Prochlorococcus marinus 2012: Difference between revisions
| (50 intermediate revisions by the same user not shown) | |||
| Line 4: | Line 4: | ||
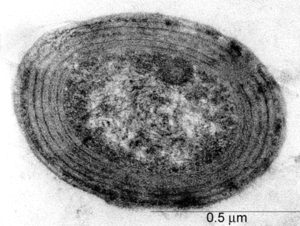
[[File: | [[File:MinerSpropic12345.gif|300px|thumb|right|Prochlorococcus Marinus SS120. Image courtesy of http://www.pnas.org/content/100/17/9647.long. ]] | ||
==Classification== | ==Classification== | ||
| Line 28: | Line 28: | ||
==Description and significance== | ==Description and significance== | ||
Prochlorococcus is a photosynthetic prokaryote. It is revealed by transmission election microscopy to have the same structure as a typical cyanacobacteria[ | Prochlorococcus is a photosynthetic prokaryote. It is revealed by transmission election microscopy to have the same structure as a typical cyanacobacteria[1]. The cytoplasm holds DNA fibrils, carboxyzomes, and glycogen granules, which can be found near or between thylakoids. There is commonly two to four thylakoids in a Prochlorococcus cell, although sometimes there can be up to six thylakoids. Thylakoids can be found running parallel to the cell membrane [2]. | ||
Many methods used to find cell sizes tend to be biased towards tiny things, including Prochlorococcus cells. It is estimated that Prochlorococcus cells sizes vary from 0.5 to 0.8 μm in length, and 0.4 to 0.6 μm in width. A Prochlorococcus cell's size van vary depending on its environment. For example, its size was shown to increase by 0.45 to 0.75 μm between the surface and at a depth of 150 meters in the Sargasso Sea [ | Many methods used to find cell sizes tend to be biased towards tiny things, including Prochlorococcus cells. It is estimated that Prochlorococcus cells sizes vary from 0.5 to 0.8 μm in length, and 0.4 to 0.6 μm in width. A Prochlorococcus cell's size van vary depending on its environment. For example, its size was shown to increase by 0.45 to 0.75 μm between the surface and at a depth of 150 meters in the Sargasso Sea [2]. Studies show that Prochlorococcus can grow quite quickly in nutrient-poor habitats. The small size allows these picoplankton to take up dissolved nutrients more efficiently than larger organisms [3]. | ||
Prochlorococcus are important due to their contribution to oxygen production. Their production is found to contribute up to 39% of primary production in the eastern and western equatorial Pacific, and up to 82% in the North Pacific Ocean at Station ALOHA. | Prochlorococcus are important due to their contribution to oxygen production. Their production is found to contribute up to 39% of primary production in the eastern and western equatorial Pacific, and up to 82% in the North Pacific Ocean at Station ALOHA. | ||
| Line 36: | Line 36: | ||
==Genome structure== | ==Genome structure== | ||
Because of its small size, Prochlorococcus marinus usually has a small | Because of its small size, Prochlorococcus marinus usually has a small genome [4]. It actually maintains the smallest genome of all cyanobacteria, with only 1.7 Mb. This may be considered an adaptation because in the ocean (environment of this organism) there is not an excess nitrogen or phosphorus, which are essential in the production of nucleotides. There are generally two strains of this bacteria found in the ocean. The low-light adapted and high- light adapted strains are a result of the difference of light levels at different depths in the ocean. P. marinus SS120, is the low light adapted form, which is composed of a singular circular chromosome of 1,751,080 bp with a C+G content of 36.4%. It also contains 1,884 predicted protein-coding genes, a single rRna operon, and 40 tRNA genes [5]. The low- light adapted strains have extra genes for the antenna complex protein, which is where the chlorophyll gets its energy from light [4]. These low-light adapted strains genetically produce chlorophyll b as opposed to chlorophyll a (high-light adapted strain) because chlorophyll b can be more helpful at a with a wider spectrum of light, which is essential when light is scarce. By having genes for more chlorophyll b and antenna protein complex, these bacteria are able to thrive even at depths of up to 200m [4]. These low-light adapted strains have a lot more variation when it comes to their genome, most likely as an adaptation for the tougher environment that the deeper ocean poses. The ocean is a very stable environment, thus these microbes have seen a decrease in the amount of stress- related and housekeeping genes, as compared to the genome of other cyanobacteria [4]. The genes for antenna protein are increased, as we discussed their characteristics that are essential to this bacteria. The genome of these bacteria are essential in the studying of photosynthetic bacteria, and their response to changing environments because of their unique and decreased genome [4]. | ||
