Clostridium tetani and Tetanus: Difference between revisions
No edit summary |
|||
| (20 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | |||
==Introduction== | ==Introduction== | ||
[[Image:TetanusCharlesBell.jpg|thumb|300px|right| [http://en.wikipedia.org/wiki/File:Opisthotonus_in_a_patient_suffering_from_tetanus_-_Painting_by_Sir_Charles_Bell_-_1809.jpg <i>Opisthotonus</i> (Tetanus) by Charles Bell (1809).]]] | [[Image:TetanusCharlesBell.jpg|thumb|300px|right| Figure 1. [http://en.wikipedia.org/wiki/File:Opisthotonus_in_a_patient_suffering_from_tetanus_-_Painting_by_Sir_Charles_Bell_-_1809.jpg <i>Opisthotonus</i> (Tetanus) by Charles Bell (1809).] The subject is suffering from the spastic paralysis of tetanus and is presenting with <i>opisthotonus</i>, or arching of the neck and back due to muscle contractions.]] | ||
<br>By Tyler Stearns<br> | <br>By Tyler Stearns<br> | ||
<i>Clostridium tetani</i> is a Gram-postive, spore-forming bacillus that causes the disease tetanus (Slonczewski & Foster 2011). <i>C. tetani</i> is ubiquitous in nature; it is primarily found in soil, but can also be found in animal and human fecal samples (Cook 2001). In one survey, <i>C. tetani</i> was found in approximately 30% of soil samples. Growth did not appear to be dependent on soil type or climate (Smith 1978). <i>C. tetani</i> is an obligate anaerobe, but its spores can survive extreme conditions, including treatment with antiseptic phenols and autoclaving at 121°C for 10-15 minutes (Pink Book). | <i>Clostridium tetani</i> is a Gram-postive, spore-forming bacillus that causes the disease tetanus (Slonczewski & Foster 2011). <i>C. tetani</i> is ubiquitous in nature; it is primarily found in soil, but can also be found in animal and human fecal samples (Cook 2001). In one survey, <i>C. tetani</i> was found in approximately 30% of soil samples. Growth did not appear to be dependent on soil type or climate (Smith 1978). <i>C. tetani</i> is an obligate anaerobe, but its spores can survive extreme conditions, including treatment with antiseptic phenols and autoclaving at 121°C for 10-15 minutes (Pink Book). | ||
Besides its pervasiveness and survival in extreme conditions, <i>C. tetani</i> also produces one of the strongest toxins known to man. The tetanus toxin (TeTx) produces clinical tetanus disease, which is characterized by spastic paralysis. TeTx has an estimated lethal dose of 2.5 nanograms per kilogram of body weight (Pink Book). Thus, a mere 175 nanograms of TeTx can kill a 70 kg (154 lb) human. | Besides its pervasiveness and survival in extreme conditions, <i>C. tetani</i> also produces one of the strongest toxins known to man. The tetanus toxin (TeTx) produces clinical tetanus disease, which is characterized by spastic paralysis (Figure 1). TeTx has an estimated lethal dose of 2.5 nanograms per kilogram of body weight (Pink Book). Thus, a mere 175 nanograms of TeTx can kill a 70 kg (154 lb) human. | ||
A tetanus antitoxin and vaccine were developed nearly a century ago and regular vaccination has become common in developed countries, lowering the number of tetanus cases in the United States to 124 between 1995 and 1997 (Hsu 2001). Despite these advances, there are still an estimated 700,000 to 1,000,000 cases of tetanus worldwide every year, mostly in developing countries where vaccines are less common (Thwaites 2003). The following material is an overview of what is currently | A tetanus antitoxin and vaccine were developed nearly a century ago and regular vaccination has become common in developed countries, thus lowering the number of tetanus cases in the United States to 124 between 1995 and 1997 (Hsu 2001). Despite these advances, there are still an estimated 700,000 to 1,000,000 cases of tetanus worldwide every year, mostly in developing countries where vaccines are less common (Thwaites 2003). The following material is an overview of what is currently known about tetanus and TeTx, and how this deadly and still far-too-common disease can be controlled and treated. | ||
==The Tetanus Toxin: Genetics and Mechanism of Action== | ==The Tetanus Toxin: Genetics and Mechanism of Action== | ||
| Line 13: | Line 14: | ||
===Genetics=== | ===Genetics=== | ||
The genome of <i>C. tetani</i> consists of a single 2,799,250-bp chromosome with 2,372 open reading frames (ORFs); the tetanus toxin (TeTx) is encoded on a 74,082-bp plasmid containing 61 ORFs (Brüggemann 2003). The genus <i>Clostridium</i> is a member of the Firmicutes phylum, which | The genome of <i>C. tetani</i> consists of a single 2,799,250-bp chromosome with 2,372 open reading frames (ORFs); the tetanus toxin (TeTx) is encoded on a 74,082-bp plasmid containing 61 ORFs (Brüggemann 2003). The genus <i>Clostridium</i> is a member of the Firmicutes phylum, which is known for a low G-C content (S & F 2011). The <i>C. tetani</i> chromosome has a G-C content of 28.6%, while the TeTx-encoding plasmid pE88 only has a G-C content of 24.5%. The G-C ratio is relatively stable in the main chromosome, indicating a lack of recent horizontal gene transfer (Brüggemann 2003). | ||
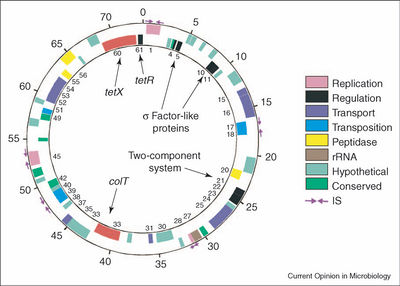
The plasmid pE88 holds the genes for TeTx (<i>tetX</i>) and its direct transcriptional regulator TetR. The <i>tetR</i> gene is located just upstream from <i>tetX</i> (Brüggemann 2005). TetR was thought to possibly be a positive | The plasmid pE88 holds the genes for TeTx (<i>tetX</i>) and its direct transcriptional regulator TetR (Figure 2). The <i>tetR</i> gene is located just upstream from <i>tetX</i> (Brüggemann 2005). TetR was thought to possibly be a positive regulator of toxin expression, but current research has shown that TetR is in fact a sigma factor of a subgroup unique to clostridial species. This research found that TetR only associated with target DNA in the presence of the RNA polymerase core enzyme. In the presence of the RNA polymerase core enzyme, TetR bound to the core enzyme and triggered transcription of the target DNA at the promoter in vitro (Raffestin 2005). Other regulatory genes are present on the plasmid. CTP05, CTP10, and CTP11 are sigma factor-like proteins, and CTP21 and CTP22 form a two-component system of unknown regulatory function. The three sigma-like proteins do not appear to be involved in the regulation of toxin production like TetR (Brüggemann 2005; Raffestin 2005). | ||
[[Image:TetanusPlasmid.jpg|thumb|400px|right|The plasmid pE88 encodes the tetanus toxin and its regulators (from Brüggemann 2005).]] | [[Image:TetanusPlasmid.jpg|thumb|400px|right|Figure 2. The plasmid pE88 encodes the tetanus toxin and its regulators (from Brüggemann 2005).]] | ||
Other virulence factors may be present on pE88, in addition to the primary tetanus toxin. The 114-kDa collagenase ColT is also encoded on the plasmid (Brüggemann 2003). Collagenase is an enzyme that degrades collagen, which makes up almost 25 to 33% of the total protein in mammalian organisms (Harrington 1996). Thus, ColT may help destroy tissue in an infected host. | Other virulence factors may be present on pE88, in addition to the primary tetanus toxin. The 114-kDa collagenase ColT is also encoded on the plasmid (Brüggemann 2003). Collagenase is an enzyme that degrades collagen, which makes up almost 25 to 33% of the total protein in mammalian organisms (Harrington 1996). Thus, ColT may help destroy tissue in an infected host. | ||
| Line 24: | Line 25: | ||
The main chromosome of <i>C. tetani</i> also possesses potential virulence factors. ORFs for tetanolysin O, hemolysin, and fibronectin-binding proteins, as well as genes for surface-layer (S-layer) proteins have been identified (Brüggemann 2003). Tetanolysin, discussed below, is one of the two exotoxins produced by C. tetani, in addition to TeTx. The S-layer has been characterized as a molecular sieve or as a possible defense against parasites. However, it also has been implicated in host cell adhesion or as a possible mechanism of evading host immune systems in another pathogenic clostridial species, <i>C. difficile</i> (Spigaglia 2011). | The main chromosome of <i>C. tetani</i> also possesses potential virulence factors. ORFs for tetanolysin O, hemolysin, and fibronectin-binding proteins, as well as genes for surface-layer (S-layer) proteins have been identified (Brüggemann 2003). Tetanolysin, discussed below, is one of the two exotoxins produced by C. tetani, in addition to TeTx. The S-layer has been characterized as a molecular sieve or as a possible defense against parasites. However, it also has been implicated in host cell adhesion or as a possible mechanism of evading host immune systems in another pathogenic clostridial species, <i>C. difficile</i> (Spigaglia 2011). | ||
