic media were thought to be pure cultures, however were actually contaminated with members of the genus Acidiphilium (16). This prompted scientists to study a possible symbiosis between the two organisms. Acidiphilium was believed to aide A. ferrooxidans growth in unfavorable conditions by metabolizing organic materials. It is well documented that A. ferrooxidans is notably intolerant of low molecular weight organic acids (17). Cultivation of A. ferrooxidans is unfavorable on acidic ferrous iron agar because growth becomes inhibited by agar hydrolyzed products like: pyruvic acid, citric acid, oxaloacetic acid and glucose (18). Members of the genus Acidiphilium are oligotrophs, preferring low concentrations of organic compounds in growth media. Common heterotrophic media (eutrophic) is rich in glucose and other organic compounds that make it difficult for Acidiphilium cryptum to grow at all. This could explain the reason that these two microbes are commonly found together in co-culture. The Acidiphilia scavenge for organic compounds excreted by A. ferrooxidans such as: pyruvate, glutamate, aspartate, serine, and glycine (18). Additionally, some members of the genus Acidiphilium are capable of iron reduction, which is the opposite metabolism performed by A. ferrooxidans. Growth is made much more favorable by the removal of organic compounds that harm A. ferrooxidans as well as the oxidation of ferrious iron. Lastly, a common oxidation product of pyrite (FeS2), thiosulfate, is very toxic to Acidiphilium. A. ferrooxidans can oxidize thiosulfate and prevent any damage that might occur to Acidiphilium. In addition, thiosulfate is very unstable and breaks down under acidic conditions, another by-product of A. ferrooxidans growth.
Bioleaching, or biohydrometallurgy, are methods performed by industries to utilize A. ferrooxidans ability to oxidize metals from ore. Essentially, A. ferrooxidans oxidation metabolism is beneficial since it can attack insoluble sulfide containing minerals (i.e. copper, lead, zinc and nickel) and covert them to soluble metal sulfates (19). Currently, bioleaching involves a microbial community, not just one organism, to extract desirable metals from unrefined ores. Traditional concentration and smelting techniques produce hazardous byproducts, whereas A. ferrooxidans are a clean alternative. Coal can contain undesirable sulfur compounds created by natural weathering processes. This undesirable sulfur can be solubilized by A. ferrooxidans and removed by washing techniques; this process is known as desulfurization (20). Furthermore, these techniques have shown significant promise in a few other areas. Recycling of computers and electronics produces copious amounts of dust-like materials that are the result of shredding and separation. These dusty residues are known to contain valuable concentrations of metals that would otherwise be incinerated or discarded. In one experiment, over 90% of the available Cu, Zn, Ni and Al was leached out of this computer circuit board dust (21).
References
1. Kelly, D. P., and A. P. Harrison. 1989. Genus Thiobacillus Beijerinck, p. 1842-1858. In J. T. Staley, M. P. Bryant, N. Pfennig, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 3. The Williams & Wilkins Co., Baltimore.
2. Mackintosh, M. E. 1978. Nitrogen fixation by Thiobacillus ferrooxidans. J. Gen. Microbiol. 105:215-218.
3. Pronk, J. T., K. Liem, P. Bos, and J. G. Kuenen. 1991. Energy transduction by anaerobic ferric iron respiration in Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 57:2063-2068.
4. Corbet, C. M., and W. J. Ingledew. 1987. Is Fe3+'2+ cycling an intermediate in sulphur oxidation by Fe2+-grown Thiobacillus ferrooxidans? FEMS Microbiol. Lett. 41:1-6.
5. Sugio, T., K. J. White, E. Shute, D. Choate, and R. C. Blake. 1992. Existence of a hydrogen sulfide:ferric ion oxidoreductase in iron-oxidizing bacteria. Appl. Environ. Microbiol. 58:431-433.
6. Sugio, T., C. Domatsu, 0. Munakata, T. Tano, and K. Imai. 1985.
7. Role of a ferric ion-reducing system in sulfur oxidation of Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 49:1401-1406.
8. Rawlings, D. E., I.-M. Pretorius, and D. R. Woods. 1986. Expression of Thiobacillus ferrooxidans plasmid functions and the development of genetic systems for the thiobacilli. Biotechnol. Bioeng. Symp. 16:281-287.
9. Colmer, A. R., and M. E. Hinkle. 1947. The role of microorganisms in acid mine drainage: a preliminary report. Science 106:253–256.
10. Yu Yang, Min-xi Wan, Wu-yang Shi, Hong Peng, Guan-zhou Qiu, Ji-zhong Zhou, Xue-duan Liu. 2007. Bacterial diversity and community structure in acid mine drainage from Dabaoshan Mine, China. AQUATIC MICROBIAL ECOLOGY Vol. 47: 141-151
11. Brierley, C. L. 1982. Microbiological mining. Sci. Am. 247(2):42-51.
12. Merson, J. 1992. Mining with microbes. New Sci. 133:17-19.
13. Sato, A., Y. Fukumori, T. Yano, M. Kai, and T. Yamanaka. 1989. Thiobacillus ferrooxidans cytochrome c-552: purification and some of its molecular features. Biochim. Biophys. Acta 976:129-134.
14. Cox, J. C., and M. D. Brand. 1984. Iron oxidation and energy conservation in the chemoautotroph Thiobacillus ferrooxidans, p.31-46. In W. R. Strohl and 0. H. Touvinen (ed.), Microbial chemoautotrophy. Ohio State University Press, Columbus, Ohio.
15. Valdés, J., et. al. 2008. Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications, BMC Genomics 9:597
16. Guay, R., and Silver, M. 1975. “Thiobacillus acidophilus sp. Nov.; isolation and some physiological characteristics”. Can. J. Microbiol. 21(3): 281-288
17. Cabrera, G., J.M. Gómez and D. Cantero. 2005. “Influence of heavy metals on growth and ferrous sulphate oxidation by Acidithiobacillus ferrooxidans in pure and mixed cultures”. Process Biochemistry. 40: 2683-2687
18. Gurung , Anirudra and Ranadhir Chakraborty. 2009. “The role of Acidithiobacillus ferrooxidans in alleviating the inhibitory effect of thiosulfate on the growth of acidophilic Acidiphilium species isolated from acid mine drainage samples from Garubathan, India”. Can. J. Microbiol. 55: 1040-1048.
19. Tuovinen, Olli H. and Ilona J. Fry. 1993. “Bioleaching and mineral biotechnology”. Current Opinion in Biotechnology. 4: 344-355.
20. Juszczak, Anna, Florian Domka, Mieczyslaw Kozlowski and Helena Wachowska. 1994. “Microbial desulfurization of coal with thiobacillus ferrooxidans bacteria”. Fuel. Vol. 74 No. 5 pp. 725-728.
21. Brandl, H., R. Bosshard and M. Wegmann. 2000. “Computer-munching microbes: metal leaching from electronic scrap by bacteria and fungi”. Hydrometallurgy. 59: 319-326.
Authors
Page authored by Scott Henderson and Matthew Hranchook, students of Prof. Jay Lennon at Michigan State University.