==Cell structure and metabolism== | ==Cell structure and metabolism== | ||
Prochlorococcus marinus has been established as one of the smallest living | Prochlorococcus marinus has been established as one of the smallest living cyanobacterium. All photosyntheic organisms must adapt to light levels in their environment in order to survive. Since it is know that prochlorococcus marinus are oxyphotobacteria that thrive in the ocean between 40 degrees north and 40 degrees south, their cellular strutres should reflect survival components of this environment. Indeed, survival in a oligotrophic environment requires adaptations such as low cellular nutrient requirement and highly efficient nutrient transport systems. The mechanisms that prochlorococcus uses to acquire and metabolize nitrogen, phosphorus, and other elements in its environment are central to its ecology [6]. Since P. marines is an autotrough it must synthesize all cellular components, including amino acids, nucleotides, and coenzymes from CO2 and salt [7]. Additionally, due to the small size and environment, P. marinus has lowered complexity of biological systems such as DNA repair, chaperones, transport systems, and nitrogen metabolism [7]. | ||
It uses a sodium dependent bicarbonate transporter as the primary inorganic carbon uptake system. P. marinus also cannot utilize nitrate, nitrite, urea or cyanate as nitrogen sources, therefore it it only utilizes ammonia and amino acids as its source of nitrogen. | |||
Also, prochlorococcus marinus is a photosyntheitic organism, whos cellular strucutre lacks phycobiliproteins. Phycobiliproteins are water-soluable proteins that are present in most cyanobacteria and act to capture light energy which can then be used during photosynthesis. Instead of these proteins, prochlorococcus uses divinyl-chlorophyll a and b, signature molecules unique to this genus, as their primary syntheitc pigments used during the photosyntheic process to gain energy [8]. This specialized pigment apparatus used to capture light is specific to prochlorococcus marinus which results in a unique light absorption spectrum of blue pigments (common to low light in deep euphotic zones), and sets it apart from other marine bacteria such as other plants and alge [9]. Prochlorococcus is the only organism known to contain this particular compliment of pigments. | |||
==Ecology== | ==Ecology== | ||
Prochlorococcus marinus is a photosynthetic organism capable of living in nutrient-poor environments. It is frequently found in the oligotrophic ocean in tropical and subtropical regions. Prochlorococcus marinus, along with many other Prochlorococcus species, are abundantly found between 40˚ N and 40˚S latitudes. Beyond 40˚, however, Prochlorococcus concentrations decrease significantly, suggesting that these picoplanktons prefer warmer temperatures [9]. In an area exposed to UV light, Prochlorococcus marinus undergo a shifted cell cycle in order to maximize cell replication [10]. | |||
Prochlorococcus marinus, as well as many other Prochlorococcus species, largely share their habitat with the cyanobacterium Synechococcus. Normal Synechococcus concentrations are very similar to average Prochlorococcus concentrations, albeit one magnitude lower [3]. Prochlorococcus is limited to the surface mixed layer, and its abundance drops quickly below the thermocline. In these cases, Synechococcus has a vertical distribution that is similar to that of Prochlorococcus and its abundance is similar or slightly higher [3]. Prochlorococcus presents a very sharp maximum concentration near the bottom of the euphotic zone, which decreases by a minimum of one order of magnitude at the surface. Also, Prochlorococcus marinus, and other Prochlorococcus species, can be distributed in locations varying from the surface to the bottom of the euphotic zone at almost constant concentrations, where they are the most widespread in oceanic waters [3]. Prochlorococcus species contribute up to 44% of gross primary production [5]. | |||