The origin of the plasmid pE88 is still unclear. Over 50% of the ORFs on the plasmid are unique to <i>C. tetani</i> (Brüggemann 2003). Toxin genes are currently—or were in their evolutionary history—part of a flexible clostridial gene pool. Many of the toxin genes in pathogenic <i>Clostridium</i> species are on plasmids or capable of transduction by phages (Brüggemann 2005). <i>C. botulinum</i>, which produces various forms of the botulinum neurotoxin (BoNT), has been shown to transfer its toxin-encoding plasmid by conjugation. In one study, a tagged BoNT-encoding plasmid was transferred from one strain of <i>C. botulinum</i> to another. Bacteriophages for transduction were not observed, and gene transfer was not inhibited by DNase, ruling out transformation of free-floating DNA (Marshall 2010). The epsilon-toxin plasmids in <i>C. perfringens</i> Type D have also been shown to be transferable to other cells by conjugation (Hughes 2007). Given this evidence, and the fact TeTx is quite similar to BoNT, it seems highly | The origin of the plasmid pE88 is still unclear. Over 50% of the ORFs on the plasmid are unique to <i>C. tetani</i> (Brüggemann 2003). Toxin genes are currently—or were in their evolutionary history—part of a flexible clostridial gene pool. Many of the toxin genes in pathogenic <i>Clostridium</i> species are on plasmids or capable of transduction by phages (Brüggemann 2005). <i>C. botulinum</i>, which produces various forms of the botulinum neurotoxin (BoNT), has been shown to transfer its toxin-encoding plasmid by conjugation. In one study, a tagged BoNT-encoding plasmid was transferred from one strain of <i>C. botulinum</i> to another. Bacteriophages for transduction were not observed, and gene transfer was not inhibited by DNase, ruling out transformation of free-floating DNA (Marshall 2010). The epsilon-toxin plasmids in <i>C. perfringens</i> Type D have also been shown to be transferable to other cells by conjugation (Hughes 2007). Given this evidence, and the fact TeTx is quite similar to BoNT, it seems highly probable that the TeTx plasmid was acquired via some form of horizontal gene transfer, though it has clearly undergone its own divergent evolution since that point. | ||
===Infection=== | ===Infection=== | ||
Tetanus infections most commonly occur after deep-tissue puncture wounds | Tetanus infections most commonly occur after deep-tissue puncture wounds are exposed to <i>C. tetani</i>. Contamination of the wound often involves contact with soil, fecal matter, or rusty metal that contain <i>C. tetani</i> (Cook 2001; Campbell 2009). <i>C. tetani</i> is an obligate anaerobe. The conditions in a wound are particularly well suited to the bacteria's anaerobic needs (Campbell 2009). Tetanolysin is one of the two exotoxins excreted by <i>C. tetani</i>. Tetanolysin damages tissue surrounding an infection, optimizing conditions within the wound (Cook 2001; Campbell 2009). Bacteria grow and ferment in the wound, releasing in small quantities the actual causative agent of tetanus disease, the tetanospasmin protein (also referred to as tetanus toxin, TeTx, or TeNT). The toxin is mainly released during the stationary phase of growth and a significant majority of the toxin is not freed until a cell lyses, releasing its contents into the body of the host (Mellanby 1981). TeTx is distributed to motor neurons and in the bloodstream to other pre-synaptic nerve terminals in the peripheral nervous system (Kerr 1979). | ||
===Cell Entry=== | ===Cell Entry=== | ||
| Line 34: | Line 35: | ||
Tetanospasmin must enter nerve cells in order to reach its primary site of action and produce clinical tetanus disease. The toxin can have some effect on motor neurons near the site of infection, but the main syndrome is produced after TeTx reaches the spinal cord. Once the toxin has entered neurons near the site of infection or elsewhere in the peripheral nervous system, it can move retrogradely within axons, to the cell body of the neuron, then subsequently to other neurons, and thus reach the spinal cord via intra-axonal transport (Erdmann 1975). | Tetanospasmin must enter nerve cells in order to reach its primary site of action and produce clinical tetanus disease. The toxin can have some effect on motor neurons near the site of infection, but the main syndrome is produced after TeTx reaches the spinal cord. Once the toxin has entered neurons near the site of infection or elsewhere in the peripheral nervous system, it can move retrogradely within axons, to the cell body of the neuron, then subsequently to other neurons, and thus reach the spinal cord via intra-axonal transport (Erdmann 1975). | ||
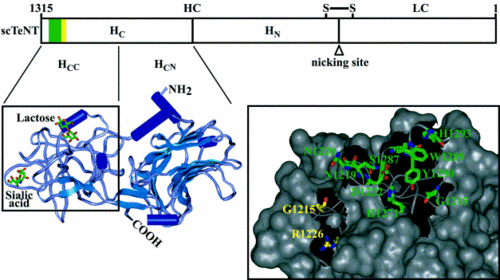
The TeTx protein is comprised of two components, both of which are necessary for intoxication but have different functions in pathogenesis. TeTx is initially synthesized as a ~150-kDa chain, but is subsequently cleaved into a two-part chain linked by a single disulfide bond (Chen 2009). The final two components are the light chain (LC; ~50 kDa) and the heavy chain (HC; ~100 kDa). The LC is the active constituent of TeTx, but the HC is required for entry into the cell, where LCs function to cause disease (Binz 2009). | The TeTx protein is comprised of two components, both of which are necessary for intoxication but have different functions in pathogenesis. TeTx is initially synthesized as a ~150-kDa chain, but is subsequently cleaved into a two-part chain linked by a single disulfide bond (Chen 2009). The final two components are the light chain (LC; ~50 kDa) and the heavy chain (HC; ~100 kDa) (Figure 3). The LC is the active constituent of TeTx, but the HC is required for entry into the cell, where LCs function to cause disease (Binz 2009). | ||
[[Image:TeTxDiagram.gif|thumb|500px|right|Diagram of TeTx. Regions forming the sialic acid and lactose-binding sites are highlighted by yellow and green bars, respectively. Lower left panel: secondary structure presentation of the TeTx H<sub>C</sub>-fragment. a-Helices are presented as dark blue cylinders, b-strands as light blue arrows, and the sialic acid and lactose bound to the H<sub>C</sub>-fragment. Lower right panel: magnified surface plot of the H<sub>CC</sub>-domain. Amino acid residues that presumably interact with sialic acid or lactose are shown in the stick model in yellow or green, respectively | [[Image:TeTxDiagram.gif|thumb|500px|right|Figure 3. Diagram of TeTx. Regions forming the sialic acid and lactose-binding sites are highlighted by yellow and green bars, respectively. Lower left panel: secondary structure presentation of the TeTx H<sub>C</sub>-fragment. a-Helices are presented as dark blue cylinders, b-strands as light blue arrows, and the sialic acid and lactose bound to the H<sub>C</sub>-fragment. Lower right panel: magnified surface plot of the H<sub>CC</sub>-domain. Amino acid residues that presumably interact with sialic acid or lactose are shown in the stick model in yellow or green, respectively (from Rummel 2003).]] | ||
The HCs of TeTx interact with gangliosides on the surface of neuronal membranes. Gangliosides are sialic acid-containing glycosphingolipids that may be involved in signal transduction and are found in the greatest concentrations on the outer layers of membranes in the nervous system (Yu 2008). In preparations of mouse neurons where production of gangliosides has been inhibited there is no sign of TeTx binding to cells and the toxin does not prevent the release of neurotransmitter from those cells (Williamson 1999). Just as TeTx is divided into two domains, HC can also be divided into two functionally distinct domains. The amino-terminal domain H<sub>N</sub> is responsible for translocating the LC across the plasma membrane, whereas the carboxyl-terminal domain H<sub>C</sub> is responsible for the binding of TeTx to gangliosides on neurons (Rummel 2003). | The HCs of TeTx interact with gangliosides on the surface of neuronal membranes. Gangliosides are sialic acid-containing glycosphingolipids that may be involved in signal transduction and are found in the greatest concentrations on the outer layers of membranes in the nervous system (Yu 2008). In preparations of mouse neurons where production of gangliosides has been inhibited there is no sign of TeTx binding to cells and the toxin does not prevent the release of neurotransmitter from those cells (Williamson 1999). Just as TeTx is divided into two domains, HC can also be divided into two functionally distinct domains. The amino-terminal domain H<sub>N</sub> is responsible for translocating the LC across the plasma membrane, whereas the carboxyl-terminal domain H<sub>C</sub> is responsible for the binding of TeTx to gangliosides on neurons (Rummel 2003). | ||