==Pathology== | ==Pathology== | ||
Prochlorococcus marinus is not considered a pathogenic organism. It is a free-floating cyanobacteria, which means it uses the sun's rays for energy [9]. It is completely dependent on the sun's light for energy, thus it needs no other source of food, such as another organism [4]. | |||
==Current Research== | ==Current Research== | ||
1. Prochlorococcus marinus is a cyanobacteria that relies on the light from the sun. In today’s world the threat of excess UV rays as a mutating factor is constantly being addressed. Prochlorococcus is constantly being bombarded by these UV rays and thus this research article describes the techniques that this bacteria has to prevent against damaging mutations. These bacteria were observed in the absence and presence of UV radiation [11]. This allows for the observation of the DNA repair, and cell cycle techniques that are in place by the cell to prevent against damaging mutations. The different layers of the ocean provide different amounts of light to these organisms, and thus different amounts of ultraviolet radiation. The genes that are most affected by UV radiation are the ones that allow for smooth DNA replication [11]. The S phase, in which the cell replicates its DNA is one of the most affected phases of replication. To combat this problem, these cells are known to delay there S phase when UV radiation is high. This delay, caused by a repression of dnaA, allows more time for oligonucleotide removal by nucleotide excision repair, and allows DNA photylase to repair UV- caused mutations. These adaptations allow for the cells to survive in environments dominated by DNA- mutating ultraviolet light [11]. | |||
2. Once Prochlorococcus marinus was discovered in 1986, an immense amount of studies have begun on this species since it is one of the main contributors to our worlds carbon source. Most recently, two new uncharacterized Prochlorococcus clades have been detected in samples from the Atlantic, Pacific, and Indian Oceans. Douglas B. Rusch and his team of researchers have conducted a phylogenetic analysis using different genetic markers, which resulted in the unknown clades producing lineages adapted to high-light environments [12]. Consistent with other high light adapted Prochlorococcus, these new clades reside in high-temperature surface water with low-iron levels. His research showed that they are genetically distinct from one another and other strains of the species Prochlorococcus, and one of their main functions is to reduce an organisms iron quota through the loss of iron-containing proteins. This could explain why some Prochlorococcus species from iron depleted regions do not respond to iron fertilization experiments [12]. Additionally, this new research will help further the understanding of how marine organisms adapt to variations in nutrient availability. | |||
3. Prochlorococcus marinus, along with many other species of this genus, contribute greatly to gross primary production with their neighbors Synechococcus spp. Current research investigates production of the toxic chemical iodomethane, which can be found in pesticides. The research done in this study is not aimed towards assessing P. marinus' contribution to iodomethane's harmful effects. Rather, researchers have found that production of iodomethane is due to the compromised conditions of P. marinus cells' physiology. P. marinus were found to produce iodomethane depending on their loss rate, their abundance, and their cellular physiological states. Recently, there have been discrepancies in iodomethane production compared to 2006. This research can assist future researchers and scientists in developing ways to resolve possible overproduction of iodomethane[13]. | |||
==Cool Factor== | ==Cool Factor== | ||
1. The DNA replication of these bacteria occurs mostly at night to prevent against Ultraviolet mutations during these processes (during the night there is less radiation) [10]. | |||