The H<sub>C</sub> domain can be broken down even further into the H<sub>CN</sub> and H<sub>CC</sub> subunits. The H<sub>CC</sub> fragment has been shown to bind the gangliosides and helps in retrograde transport along the axon so that TeTx can reach the spinal cord (Rummel 2003). The H<sub>CN</sub> domain does not bind gangliosides (Figueiredo 1995) and its ultimate role remains unclear. The H<sub>CC</sub> domain has two important binding sites associated with it. These pockets are termed the lactose binding site and the sialic acid binding site (Binz 2009). In one set of experiments, researchers mutated these binding pockets by changing certain amino acids. When only the sialic acid binding site was mutated, toxicity was reduced by almost 99%; toxicity levels were almost undetectable after mutation of the lactose site (Rummel 2003). Furthermore, the experiments were performed on GT1b-type gangliosides, which have the highest affinity for TeTx. Another study used gangliosides of the type GM1a, which binds only the lactose pocket, and GD3, which binds only the sialic acid pocket, in order to explicate the role of these pockets in TeTx binding. They confirmed again that a mutated H<sub>CC</sub> fragment lacking one pocket or the other did not bind TeTx. In the presence of only one of the gangliosides—either GM1a or GD3—toxin was unable to enter the cell (Chen 2009). The results from both investigations show that both binding sites within the H<sub>CC</sub> domain are necessary for TeTx to enter the cell. In addition, it appears as though two separate gangliosides are needed to bind H<sub>CC</sub>. | The H<sub>C</sub> domain can be broken down even further into the H<sub>CN</sub> and H<sub>CC</sub> subunits. The H<sub>CC</sub> fragment has been shown to bind the gangliosides and helps in retrograde transport along the axon so that TeTx can reach the spinal cord (Rummel 2003). The H<sub>CN</sub> domain does not bind gangliosides (Figueiredo 1995) and its ultimate role remains unclear. The H<sub>CC</sub> domain has two important binding sites associated with it. These pockets are termed the lactose binding site and the sialic acid binding site (Figure 3; Binz 2009). In one set of experiments, researchers mutated these binding pockets by changing certain amino acids. When only the sialic acid binding site was mutated, toxicity was reduced by almost 99%; toxicity levels were almost undetectable after mutation of the lactose site (Rummel 2003). Furthermore, the experiments were performed on GT1b-type gangliosides, which have the highest affinity for TeTx. Another study used gangliosides of the type GM1a, which binds only the lactose pocket, and GD3, which binds only the sialic acid pocket, in order to explicate the role of these pockets in TeTx binding. They confirmed again that a mutated H<sub>CC</sub> fragment lacking one pocket or the other did not bind TeTx. In the presence of only one of the gangliosides—either GM1a or GD3—toxin was unable to enter the cell (Chen 2009). The results from both investigations show that both binding sites within the H<sub>CC</sub> domain are necessary for TeTx to enter the cell. In addition, it appears as though two separate gangliosides are needed to bind H<sub>CC</sub>. | ||
Once the H<sub>C</sub> domain has bound to the gangliosides, the H<sub>N</sub> domain then allows the LC to cross into the cytoplasm of the cell, where it can act on neurotransmitter vesicles. It is currently unknown how exactly H<sub>N</sub> helps the LC gain access to the cytoplasm. Models have been proposed for BoNT, which is structurally similar to TeTx. In these models, the low pH of the endosome may cause a structural change in the HC causing the H<sub>N</sub> domain to insert into the endosome membrane and form a channel (Brunger 2007). The H<sub>N</sub> channel may also act as a chaperone, unfolding the LC, pulling it across the endosomal transmembrane channel, and then refolding it in the cytoplasm of the neuron (Binz 2009). | Once the H<sub>C</sub> domain has bound to the gangliosides, the H<sub>N</sub> domain then allows the LC to cross into the cytoplasm of the cell, where it can act on neurotransmitter vesicles. It is currently unknown how exactly H<sub>N</sub> helps the LC gain access to the cytoplasm. Models have been proposed for BoNT, which is structurally similar to TeTx. In these models, the low pH of the endosome may cause a structural change in the HC, causing the H<sub>N</sub> domain to insert into the endosome membrane and form a channel (Brunger 2007). The H<sub>N</sub> channel may also act as a chaperone, unfolding the LC, pulling it across the endosomal transmembrane channel, and then refolding it in the cytoplasm of the neuron (Binz 2009). | ||
===Mechanism of Action within the Cell=== | ===Mechanism of Action within the Cell=== | ||
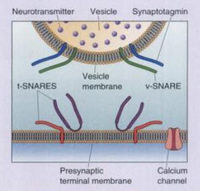
[[Image:BearSNARE.jpg|thumb|200px|right|SNARE proteins dock and fuse neurotransmitter vesicles with the neuronal membrane for exocytosis (from Bear 2007).]] | [[Image:BearSNARE.jpg|thumb|200px|right|Figure 4. SNARE proteins dock and fuse neurotransmitter vesicles with the neuronal membrane for exocytosis (from Bear 2007).]] | ||
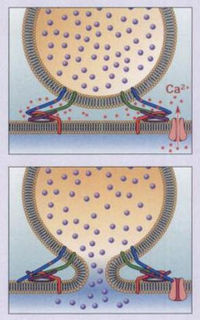
[[Image:BearSNARE2.jpg|thumb|200px|right|A neurotransmitter vesicle binding to a cell membrane. An action potential produces an influx of Ca<sup>2+</sup>, which causes SNAREs to bind with one another, allowing the vesicle to dock and fuse with the neuronal membrane, and thus release neurotransmitter into the synaptic cleft. TeTx blocks this process (from Bear 2007).]] | [[Image:BearSNARE2.jpg|thumb|200px|right|Figure 5. A neurotransmitter vesicle binding to a cell membrane. An action potential produces an influx of Ca<sup>2+</sup>, which causes SNAREs to bind with one another, allowing the vesicle to dock and fuse with the neuronal membrane, and thus release neurotransmitter into the synaptic cleft. TeTx blocks this process (from Bear 2007).]] | ||
Once in the cytoplasm, the LC, which is the active component of the tetanus toxin, begins interrupting release of neurotransmitters. When TeTx reaches the spinal cord, it leaves the motor neuron and moves to the synaptic nerve terminals of spinal neurons. Primarily neurons with inhibitory neurotransmitters are affected, leading to the symptoms of clinical tetanus. | Once in the cytoplasm, the LC, which is the active component of the tetanus toxin, begins interrupting release of neurotransmitters. When TeTx reaches the spinal cord, it leaves the motor neuron and moves to the synaptic nerve terminals of spinal neurons. Primarily, neurons with inhibitory neurotransmitters are affected, leading to the symptoms of clinical tetanus. | ||
The LC of TeTx is an endoprotease, meaning it is capable of cleaving peptide bonds away from the amino- and carboxyl-terminal ends and breaking apart proteins (Binz 2009; Lebrun 2009). Most proteases in bacteria are used for food acquisition for the cell. The protease breaks down long peptide chains into shorter ones to enable absorption by the bacteria (Lebrun 2009). Metabolic functions like this have not been shown in TeTx, however. The toxin-encoding plasmid is not found in every strain of <i>C. tetani</i>, and therefore it appears as though the toxin—along with its proteolytic capabilities—may have evolved primarily as a defense mechanism or a virulence factor (Campbell 2009). | The LC of TeTx is an endoprotease, meaning it is capable of cleaving peptide bonds away from the amino- and carboxyl-terminal ends and breaking apart proteins (Binz 2009; Lebrun 2009). Most proteases in bacteria are used for food acquisition for the cell. The protease breaks down long peptide chains into shorter ones to enable absorption by the bacteria (Lebrun 2009). Metabolic functions like this have not been shown in TeTx, however. The toxin-encoding plasmid is not found in every strain of <i>C. tetani</i>, and therefore it appears as though the toxin—along with its proteolytic capabilities—may have evolved primarily as a defense mechanism or a virulence factor (Campbell 2009). | ||
The LC can be further classified as a zinc metalloprotease. A Zn<sup>2+</sup> ion interacts with two histidine residues in the LC. Early research into TeTx's mechanism of action showed that zinc was necessary for inhibiting evoked neurotransmitter release in Aplysia californica (Schiavo 1992a). If LCs were depleted of Zn<sup>2+</sup> and treated with metal chelators to remove extra Zn<sup>2+</sup> ions, the LCs alone did not cause much inhibition of neurotransmitter release. Thus, it was shown that the active domain of TeTx, the LC, is a zinc-dependent metalloprotease. | The LC can be further classified as a zinc metalloprotease. A Zn<sup>2+</sup> ion interacts with two histidine residues in the LC. Early research into TeTx's mechanism of action showed that zinc was necessary for inhibiting evoked neurotransmitter release in <i>Aplysia californica</i> (Schiavo 1992a). If LCs were depleted of Zn<sup>2+</sup> and treated with metal chelators to remove extra Zn<sup>2+</sup> ions, the LCs alone did not cause much inhibition of neurotransmitter release. Thus, it was shown that the active domain of TeTx, the LC, is a zinc-dependent metalloprotease. | ||
TeTx primarily prevents the release of the inhibitory neurotransmitters gamma-aminobutyric acid (GABA) and glycine in the spinal cord (Collingridge 1982; Cook 2001). The LC cleaves synaptobrevin-2 (also known as VAMP2), a SNARE protein involved in the fusion of neurotransmitter vesicles with the neuronal membrane for exocytosis. An action potential in a neuron usually causes Ca<sup>2+</sup> to enter into the cell. Under normal conditions, Ca<sup>2+</sup> causes v-SNAREs on neurotransmitter vesicles (such as synaptobrevin) to interact with t-SNAREs on the membrane of the neuron. The SNAREs bind with one another, allowing the vesicle to dock and fuse with the neuronal membrane, and thus release neurotransmitter into the synaptic cleft (Bear 2007). In the presence of TeTx, however, the LC hydrolyzes the bond between amino acid residues Gln<sub>76</sub> and Phe<sub>77</sub> on synaptobrevin-2. Synaptobrevin-1, which has a valine at the corresponding position on synaptobrevin-2, is not cleaved by TeTx (Schiavo 1992b). The result is that vesicles containing GABA and glycine are not allowed to dock, and therefore cannot be released. | TeTx primarily prevents the release of the inhibitory neurotransmitters gamma-aminobutyric acid (GABA) and glycine in the spinal cord (Collingridge 1982; Cook 2001). The LC cleaves synaptobrevin-2 (also known as VAMP2), a SNARE protein involved in the fusion of neurotransmitter vesicles with the neuronal membrane for exocytosis (Figure 4). An action potential in a neuron usually causes Ca<sup>2+</sup> to enter into the cell. Under normal conditions, Ca<sup>2+</sup> causes v-SNAREs on neurotransmitter vesicles (such as synaptobrevin) to interact with t-SNAREs on the membrane of the neuron (Figure 5). The SNAREs bind with one another, allowing the vesicle to dock and fuse with the neuronal membrane, and thus release neurotransmitter into the synaptic cleft (Bear 2007). In the presence of TeTx, however, the LC hydrolyzes the bond between amino acid residues Gln<sub>76</sub> and Phe<sub>77</sub> on synaptobrevin-2. Synaptobrevin-1, which has a valine at the corresponding position on synaptobrevin-2, is not cleaved by TeTx (Schiavo 1992b). The result of cleaving synaptobrevin-2 is that vesicles containing GABA and glycine are not allowed to dock, and therefore cannot be released. | ||
==Clinical Tetanus== | ==Clinical Tetanus== | ||
| Line 62: | Line 63: | ||
===Symptoms=== | ===Symptoms=== | ||
[[Image:RisusSardonicus.jpg|thumb|200px|left|A tetanus patient with <i>risus sardonicus</i> and contraction of the neck muscles ([http://phil.cdc.gov/phil/home.asp Image #2857 from the CDC's Public Health Image Library (PHIL).]]] | [[Image:RisusSardonicus.jpg|thumb|200px|left|Figure 6. A tetanus patient with <i>risus sardonicus</i> and contraction of the neck muscles ([http://phil.cdc.gov/phil/home.asp Image #2857 from the CDC's Public Health Image Library (PHIL).]]] | ||
Tetanus is characterized by a clinical triad of rigidity, muscles spasms, and, in more severe cases, autonomic dysfunction (Cook 2001). In local tetanus, the symptoms are restricted to areas around the site of infection. Generalized tetanus is more common, however, and produces symptoms throughout the entire body (Cook 2001). Generalized tetanus usually presents with rigidity and spasms in the head and neck area, and then progresses down the entire body. One of the first signs of tetanus is trismus, or lockjaw. <i>Risus sardonicus</i>, a grin-like facial expression, also appears as the result of facial muscle spasms. When the spasms progress caudally down the body they can cause opisthotonos, or back arching. Prolonged contractions, especially of agonist and antagonist muscle groups, can be quite painful and are powerful enough to cause fractures and muscle tears (PubMed Health 2010). Autonomic dysfunction ranges from more minor ailments like drooling, excessive sweating, and problems with waste excretion, to much more severe symptoms such as cardiac problems and inability to regulate blood pressure (Cook 2001; PubMed Health 2010). | Tetanus is characterized by a clinical triad of rigidity, muscles spasms, and, in more severe cases, autonomic dysfunction (Cook 2001). In local tetanus, the symptoms are restricted to areas around the site of infection. Generalized tetanus is more common, however, and produces symptoms throughout the entire body (Cook 2001). Generalized tetanus usually presents with rigidity and spasms in the head and neck area, and then progresses down the entire body. One of the first signs of tetanus is trismus, or lockjaw. <i>Risus sardonicus</i>, a grin-like facial expression, also appears as the result of facial muscle spasms (Figure 6). When the spasms progress caudally down the body they can cause opisthotonos, or back arching. Prolonged contractions, especially of agonist and antagonist muscle groups, can be quite painful and are powerful enough to cause fractures and muscle tears (PubMed Health 2010). Autonomic dysfunction ranges from more minor ailments like drooling, excessive sweating, and problems with waste excretion, to much more severe symptoms such as cardiac problems and inability to regulate blood pressure (Cook 2001; PubMed Health 2010). | ||
These signs and symptoms of clinical tetanus occur when inhibitory neurons in the spinal cord and brain stem are affected. The spastic paralysis seen in tetanus is the result of excessive acetylcholine release caused by the disinhibition of motor neurons (Cook 2001). Alpha motor neurons normally receive both excitatory and inhibitory signals. Interneurons in the spinal cord provide important inhibition of alpha motor neurons, especially in coordinating reflexes and movement of antagonistic muscle groups (Bear 2007). When the inhibition is blocked, alpha motor neurons can send unregulated excitatory signals, even to muscles with opposing functions. | These signs and symptoms of clinical tetanus occur when inhibitory neurons in the spinal cord and brain stem are affected. The spastic paralysis seen in tetanus is the result of excessive acetylcholine release caused by the disinhibition of motor neurons (Cook 2001). Alpha motor neurons normally receive both excitatory and inhibitory signals. Interneurons in the spinal cord provide important inhibition of alpha motor neurons, especially in coordinating reflexes and movement of antagonistic muscle groups (Bear 2007). When the inhibition is blocked, alpha motor neurons can send unregulated excitatory signals, even to muscles with opposing functions. | ||
Autonomic dysfunction is caused by hyperactivity of the sympathetic nervous system (SNS). The SNS is the division of the autonomic nervous system that is responsible for the so-called “fight-or-flight” response. Under normal conditions, signals from the brain stem and spinal cord stimulate postganglionic nerve fibers with acetylcholine. The postganglionic fibers of the SNS then modulate heart rate, vasoconstriction, and other visceral responses. These postganglionic fibers of the SNS use the catecholamine neurotransmitter norepinephrine to send signals (Bear 2007). In tetanus, the SNS, just like the spinal motor neurons, is disinhibited. Prolonged stimulation of the SNS or continuous release of catecholamines can cause myocardial and vascular damage, similar to that found in tetanus. The sustained hypertension, tachycardia, increased oxygen consumption, peripheral vasoconstriction, and increased levels of catecholamines present in urine seen in severe tetanus patients | Autonomic dysfunction is caused by hyperactivity of the sympathetic nervous system (SNS). The SNS is the division of the autonomic nervous system that is responsible for the so-called “fight-or-flight” response. Under normal conditions, signals from the brain stem and spinal cord stimulate postganglionic nerve fibers with acetylcholine. The postganglionic fibers of the SNS then modulate heart rate, vasoconstriction, and other visceral responses. These postganglionic fibers of the SNS use the catecholamine neurotransmitter norepinephrine to send signals (Bear 2007). In tetanus, the SNS, just like the spinal motor neurons, is disinhibited. Prolonged stimulation of the SNS or continuous release of catecholamines can cause myocardial and vascular damage, similar to that found in tetanus. The sustained hypertension, tachycardia, increased oxygen consumption, peripheral vasoconstriction, and increased levels of catecholamines present in urine seen in severe tetanus patients point to this hyperactivity of the SNS (Tsueda 1974). | ||
===Prevention and Treatment=== | ===Prevention and Treatment=== | ||
| Line 85: | Line 86: | ||
====Treating the Infection==== | ====Treating the Infection==== | ||
The first two principles of tetanus treatment involve neutralizing unbound toxin and removing the source of infection. | The first two principles of tetanus treatment involve neutralizing the unbound toxin and removing the source of infection. | ||
The unbound toxin is neutralized using human tetanus immunoglobulin (HTIG). HTIG is injected intramuscularly in doses ranging from 500-10,000 IU, though 500 IU is now the preferred dose (Hsu 2001). Due to the high binding affinity of TeTx to substrates in neurons, HTIG can only neutralize the unbound toxin circulating outside of neurons. | |||
The source of infection must be eliminated in order to prevent the release of more TeTx. If a wound is present it should be cleaned and all devitalized tissue should be removed. Cleaning the wound decreases the bacterial load directly, and, as <i>C. tetani</i> is an obligate anaerobe, exposing the infection to the air will help kill some bacteria (Cook 2001; Hsu 2001). Antibiotics must also be used to destroy any more <i>C. tetani</i> that may produce toxin. Penicillin, metronidazole, erythromycin, tetracycline, chloramphenicol, and clindamycin are all acceptable for use | The source of infection must be eliminated in order to prevent the release of more TeTx. If a wound is present it should be cleaned and all devitalized tissue should be removed. Cleaning the wound decreases the bacterial load directly, and, as <i>C. tetani</i> is an obligate anaerobe, exposing the infection to the air will help kill some bacteria (Cook 2001; Hsu 2001). Antibiotics must also be used to destroy any more <i>C. tetani</i> that may produce toxin. Penicillin, metronidazole, erythromycin, tetracycline, chloramphenicol, and clindamycin are all acceptable for use. | ||