2. As cell physiology declines, production of iodomethane (CH3I) by P. marinus increases. Researchers have calculated CH3I levels in the tropical Atlantic, and have suggested that Prochlorococcus could be a major contributor in that region [13]. | |||
3. It is one of the most abundant organisms on Earth - about 20,000 cells in 1 mL of seawater, and several octillion (10^-27) organisms worldwide [14]. | |||
==References== | ==References== | ||
1. | 1. Partensky, F., W. R. Hess, and D. Vaulot. "Prochlorococcus, a Marine Photosynthetic Prokaryote of Global Significance." Microbiology and Molecular Biology Reviews 63.1 (1999): 106-27. | ||
2. Campbell, Lisa, H. A. Nolla, and Daniel Vaulot. "The Importance of Prochlorococcus to Community Structure in the Central North Pacific Ocean." Limnology and Oceanography 39.4 (1994): 954-61. | |||
3. Liu, Hongbin, Hector A. Nolla, and Lisa Campbell. "Prochlorococcus Growth Rate and Contribution to Primary Production in the Equatorial and Subtropical North Pacific Ocean." Aquatic Microbial Ecology 12 (1997): 39-47. | |||
4. [http://www.cns.fr/spip/Prochlorococcus-marinus-smallest.html "One of the Smallest Photosynthetic Organisms Known." Site Du Genoscope. Genoscope, 16 Jan. 2008. Web. 02 Feb. 2012.] | |||
5. Dufresne, A. et al. 'Genome sequence of the cyanobacterium Prochlorococcus marinus SS120, a nearly minimal oxyphototrophic genome.' PNAS 19 August 2003 vol. 100 no. 17 p10020–10025 | |||
6. Tolonen, Andrew Carl. "Prochlorococcus genetic transformation and genomics of nitrogen metabolism". Woods Hole Oceanographic Institution. Massachusetts Institute of Technology. 2005. | |||
7. Bryant, Donald A. 'The beauty in small things revealed'. PNAS. 19 August 2003. vol. 100 no. 17. pp. 9647-9649. | |||
8. Chisholm, Sallie W., Sheila L. Frankel, Ralf Goericke et al. "Prochlorococcus marinus nov. gen. nov. sp.: an oxyphototrophic marine prokaryote containing divinyl chlorophyll a and b." Archives of Microbiology 157: 3 (1992): 297-300. | |||
9. Steglich, Caludia., Matthias Futschik, Trent Rectro, Robert Steen, and Sallie W. Chisholm. "Genome-Wide Analysis of Light Sensing ni Prochlorococcus." Journal of Bacteriology 188: 22. (Nov. 2006): 7796-7806. | |||
10. Moore, L. R., R. Goericke, and S. W. Chisholm. 1995. Comparative physiology of Synechococcus and Prochlorococcus: influence of light and temperature | |||
on growth, pigments, fluorescence and absorptive properties. Mar. Ecol. Prog. Ser. 116:259–275. | |||
11. Kolowrat, Christian; Partensky, Frédéric; Mella-Flores, Daniella; Corguillé, Gildas Le; Boutte, Christophe; Blot, Nicolas; Ratin, Morgane; Ferréol, Martial; Lecomte, Xavier; Gourvil, Priscillia; Lennon, Jean-François; Kehoe, David M.; Garczarek, Laurence. "Ultraviolet stress delays chromosome replication in light/dark synchronized cells of the marine cyanobacterium Prochlorococcus marinus." BMC Microbiology, 2010, Vol. 10, p204-227 | |||
12. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2941326/?tool=pubmed Rusch DB, Martiny AC, Dupont CL, Halpern AL, Venter JC. Characterization of Prochlorococcus clades from iron-depleted oceanic regions. Proc Natl Acad Sci USA. 2010;107:16184–16189.] | |||
13.Hughes, Claire, and Et Al. "Iodomethane Production by Two Important Marine Cyanobacteria: Prochlorococcus Marinus (CCMP 2389) and Synechococcus Sp. (CCMP 2370)." Elsevier 125.1-4 (2011): 19-25. | |||
14. [http://www.scientificamerican.com/article.cfm?id=the-cells-that-rule-the-s. Nadis, Steve. 'The cells that rule the sea'. Scientific American Magazine. 2003.] | |||
Latest revision as of 06:57, 28 February 2012
A Microbial Biorealm page on the genus Prochlorococcus marinus 2012 Prochlorococcus
Classification
Higher order taxa
Bacteria;Cyanobacteria; Prochlorales; Prochlorococcaceae; Prochlorococcus
|
NCBI: Taxonomy |
Species
|
NCBI: Genome |
Prochlorococcus marinus
Description and significance
Prochlorococcus is a photosynthetic prokaryote. It is revealed by transmission election microscopy to have the same structure as a typical cyanacobacteria[1]. The cytoplasm holds DNA fibrils, carboxyzomes, and glycogen granules, which can be found near or between thylakoids. There is commonly two to four thylakoids in a Prochlorococcus cell, although sometimes there can be up to six thylakoids. Thylakoids can be found running parallel to the cell membrane [2].