In one of the few studies to directly test antibiotic sensitivity in <i>C. tetani</i> cultures, it was shown that strains taken from dozens of clinical tetanus patients were sensitive to penicillin, metronidazole, chloramphenicol, erythromycin, and ofloxacin, but were resistant to trimethoprim/sulfamethoxazole (Figure 7; Campbell 2009). As seen in Figure 7, penicillin and metronidazole had the largest clearings at 42 mm. Erythromycin produced the largest minimum clearing around the antibiotic disk, but both penicillin and metronidazole still had relatively large minimum clearings. These results, taken with historical treatment practices, led the researchers to conclude that penicillin is an appropriate treatment against <i>C. tetani</i>, with metronidazole being a suitable alternative. | |||
[[Image:AntibioticTetanus.jpg|thumb|750px|center|Figure 7. Antibiotic sensitivities of 45 clinical <i>C. tetani</i> isolates. Overall, the <i>C. tetani</i> strains were most sensitive to penicillin and metronidazole, and were only resistant to the trimethoprim/sulfamethoxazole mix (from Campbell 2009).]] | |||
Though penicillin was the drug of choice to treat tetanus infection in the past, other researchers recommend the use of metronidazole (Cook 2001; Hsu 2001). Metronidazole is associated with a better recovery time and a lower mortality rate than penicillin. Penicillin requires adequate blood flow to the site of infection in order to reach effective concentrations. The anaerobic wounds where <i>C. tetani</i> thrive often have become devitalized and do not receive enough blood flow. Metronidazole can penetrate devitalized tissue in wounds that penicillin cannot normally reach (Ahamdsyah 1985). Thus, while penicillin and metronidazole may have similar effectiveness in <i>C. tetani</i> cultures, they can produce different results in actual patients. Campbell et al. (2009) have suggested the use of topical instead of intravenous penicillin to avoid these vascularization issues. However, penicillin still has the added disadvantage of being a GABA<sub>A</sub> receptor antagonist; given that tetanus acts mainly by antagonizing the release of GABA in the CNS, it would seem unwise to compound that effect during treatment (Bleck 1994). With greater effectiveness and fewer side effects, metronidazole appears to be a better treatment against <i>C. tetani</i> infection in tetanus patients. | |||
====Treating the Symptoms==== | ====Treating the Symptoms==== | ||
| Line 103: | Line 108: | ||
==Conclusion== | ==Conclusion== | ||
Despite an ever-increasing amount of knowledge related to tetanus, TeTx, and C. tetani, there are still hundreds of thousands of cases each year, many ending in death. The global mortality rate of tetanus is estimated to be 30-50%. In the United States, this has been reduced to approximately 11% (Hsu 2001). One particular hospital in Venezuela found that patients treated in the intensive care unit (ICU) had only an 11% mortality rate, whereas those treated conservatively in the general wards of the hospital had a mortality rate above 40% (Trujillo 1987). Cause of death in acute tetanus is associated with respiratory failure. With the introduction of ICUs and mechanical ventilation, sudden cardiac failure linked to autonomic dysfunction | Despite an ever-increasing amount of knowledge related to tetanus, TeTx, and <i>C. tetani</i>, there are still hundreds of thousands of cases of tetanus each year, many ending in death. The global mortality rate of tetanus is estimated to be 30-50%. In the United States, this has been reduced to approximately 11% (Hsu 2001). One particular hospital in Venezuela found that patients treated in the intensive care unit (ICU) had only an 11% mortality rate, whereas those treated conservatively in the general wards of the hospital had a mortality rate above 40% (Trujillo 1987). Cause of death in acute tetanus is associated with respiratory failure. With the introduction of ICUs and mechanical ventilation, sudden cardiac failure linked to autonomic dysfunction became the main cause of death (Thwaites 2003). | ||
New developments in our understanding of the tetanus toxin and the <i>C. tetani</i> organism can help lead to better treatments, and, hopefully, a reduction in the mortality rates of clinical tetanus. Even more importantly, if vaccination becomes more widespread and consistent, tetanus incidence rates should drop like they did in the United States—almost 96% since the late 1940s (Roper 2004). | New developments in our understanding of the tetanus toxin and the <i>C. tetani</i> organism can help lead to better treatments, and, hopefully, a reduction in the mortality rates of clinical tetanus. Even more importantly, if vaccination becomes more widespread and consistent, tetanus incidence rates should drop like they did in the United States—almost 96% since the late 1940s (Roper 2004). | ||
==References== | ==References== | ||
Ahmadsyah, I., & Salim, A. (1985). Treatment of tetanus: an open study to compare the efficacy of procaine penicillin and metronidazole. British Medical Journal (Clinical research ed.), 291(6496), 648-650. | |||
Bear, M. (2007). Neuroscience : exploring the brain (3rd ed.). Philadelphia PA: Lippincott Williams & Wilkins. | Bear, M. (2007). Neuroscience : exploring the brain (3rd ed.). Philadelphia PA: Lippincott Williams & Wilkins. | ||
| Line 183: | Line 190: | ||
Yu, R. K., Nakatani, Y., & Yanagisawa, M. (2008). The role of glycosphingolipid metabolism in the developing brain. The Journal of Lipid Research, 50(Supplement), S440-S445. doi:10.1194/jlr.R800028-JLR200 | Yu, R. K., Nakatani, Y., & Yanagisawa, M. (2008). The role of glycosphingolipid metabolism in the developing brain. The Journal of Lipid Research, 50(Supplement), S440-S445. doi:10.1194/jlr.R800028-JLR200 | ||
Edited by student of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol238/biol238syl10.html BIOL 238 Microbiology], 2011, [http://www.kenyon.edu/index.xml Kenyon College]. | Edited by [mailto:stearnstj@gmail.com Tyler Stearns], a student of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol238/biol238syl10.html BIOL 238 Microbiology], 2011, [http://www.kenyon.edu/index.xml Kenyon College]. | ||
<!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | <!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | ||
Latest revision as of 14:29, 23 July 2011
Introduction

By Tyler Stearns
Clostridium tetani is a Gram-postive, spore-forming bacillus that causes the disease tetanus (Slonczewski & Foster 2011). C. tetani is ubiquitous in nature; it is primarily found in soil, but can also be found in animal and human fecal samples (Cook 2001). In one survey, C. tetani was found in approximately 30% of soil samples. Growth did not appear to be dependent on soil type or climate (Smith 1978). C. tetani is an obligate anaerobe, but its spores can survive extreme conditions, including treatment with antiseptic phenols and autoclaving at 121°C for 10-15 minutes (Pink Book).
Besides its pervasiveness and survival in extreme conditions, C. tetani also produces one of the strongest toxins known to man. The tetanus toxin (TeTx) produces clinical tetanus disease, which is characterized by spastic paralysis (Figure 1). TeTx has an estimated lethal dose of 2.5 nanograms per kilogram of body weight (Pink Book). Thus, a mere 175 nanograms of TeTx can kill a 70 kg (154 lb) human.
A tetanus antitoxin and vaccine were developed nearly a century ago and regular vaccination has become common in developed countries, thus lowering the number of tetanus cases in the United States to 124 between 1995 and 1997 (Hsu 2001). Despite these advances, there are still an estimated 700,000 to 1,000,000 cases of tetanus worldwide every year, mostly in developing countries where vaccines are less common (Thwaites 2003). The following material is an overview of what is currently known about tetanus and TeTx, and how this deadly and still far-too-common disease can be controlled and treated.
The Tetanus Toxin: Genetics and Mechanism of Action
Genetics
The genome of C. tetani consists of a single 2,799,250-bp chromosome with 2,372 open reading frames (ORFs); the tetanus toxin (TeTx) is encoded on a 74,082-bp plasmid containing 61 ORFs (Brüggemann 2003). The genus Clostridium is a member of the Firmicutes phylum, which is known for a low G-C content (S & F 2011). The C. tetani chromosome has a G-C content of 28.6%, while the TeTx-encoding plasmid pE88 only has a G-C content of 24.5%. The G-C ratio is relatively stable in the main chromosome, indicating a lack of recent horizontal gene transfer (Brüggemann 2003).
The plasmid pE88 holds the genes for TeTx (tetX) and its direct transcriptional regulator TetR (Figure 2). The tetR gene is located just upstream from tetX (Brüggemann 2005). TetR was thought to possibly be a positive regulator of toxin expression, but current research has shown that TetR is in fact a sigma factor of a subgroup unique to clostridial species. This research found that TetR only associated with target DNA in the presence of the RNA polymerase core enzyme. In the presence of the RNA polymerase core enzyme, TetR bound to the core enzyme and triggered transcription of the target DNA at the promoter in vitro (Raffestin 2005). Other regulatory genes are present on the plasmid. CTP05, CTP10, and CTP11 are sigma factor-like proteins, and CTP21 and CTP22 form a two-component system of unknown regulatory function. The three sigma-like proteins do not appear to be involved in the regulation of toxin production like TetR (Brüggemann 2005; Raffestin 2005).
Other virulence factors may be present on pE88, in addition to the primary tetanus toxin. The 114-kDa collagenase ColT is also encoded on the plasmid (Brüggemann 2003). Collagenase is an enzyme that degrades collagen, which makes up almost 25 to 33% of the total protein in mammalian organisms (Harrington 1996). Thus, ColT may help destroy tissue in an infected host.
The main chromosome of C. tetani also possesses potential virulence factors. ORFs for tetanolysin O, hemolysin, and fibronectin-binding proteins, as well as genes for surface-layer (S-layer) proteins have been identified (Brüggemann 2003). Tetanolysin, discussed below, is one of the two exotoxins produced by C. tetani, in addition to TeTx. The S-layer has been characterized as a molecular sieve or as a possible defense against parasites. However, it also has been implicated in host cell adhesion or as a possible mechanism of evading host immune systems in another pathogenic clostridial species, C. difficile (Spigaglia 2011).
The origin of the plasmid pE88 is still unclear. Over 50% of the ORFs on the plasmid are unique to C. tetani (Brüggemann 2003). Toxin genes are currently—or were in their evolutionary history—part of a flexible clostridial gene pool. Many of the toxin genes in pathogenic Clostridium species are on plasmids or capable of transduction by phages (Brüggemann 2005). C. botulinum, which produces various forms of the botulinum neurotoxin (BoNT), has been shown to transfer its toxin-encoding plasmid by conjugation. In one study, a tagged BoNT-encoding plasmid was transferred from one strain of C. botulinum to another. Bacteriophages for transduction were not observed, and gene transfer was not inhibited by DNase, ruling out transformation of free-floating DNA (Marshall 2010). The epsilon-toxin plasmids in C. perfringens Type D have also been shown to be transferable to other cells by conjugation (Hughes 2007). Given this evidence, and the fact TeTx is quite similar to BoNT, it seems highly probable that the TeTx plasmid was acquired via some form of horizontal gene transfer, though it has clearly undergone its own divergent evolution since that point.
Infection
Tetanus infections most commonly occur after deep-tissue puncture wounds are exposed to C. tetani. Contamination of the wound often involves contact with soil, fecal matter, or rusty metal that contain C. tetani (Cook 2001; Campbell 2009). C. tetani is an obligate anaerobe. The conditions in a wound are particularly well suited to the bacteria's anaerobic needs (Campbell 2009). Tetanolysin is one of the two exotoxins excreted by C. tetani. Tetanolysin damages tissue surrounding an infection, optimizing conditions within the wound (Cook 2001; Campbell 2009). Bacteria grow and ferment in the wound, releasing in small quantities the actual causative agent of tetanus disease, the tetanospasmin protein (also referred to as tetanus toxin, TeTx, or TeNT). The toxin is mainly released during the stationary phase of growth and a significant majority of the toxin is not freed until a cell lyses, releasing its contents into the body of the host (Mellanby 1981). TeTx is distributed to motor neurons and in the bloodstream to other pre-synaptic nerve terminals in the peripheral nervous system (Kerr 1979).
Cell Entry
Tetanospasmin must enter nerve cells in order to reach its primary site of action and produce clinical tetanus disease. The toxin can have some effect on motor neurons near the site of infection, but the main syndrome is produced after TeTx reaches the spinal cord. Once the toxin has entered neurons near the site of infection or elsewhere in the peripheral nervous system, it can move retrogradely within axons, to the cell body of the neuron, then subsequently to other neurons, and thus reach the spinal cord via intra-axonal transport (Erdmann 1975).
The TeTx protein is comprised of two components, both of which are necessary for intoxication but have different functions in pathogenesis. TeTx is initially synthesized as a ~150-kDa chain, but is subsequently cleaved into a two-part chain linked by a single disulfide bond (Chen 2009). The final two components are the light chain (LC; ~50 kDa) and the heavy chain (HC; ~100 kDa) (Figure 3). The LC is the active constituent of TeTx, but the HC is required for entry into the cell, where LCs function to cause disease (Binz 2009).
The HCs of TeTx interact with gangliosides on the surface of neuronal membranes. Gangliosides are sialic acid-containing glycosphingolipids that may be involved in signal transduction and are found in the greatest concentrations on the outer layers of membranes in the nervous system (Yu 2008). In preparations of mouse neurons where production of gangliosides has been inhibited there is no sign of TeTx binding to cells and the toxin does not prevent the release of neurotransmitter from those cells (Williamson 1999). Just as TeTx is divided into two domains, HC can also be divided into two functionally distinct domains. The amino-terminal domain HN is responsible for translocating the LC across the plasma membrane, whereas the carboxyl-terminal domain HC is responsible for the binding of TeTx to gangliosides on neurons (Rummel 2003).
The HC domain can be broken down even further into the HCN and HCC subunits. The HCC fragment has been shown to bind the gangliosides and helps in retrograde transport along the axon so that TeTx can reach the spinal cord (Rummel 2003). The HCN domain does not bind gangliosides (Figueiredo 1995) and its ultimate role remains unclear. The HCC domain has two important binding sites associated with it. These pockets are termed the lactose binding site and the sialic acid binding site (Figure 3; Binz 2009). In one set of experiments, researchers mutated these binding pockets by changing certain amino acids. When only the sialic acid binding site was mutated, toxicity was reduced by almost 99%; toxicity levels were almost undetectable after mutation of the lactose site (Rummel 2003). Furthermore, the experiments were performed on GT1b-type gangliosides, which have the highest affinity for TeTx. Another study used gangliosides of the type GM1a, which binds only the lactose pocket, and GD3, which binds only the sialic acid pocket, in order to explicate the role of these pockets in TeTx binding. They confirmed again that a mutated HCC fragment lacking one pocket or the other did not bind TeTx. In the presence of only one of the gangliosides—either GM1a or GD3—toxin was unable to enter the cell (Chen 2009). The results from both investigations show that both binding sites within the HCC domain are necessary for TeTx to enter the cell. In addition, it appears as though two separate gangliosides are needed to bind HCC.
Once the HC domain has bound to the gangliosides, the HN domain then allows the LC to cross into the cytoplasm of the cell, where it can act on neurotransmitter vesicles. It is currently unknown how exactly HN helps the LC gain access to the cytoplasm. Models have been proposed for BoNT, which is structurally similar to TeTx. In these models, the low pH of the endosome may cause a structural change in the HC, causing the HN domain to insert into the endosome membrane and form a channel (Brunger 2007). The HN channel may also act as a chaperone, unfolding the LC, pulling it across the endosomal transmembrane channel, and then refolding it in the cytoplasm of the neuron (Binz 2009).
Mechanism of Action within the Cell
Once in the cytoplasm, the LC, which is the active component of the tetanus toxin, begins interrupting release of neurotransmitters. When TeTx reaches the spinal cord, it leaves the motor neuron and moves to the synaptic nerve terminals of spinal neurons. Primarily, neurons with inhibitory neurotransmitters are affected, leading to the symptoms of clinical tetanus.
The LC of TeTx is an endoprotease, meaning it is capable of cleaving peptide bonds away from the amino- and carboxyl-terminal ends and breaking apart proteins (Binz 2009; Lebrun 2009). Most proteases in bacteria are used for food acquisition for the cell. The protease breaks down long peptide chains into shorter ones to enable absorption by the bacteria (Lebrun 2009). Metabolic functions like this have not been shown in TeTx, however. The toxin-encoding plasmid is not found in every strain of C. tetani, and therefore it appears as though the toxin—along with its proteolytic capabilities—may have evolved primarily as a defense mechanism or a virulence factor (Campbell 2009).
The LC can be further classified as a zinc metalloprotease. A Zn2+ ion interacts with two histidine residues in the LC. Early research into TeTx's mechanism of action showed that zinc was necessary for inhibiting evoked neurotransmitter release in Aplysia californica (Schiavo 1992a). If LCs were depleted of Zn2+ and treated with metal chelators to remove extra Zn2+ ions, the LCs alone did not cause much inhibition of neurotransmitter release. Thus, it was shown that the active domain of TeTx, the LC, is a zinc-dependent metalloprotease.
TeTx primarily prevents the release of the inhibitory neurotransmitters gamma-aminobutyric acid (GABA) and glycine in the spinal cord (Collingridge 1982; Cook 2001). The LC cleaves synaptobrevin-2 (also known as VAMP2), a SNARE protein involved in the fusion of neurotransmitter vesicles with the neuronal membrane for exocytosis (Figure 4). An action potential in a neuron usually causes Ca2+ to enter into the cell. Under normal conditions, Ca2+ causes v-SNAREs on neurotransmitter vesicles (such as synaptobrevin) to interact with t-SNAREs on the membrane of the neuron (Figure 5). The SNAREs bind with one another, allowing the vesicle to dock and fuse with the neuronal membrane, and thus release neurotransmitter into the synaptic cleft (Bear 2007). In the presence of TeTx, however, the LC hydrolyzes the bond between amino acid residues Gln76 and Phe77 on synaptobrevin-2. Synaptobrevin-1, which has a valine at the corresponding position on synaptobrevin-2, is not cleaved by TeTx (Schiavo 1992b). The result of cleaving synaptobrevin-2 is that vesicles containing GABA and glycine are not allowed to dock, and therefore cannot be released.