Many methods used to find cell sizes tend to be biased towards tiny things, including Prochlorococcus cells. It is estimated that Prochlorococcus cells sizes vary from 0.5 to 0.8 μm in length, and 0.4 to 0.6 μm in width. A Prochlorococcus cell's size van vary depending on its environment. For example, its size was shown to increase by 0.45 to 0.75 μm between the surface and at a depth of 150 meters in the Sargasso Sea [2]. Studies show that Prochlorococcus can grow quite quickly in nutrient-poor habitats. The small size allows these picoplankton to take up dissolved nutrients more efficiently than larger organisms [3].
Prochlorococcus are important due to their contribution to oxygen production. Their production is found to contribute up to 39% of primary production in the eastern and western equatorial Pacific, and up to 82% in the North Pacific Ocean at Station ALOHA.
Genome structure
Because of its small size, Prochlorococcus marinus usually has a small genome [4]. It actually maintains the smallest genome of all cyanobacteria, with only 1.7 Mb. This may be considered an adaptation because in the ocean (environment of this organism) there is not an excess nitrogen or phosphorus, which are essential in the production of nucleotides. There are generally two strains of this bacteria found in the ocean. The low-light adapted and high- light adapted strains are a result of the difference of light levels at different depths in the ocean. P. marinus SS120, is the low light adapted form, which is composed of a singular circular chromosome of 1,751,080 bp with a C+G content of 36.4%. It also contains 1,884 predicted protein-coding genes, a single rRna operon, and 40 tRNA genes [5]. The low- light adapted strains have extra genes for the antenna complex protein, which is where the chlorophyll gets its energy from light [4]. These low-light adapted strains genetically produce chlorophyll b as opposed to chlorophyll a (high-light adapted strain) because chlorophyll b can be more helpful at a with a wider spectrum of light, which is essential when light is scarce. By having genes for more chlorophyll b and antenna protein complex, these bacteria are able to thrive even at depths of up to 200m [4]. These low-light adapted strains have a lot more variation when it comes to their genome, most likely as an adaptation for the tougher environment that the deeper ocean poses. The ocean is a very stable environment, thus these microbes have seen a decrease in the amount of stress- related and housekeeping genes, as compared to the genome of other cyanobacteria [4]. The genes for antenna protein are increased, as we discussed their characteristics that are essential to this bacteria. The genome of these bacteria are essential in the studying of photosynthetic bacteria, and their response to changing environments because of their unique and decreased genome [4].
Cell structure and metabolism
Prochlorococcus marinus has been established as one of the smallest living cyanobacterium. All photosyntheic organisms must adapt to light levels in their environment in order to survive. Since it is know that prochlorococcus marinus are oxyphotobacteria that thrive in the ocean between 40 degrees north and 40 degrees south, their cellular strutres should reflect survival components of this environment. Indeed, survival in a oligotrophic environment requires adaptations such as low cellular nutrient requirement and highly efficient nutrient transport systems. The mechanisms that prochlorococcus uses to acquire and metabolize nitrogen, phosphorus, and other elements in its environment are central to its ecology [6]. Since P. marines is an autotrough it must synthesize all cellular components, including amino acids, nucleotides, and coenzymes from CO2 and salt [7]. Additionally, due to the small size and environment, P. marinus has lowered complexity of biological systems such as DNA repair, chaperones, transport systems, and nitrogen metabolism [7]. It uses a sodium dependent bicarbonate transporter as the primary inorganic carbon uptake system. P. marinus also cannot utilize nitrate, nitrite, urea or cyanate as nitrogen sources, therefore it it only utilizes ammonia and amino acids as its source of nitrogen.
Also, prochlorococcus marinus is a photosyntheitic organism, whos cellular strucutre lacks phycobiliproteins. Phycobiliproteins are water-soluable proteins that are present in most cyanobacteria and act to capture light energy which can then be used during photosynthesis. Instead of these proteins, prochlorococcus uses divinyl-chlorophyll a and b, signature molecules unique to this genus, as their primary syntheitc pigments used during the photosyntheic process to gain energy [8]. This specialized pigment apparatus used to capture light is specific to prochlorococcus marinus which results in a unique light absorption spectrum of blue pigments (common to low light in deep euphotic zones), and sets it apart from other marine bacteria such as other plants and alge [9]. Prochlorococcus is the only organism known to contain this particular compliment of pigments.