Clinical Tetanus
Symptoms
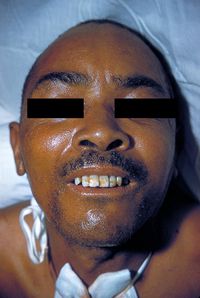
Tetanus is characterized by a clinical triad of rigidity, muscles spasms, and, in more severe cases, autonomic dysfunction (Cook 2001). In local tetanus, the symptoms are restricted to areas around the site of infection. Generalized tetanus is more common, however, and produces symptoms throughout the entire body (Cook 2001). Generalized tetanus usually presents with rigidity and spasms in the head and neck area, and then progresses down the entire body. One of the first signs of tetanus is trismus, or lockjaw. Risus sardonicus, a grin-like facial expression, also appears as the result of facial muscle spasms (Figure 6). When the spasms progress caudally down the body they can cause opisthotonos, or back arching. Prolonged contractions, especially of agonist and antagonist muscle groups, can be quite painful and are powerful enough to cause fractures and muscle tears (PubMed Health 2010). Autonomic dysfunction ranges from more minor ailments like drooling, excessive sweating, and problems with waste excretion, to much more severe symptoms such as cardiac problems and inability to regulate blood pressure (Cook 2001; PubMed Health 2010).
These signs and symptoms of clinical tetanus occur when inhibitory neurons in the spinal cord and brain stem are affected. The spastic paralysis seen in tetanus is the result of excessive acetylcholine release caused by the disinhibition of motor neurons (Cook 2001). Alpha motor neurons normally receive both excitatory and inhibitory signals. Interneurons in the spinal cord provide important inhibition of alpha motor neurons, especially in coordinating reflexes and movement of antagonistic muscle groups (Bear 2007). When the inhibition is blocked, alpha motor neurons can send unregulated excitatory signals, even to muscles with opposing functions.
Autonomic dysfunction is caused by hyperactivity of the sympathetic nervous system (SNS). The SNS is the division of the autonomic nervous system that is responsible for the so-called “fight-or-flight” response. Under normal conditions, signals from the brain stem and spinal cord stimulate postganglionic nerve fibers with acetylcholine. The postganglionic fibers of the SNS then modulate heart rate, vasoconstriction, and other visceral responses. These postganglionic fibers of the SNS use the catecholamine neurotransmitter norepinephrine to send signals (Bear 2007). In tetanus, the SNS, just like the spinal motor neurons, is disinhibited. Prolonged stimulation of the SNS or continuous release of catecholamines can cause myocardial and vascular damage, similar to that found in tetanus. The sustained hypertension, tachycardia, increased oxygen consumption, peripheral vasoconstriction, and increased levels of catecholamines present in urine seen in severe tetanus patients point to this hyperactivity of the SNS (Tsueda 1974).
Prevention and Treatment
The tetanus toxoid—a version of the toxin inactivated by formalin—was first produced in 1924, and widespread vaccination in developed countries began a few decades later. Vaccination is incredibly effective at preventing tetanus.
Those who do develop tetanus, however, must receive immediate treatment. Three management principles are used in the treatment of tetanus: C. tetani organisms present in the body should be destroyed to prevent the release of more toxin; toxin already released should be neutralized, if possible; and the effects of toxin already in the CNS should be minimized (Cook 2001).
Vaccination
There are currently four vaccine preparations containing the tetanus toxoid: DTaP, DT, Tdap, and Td (D = diphtheria, T = tetanus, and aP = acellular pertussis). Capital letters in the abbreviation stand for full-strength toxoid and lowercase letters stand for a reduced-strength toxoid. DTaP and DT are meant for children younger than 7 years old. Tdap and Td are for adolescents and adults (CDC Vaccines).
After receiving primary doses of the vaccine, antitoxin levels greatly exceed the protective levels of 0.1 IU/mL. Antitoxin levels decrease over time, however, and a booster should be given every 10 years. It is also recommended that anyone who receives a wound that is neither clean nor minor and has not had a booster in more than five years should receive one (Pink Book). The vaccine is nearly 100% effective in preventing tetanus, though it is not perfect. In one case, a man with antitetanus antibody levels of 0.22 IU/mL developed rigidity and autonomic dysfunction and responded positively to treatment for tetanus (Beltran 2007).
Treating the Infection
The first two principles of tetanus treatment involve neutralizing the unbound toxin and removing the source of infection.
The unbound toxin is neutralized using human tetanus immunoglobulin (HTIG). HTIG is injected intramuscularly in doses ranging from 500-10,000 IU, though 500 IU is now the preferred dose (Hsu 2001). Due to the high binding affinity of TeTx to substrates in neurons, HTIG can only neutralize the unbound toxin circulating outside of neurons.
The source of infection must be eliminated in order to prevent the release of more TeTx. If a wound is present it should be cleaned and all devitalized tissue should be removed. Cleaning the wound decreases the bacterial load directly, and, as C. tetani is an obligate anaerobe, exposing the infection to the air will help kill some bacteria (Cook 2001; Hsu 2001). Antibiotics must also be used to destroy any more C. tetani that may produce toxin. Penicillin, metronidazole, erythromycin, tetracycline, chloramphenicol, and clindamycin are all acceptable for use.
In one of the few studies to directly test antibiotic sensitivity in C. tetani cultures, it was shown that strains taken from dozens of clinical tetanus patients were sensitive to penicillin, metronidazole, chloramphenicol, erythromycin, and ofloxacin, but were resistant to trimethoprim/sulfamethoxazole (Figure 7; Campbell 2009). As seen in Figure 7, penicillin and metronidazole had the largest clearings at 42 mm. Erythromycin produced the largest minimum clearing around the antibiotic disk, but both penicillin and metronidazole still had relatively large minimum clearings. These results, taken with historical treatment practices, led the researchers to conclude that penicillin is an appropriate treatment against C. tetani, with metronidazole being a suitable alternative.
Though penicillin was the drug of choice to treat tetanus infection in the past, other researchers recommend the use of metronidazole (Cook 2001; Hsu 2001). Metronidazole is associated with a better recovery time and a lower mortality rate than penicillin. Penicillin requires adequate blood flow to the site of infection in order to reach effective concentrations. The anaerobic wounds where C. tetani thrive often have become devitalized and do not receive enough blood flow. Metronidazole can penetrate devitalized tissue in wounds that penicillin cannot normally reach (Ahamdsyah 1985). Thus, while penicillin and metronidazole may have similar effectiveness in C. tetani cultures, they can produce different results in actual patients. Campbell et al. (2009) have suggested the use of topical instead of intravenous penicillin to avoid these vascularization issues. However, penicillin still has the added disadvantage of being a GABAA receptor antagonist; given that tetanus acts mainly by antagonizing the release of GABA in the CNS, it would seem unwise to compound that effect during treatment (Bleck 1994). With greater effectiveness and fewer side effects, metronidazole appears to be a better treatment against C. tetani infection in tetanus patients.
Treating the Symptoms
The third major part of treatment for tetanus is controlling the effects produced by toxin already bound to substrates in the CNS. The three main categories of symptoms—rigidity, muscle spasms, and autonomic dysfunction—must all be treated to avoid potentially fatal complications and to improve the general well-being of patients.
Muscle spasms and rigidity can often be controlled with the use of drugs that agonize GABAergic neurons and systems. Benzodiazepines are the drug of choice for control of muscle spasms. Drugs such as diazepam and midazolam have been used successfully to reduce muscle spasms and induce sedation (Hsu 2001; Cook 2001). Benzodiazepines bind to GABA receptors and facilitate the binding of GABA, which helps increase the action of GABA on post-synaptic target neurons (Julien 2008). If benzodiazepines are not sufficient, neuromuscular blocking agents, such as vecuronium, may be required. Paralytics like these require mechanical ventilation (Hsu 2001; Cook 2001). Additional sedation can be achieved with the barbiturate phenobarbital or the typical antipsychotic chlorpromazine (Cook 2001). Barbiturates, like benzodiazepines, are GABA agonists. Unlike benzodiazepines, however, barbiturates not only facilitate the binding of GABA with its receptor, but they can also open GABA chloride ion channels in the absence of the neurotransmitter itself (Julien 2008). Because GABA release is being blocked by TeTx, it may be useful to use drugs that can control muscle spasms in the absence of GABA.
Autonomic dysfunction has proven more difficult to control. Alpha- and beta-adrenergic blockers have been used to counter hypertension and cardiac problems. Both classes of drugs, however, have been associated with worsening symptoms or even sudden death (Cook 2001). Other agents that modulate output from the SNS have been used with mixed results. Clonidine, an alpha-adrenergic agonist, may reduce sympathetic output and thus reduce arterial pressure, heart rate, and catecholamine release, but case reports show conflicting results (Cook 2001). Magnesium may also help by blocking release of catecholamines, though the results again are mixed (Hsu 2001). One study found that magnesium sulfate treatment did not lower the number of patients requiring mechanical ventilation, but it did lower the amount of other drugs needed to control muscle spasms and cardiovascular instability, such as midazolam and verapamil (Thwaites 2006). The opioid-analgesic drugs morphine and fentanyl may also help decrease output of the SNS by modulating central controls (Hsu 2001; Cook 2001). Opioids decrease blood pressure and cause peripheral vasodilation (Julien 2008).
Conclusion
Despite an ever-increasing amount of knowledge related to tetanus, TeTx, and C. tetani, there are still hundreds of thousands of cases of tetanus each year, many ending in death. The global mortality rate of tetanus is estimated to be 30-50%. In the United States, this has been reduced to approximately 11% (Hsu 2001). One particular hospital in Venezuela found that patients treated in the intensive care unit (ICU) had only an 11% mortality rate, whereas those treated conservatively in the general wards of the hospital had a mortality rate above 40% (Trujillo 1987). Cause of death in acute tetanus is associated with respiratory failure. With the introduction of ICUs and mechanical ventilation, sudden cardiac failure linked to autonomic dysfunction became the main cause of death (Thwaites 2003).
New developments in our understanding of the tetanus toxin and the C. tetani organism can help lead to better treatments, and, hopefully, a reduction in the mortality rates of clinical tetanus. Even more importantly, if vaccination becomes more widespread and consistent, tetanus incidence rates should drop like they did in the United States—almost 96% since the late 1940s (Roper 2004).
References
Ahmadsyah, I., & Salim, A. (1985). Treatment of tetanus: an open study to compare the efficacy of procaine penicillin and metronidazole. British Medical Journal (Clinical research ed.), 291(6496), 648-650.
Bear, M. (2007). Neuroscience : exploring the brain (3rd ed.). Philadelphia PA: Lippincott Williams & Wilkins.
Beltran, A., Go, E., Haq, M., Clarke, H. B., Zaman, M., & Recco, R. A. (2007). A Case of Clinical Tetanus in a Patient with Protective Antitetanus Antibody Level. Southern Medical Journal, 100(1), 83. doi:10.1097/SMJ.0b013e31802e2717
Binz, Thomas, & Rummel, Andreas. (2009). Cell entry strategy of clostridial neurotoxins. Journal of Neurochemistry, 109(6), 1584-1595.
Bleck, T. P. (1994). Management of tetanus. Journal of the Royal Society of Medicine, 87(11), 719-720.
Brüggemann, H. (2005). Genomics of clostridial pathogens: implication of extrachromosomal elements in pathogenicity. Current Opinion in Microbiology, 8(5), 601-605.
Brüggemann, H., Bäumer, S., Fricke, W. F., Wiezer, A., Liesegang, H., Decker, I., Herzberg, C., et al. (2003). The Genome Sequence of Clostridium tetani, the Causative Agent of Tetanus Disease. Proceedings of the National Academy of Sciences of the United States of America, 100(3), 1316-1321.
Campbell, J. I., Yen, L. T. M., Loan, H. T., Diep, T. S., Nga, T. T. T., Van Minh Hoang, N., Son, L. T., et al. (2009). Microbiologic Characterization and Antimicrobial Susceptibility of Clostridium tetani Isolated from Wounds of Patients with Clinically Diagnosed Tetanus. Am J Trop Med Hyg, 80(5), 827-831.
Chen, C., Fu, Z., Kim, J.-J. P., Barbieri, J. T., & Baldwin, M. R. (2009). Gangliosides as High Affinity Receptors for Tetanus Neurotoxin. Journal of Biological Chemistry, 284(39), 26569 -26577. doi:10.1074/jbc.M109.027391
Collingridge, G. L., & Davies, J. (1982). The in vitro inhibition of GABA release by tetanus toxin. Neuropharmacology, 21(9), 851-855.
Cook, T. M., Protheroe, R. T., & Handel, J. M. (2001). Tetanus: a review of the literature. BJA: International Journal of Anaesthesia, 87(3), 477-487.
Erdmann, G., Wiegand, H., & Wellhöner, H. H. (1975). Intraaxonal and extraaxonal transport of 125I-tetanus toxin in early local tetanus. Naunyn-Schmiedeberg’s Archives of Pharmacology, 290(4), 357 - 373.
Figueiredo, D., Turcotte, C., Frankel, G., Li, Y., Dolly, O., Wilkin, G., Marriott, D., et al. (1995). Characterization of recombinant tetanus toxin derivatives suitable for vaccine development. Infection and Immunity, 63(8), 3218-3221.
Harrington, D. J. (1996). Bacterial collagenases and collagen-degrading enzymes and their potential role in human disease. Infection and Immunity, 64(6), 1885-1891.
Hsu, S. S., & Groleau, G. (2001). Tetanus in the emergency department: A current review. Journal of Emergency Medicine, 20(4), 357-365.
Hughes, M. L., Poon, R., Adams, V., Sayeed, S., Saputo, J., Uzal, F. A., McClane, B. A., et al. (2007). Epsilon-Toxin Plasmids of Clostridium perfringens Type D Are Conjugative. J. Bacteriol., 189(21), 7531-7538. doi:
10.1128/JB.00767-07
Julien, R. M. (2007). A Primer of Drug Action (Eleventh ed.). Worth Publishers.
Kerr, J. (1979). Current topics in tetanus. Intensive Care Medicine, 5(3), 105 - 110.
Lebrun, I., Marques-Porto, R., Pereira, A. S., & Perpetuo, E. A. (2009). Bacterial toxins: an overview on bacterial proteases and their action as virulence factors. Mini-Reviews in Medicinal Chemistry, 9(7), 820-828.
Marshall, K. M., Bradshaw, M., & Johnson, E. A. (2010). Conjugative Botulinum Neurotoxin-Encoding Plasmids in Clostridium botulinum. PLoS ONE, 5(6), e11087. doi:10.1371/journal.pone.0011087
Mellanby, J., & Green, J. (1981). How does tetanus toxin act? Neuroscience, 6(3), 281-300. doi:10.1016/0306-4522(81)90123-8
PubMed Health. (2010). Tetanus. Retrieved April 20, 2011.
Raffestin, S., phanie, Dupuy, B., Marvaud, J. C., & Popoff, M. R. (2005). BotR/A and TetR are alternative RNA polymerase sigma factors controlling the expression of the neurotoxin and associated protein genes in Clostridium botulinum type A and Clostridium tetani. Molecular Microbiology, 55(1), 235-249.
Roper, M. H. (2004). Tetanus prophylaxis in the emergency department. Annals of Emergency Medicine, 43(3), 315-317.
Rummel, A., Bade, S., Alves, J., Bigalke, H., & Binz, T. (2003). Two Carbohydrate Binding Sites in the HCC-domain of Tetanus Neurotoxin are Required for Toxicity. Journal of Molecular Biology, 326(3), 835-847.
Schiavo, G., Poulain, B, Rossetto, O, Benfenati, F, Tauc, L., & Montecucco, C. (1992a). Tetanus toxin is a zinc protein and its inhibition of neurotransmitter release and protease activity depend on zinc. The EMBO Journal, 11(10), 3577-3583.
Schiavo, G. G., Benfenati, Fabio, Poulain, Bernard, Rossetto, Ornella, de Laureto, P. P., DasGupta, B. R., & Montecucco, Cesare. (1992b). Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature, 359(6398), 832-835. doi:10.1038/359832a0
Slonczewski, J. L., & Foster, J. W. (2010). Microbiology: An Evolving Science. W. W. Norton & Company, Incorporated.
Smith, L. D. (1978). The occurrence of Clostridium botulinum and Clostridium tetani in the soil of the United States. Health Laboratory Science, 15(2), 74-80.
Spigaglia, P. (2011). Surface Layer Protein A Variant of Clostridium difficile PCR-Ribotype 027. Emerging Infectious Diseases. doi:10.3201/eid1702.100355
Thwaites, C. L., & Farrar, J J. (2003). Preventing and treating tetanus. BMJ : British Medical Journal, 326(7381), 117-118.
Thwaites, C., Yen, L., Loan, H., Thuy, T., Thwaites, G., Stepniewska, K., Soni, N., et al. (2006). Magnesium sulphate for treatment of severe tetanus: a randomised controlled trial. The Lancet, 368(9545), 1436-1443. doi:10.1016/S0140-6736(06)69444-0
Trujillo, M. H., Castillo, A., España, J., Manzo, A., & Zerpa, R. (1987). Impact of intensive care management on the prognosis of tetanus. Analysis of 641 cases. Chest, 92(1), 63-65. doi:10.1378/chest.92.1.63
Tsueda, K., Oliver, P. B., & Richter, R. W. (1974). Cardiovascular manifestations of tetanus. Anesthesiology, 40(6), 588-592.
Williamson, L. C., Bateman, K. E., Clifford, J. C. M., & Neale, E. A. (1999). Neuronal Sensitivity to Tetanus Toxin Requires Gangliosides. Journal of Biological Chemistry, 274(35), 25173 -25180. doi:10.1074/jbc.274.35.25173
Yu, R. K., Nakatani, Y., & Yanagisawa, M. (2008). The role of glycosphingolipid metabolism in the developing brain. The Journal of Lipid Research, 50(Supplement), S440-S445. doi:10.1194/jlr.R800028-JLR200
Edited by Tyler Stearns, a student of Joan Slonczewski for BIOL 238 Microbiology, 2011, Kenyon College.