Ecology
Prochlorococcus marinus is a photosynthetic organism capable of living in nutrient-poor environments. It is frequently found in the oligotrophic ocean in tropical and subtropical regions. Prochlorococcus marinus, along with many other Prochlorococcus species, are abundantly found between 40˚ N and 40˚S latitudes. Beyond 40˚, however, Prochlorococcus concentrations decrease significantly, suggesting that these picoplanktons prefer warmer temperatures [9]. In an area exposed to UV light, Prochlorococcus marinus undergo a shifted cell cycle in order to maximize cell replication [10].
Prochlorococcus marinus, as well as many other Prochlorococcus species, largely share their habitat with the cyanobacterium Synechococcus. Normal Synechococcus concentrations are very similar to average Prochlorococcus concentrations, albeit one magnitude lower [3]. Prochlorococcus is limited to the surface mixed layer, and its abundance drops quickly below the thermocline. In these cases, Synechococcus has a vertical distribution that is similar to that of Prochlorococcus and its abundance is similar or slightly higher [3]. Prochlorococcus presents a very sharp maximum concentration near the bottom of the euphotic zone, which decreases by a minimum of one order of magnitude at the surface. Also, Prochlorococcus marinus, and other Prochlorococcus species, can be distributed in locations varying from the surface to the bottom of the euphotic zone at almost constant concentrations, where they are the most widespread in oceanic waters [3]. Prochlorococcus species contribute up to 44% of gross primary production [5].
Pathology
Prochlorococcus marinus is not considered a pathogenic organism. It is a free-floating cyanobacteria, which means it uses the sun's rays for energy [9]. It is completely dependent on the sun's light for energy, thus it needs no other source of food, such as another organism [4].
Current Research
1. Prochlorococcus marinus is a cyanobacteria that relies on the light from the sun. In today’s world the threat of excess UV rays as a mutating factor is constantly being addressed. Prochlorococcus is constantly being bombarded by these UV rays and thus this research article describes the techniques that this bacteria has to prevent against damaging mutations. These bacteria were observed in the absence and presence of UV radiation [11]. This allows for the observation of the DNA repair, and cell cycle techniques that are in place by the cell to prevent against damaging mutations. The different layers of the ocean provide different amounts of light to these organisms, and thus different amounts of ultraviolet radiation. The genes that are most affected by UV radiation are the ones that allow for smooth DNA replication [11]. The S phase, in which the cell replicates its DNA is one of the most affected phases of replication. To combat this problem, these cells are known to delay there S phase when UV radiation is high. This delay, caused by a repression of dnaA, allows more time for oligonucleotide removal by nucleotide excision repair, and allows DNA photylase to repair UV- caused mutations. These adaptations allow for the cells to survive in environments dominated by DNA- mutating ultraviolet light [11].
2. Once Prochlorococcus marinus was discovered in 1986, an immense amount of studies have begun on this species since it is one of the main contributors to our worlds carbon source. Most recently, two new uncharacterized Prochlorococcus clades have been detected in samples from the Atlantic, Pacific, and Indian Oceans. Douglas B. Rusch and his team of researchers have conducted a phylogenetic analysis using different genetic markers, which resulted in the unknown clades producing lineages adapted to high-light environments [12]. Consistent with other high light adapted Prochlorococcus, these new clades reside in high-temperature surface water with low-iron levels. His research showed that they are genetically distinct from one another and other strains of the species Prochlorococcus, and one of their main functions is to reduce an organisms iron quota through the loss of iron-containing proteins. This could explain why some Prochlorococcus species from iron depleted regions do not respond to iron fertilization experiments [12]. Additionally, this new research will help further the understanding of how marine organisms adapt to variations in nutrient availability.
3. Prochlorococcus marinus, along with many other species of this genus, contribute greatly to gross primary production with their neighbors Synechococcus spp. Current research investigates production of the toxic chemical iodomethane, which can be found in pesticides. The research done in this study is not aimed towards assessing P. marinus' contribution to iodomethane's harmful effects. Rather, researchers have found that production of iodomethane is due to the compromised conditions of P. marinus cells' physiology. P. marinus were found to produce iodomethane depending on their loss rate, their abundance, and their cellular physiological states. Recently, there have been discrepancies in iodomethane production compared to 2006. This research can assist future researchers and scientists in developing ways to resolve possible overproduction of iodomethane[13].
Cool Factor
1. The DNA replication of these bacteria occurs mostly at night to prevent against Ultraviolet mutations during these processes (during the night there is less radiation) [10].
2. As cell physiology declines, production of iodomethane (CH3I) by P. marinus increases. Researchers have calculated CH3I levels in the tropical Atlantic, and have suggested that Prochlorococcus could be a major contributor in that region [13].
3. It is one of the most abundant organisms on Earth - about 20,000 cells in 1 mL of seawater, and several octillion (10^-27) organisms worldwide [14].
References
1. Partensky, F., W. R. Hess, and D. Vaulot. "Prochlorococcus, a Marine Photosynthetic Prokaryote of Global Significance." Microbiology and Molecular Biology Reviews 63.1 (1999): 106-27.
2. Campbell, Lisa, H. A. Nolla, and Daniel Vaulot. "The Importance of Prochlorococcus to Community Structure in the Central North Pacific Ocean." Limnology and Oceanography 39.4 (1994): 954-61.
3. Liu, Hongbin, Hector A. Nolla, and Lisa Campbell. "Prochlorococcus Growth Rate and Contribution to Primary Production in the Equatorial and Subtropical North Pacific Ocean." Aquatic Microbial Ecology 12 (1997): 39-47.
5. Dufresne, A. et al. 'Genome sequence of the cyanobacterium Prochlorococcus marinus SS120, a nearly minimal oxyphototrophic genome.' PNAS 19 August 2003 vol. 100 no. 17 p10020–10025
6. Tolonen, Andrew Carl. "Prochlorococcus genetic transformation and genomics of nitrogen metabolism". Woods Hole Oceanographic Institution. Massachusetts Institute of Technology. 2005.
7. Bryant, Donald A. 'The beauty in small things revealed'. PNAS. 19 August 2003. vol. 100 no. 17. pp. 9647-9649.
8. Chisholm, Sallie W., Sheila L. Frankel, Ralf Goericke et al. "Prochlorococcus marinus nov. gen. nov. sp.: an oxyphototrophic marine prokaryote containing divinyl chlorophyll a and b." Archives of Microbiology 157: 3 (1992): 297-300.
9. Steglich, Caludia., Matthias Futschik, Trent Rectro, Robert Steen, and Sallie W. Chisholm. "Genome-Wide Analysis of Light Sensing ni Prochlorococcus." Journal of Bacteriology 188: 22. (Nov. 2006): 7796-7806.
10. Moore, L. R., R. Goericke, and S. W. Chisholm. 1995. Comparative physiology of Synechococcus and Prochlorococcus: influence of light and temperature on growth, pigments, fluorescence and absorptive properties. Mar. Ecol. Prog. Ser. 116:259–275.
11. Kolowrat, Christian; Partensky, Frédéric; Mella-Flores, Daniella; Corguillé, Gildas Le; Boutte, Christophe; Blot, Nicolas; Ratin, Morgane; Ferréol, Martial; Lecomte, Xavier; Gourvil, Priscillia; Lennon, Jean-François; Kehoe, David M.; Garczarek, Laurence. "Ultraviolet stress delays chromosome replication in light/dark synchronized cells of the marine cyanobacterium Prochlorococcus marinus." BMC Microbiology, 2010, Vol. 10, p204-227
13.Hughes, Claire, and Et Al. "Iodomethane Production by Two Important Marine Cyanobacteria: Prochlorococcus Marinus (CCMP 2389) and Synechococcus Sp. (CCMP 2370)." Elsevier 125.1-4 (2011): 19-25.
14. Nadis, Steve. 'The cells that rule the sea'. Scientific American Magazine. 2003.
