Denitrovibrio acetiphilus: Difference between revisions
No edit summary |
|||
| (58 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
==Classification== | ==Classification== | ||
Domain: Bacteria; | Domain: Bacteria; | ||
| Line 15: | Line 13: | ||
Family: <i>Deferribacteraceae</i> | Family: <i>Deferribacteraceae</i> | ||
Genus: <i>Denitrovibrio</i> | Genus: <i>Denitrovibrio</i> [1] | ||
===Species=== | ===Species=== | ||
Species: acetiphilus | Species: <i>acetiphilus</i> [2] | ||
| Line 33: | Line 31: | ||
==Description and Significance== | ==Description and Significance== | ||
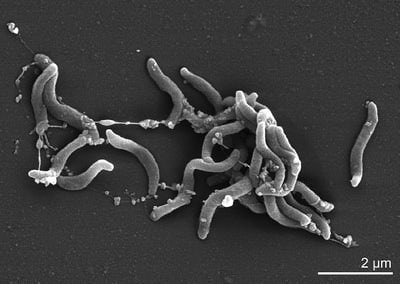
[[Image:89-2228-1-PB.jpg|400px|thumb|right|Scanning electron micrograph of <i>D. acetiphilus</i> strain N2460T]] | [[Image:89-2228-1-PB.jpg|400px|thumb|right|Figure 1. Scanning electron micrograph of <i>D. acetiphilus</i> strain N2460T [3]]] | ||
<i>Denitrovibrio acetiphilus</i> is a marine, mesophilic, obligately anerobic organism | <i>Denitrovibrio acetiphilus</i> is a Gram-negative, marine, mesophilic, obligately anerobic organism that respires through nitrate reduction.[3] | ||
<i>Denitrovibrio acetiphilus</i> is of phylogenetic interest because there are currently only six genera described in the family Deferribacteraceae. The organism was found | <i>Denitrovibrio acetiphilus</i> is of phylogenetic interest because there are currently only six genera described in the family <i>Deferribacteraceae</i>. The organism was found and isolated from a laboratory column reducing nitrate to ammonia. The column simulated off-shore oil drilling conditions, and the presence of this organism will prove beneficial to stopping the process of undesired hydrogen sulfide formation. Addition of nitrate to the system stimulates the replacement of sulfate reducing populations with nitrate reducing bacteria, therefore reducing hydrogen sulfide production. The ability of this organism to reduce nitrate is of economic significance by eliminating the need for expensive biocides currently used in the treatment of oil reserves. [3] | ||
==Genome Structure== | ==Genome Structure== | ||
[[Image:Family.png|thumb|600px|right| | [[Image:Family.png|thumb|600px|right|Figure 2. Phylogenetic tree highlighting the position of D. acetiphilus strain N2460T relative to the other species within the phylum Deferribacteres [3].]] | ||
<i>Denitrovibrio acetiphilus</i> has two known completed genome sequences, of the order Deferribacterales and the class Deferribacteres. This is the sole class in the phylum Deferribacteres. The 3,222,077 | <i>Denitrovibrio acetiphilus</i> has two known completed genome sequences, of the order <i>Deferribacterales</i> and the class <i>Deferribacteres</i>. This is the sole class in the phylum <i>Deferribacteres</i>. The genome is 3,222,077 base pairs in length and comprises one main circular chromosome. There are 3,085 total genes from which 3,034 are protein-coding and 51 are RNA. Research on the genome of <i>Denitrovibrio acetiphilus</i> is part of the Genomic Encyclopedia of Bacteria and Archaea project (GEBA) [3]. 16S rRNA gene analysis shows the strain N2460<sup>T</sup> <i>D. acetiphilus</i> is a bacterium, with the closest relatives being ‘<i>Geovibrio ferrieducens</i>’, ‘<i>Deferribacter thermophilus</i>’, and ‘<i>Flexistipes sinusarabici</i>’ [2] (See Figure 2). | ||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
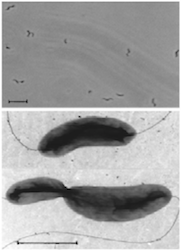
[[Image:vibrio5.png|thumb|600px|right| | [[Image:vibrio5.png|thumb|600px|right| Figure 3. Micrograph of <i>Denitrovibrio acetiphilus</i> cell morphology using phase contrast microscopy [2].]] | ||
<i>D. acetiphilus</i> is a gram negative bacterium that possesses a curved rod shaped structure | <i>D. acetiphilus</i> is a gram negative bacterium that possesses a curved rod shaped structure (See Figure 1) similar to those in the genus, Vibrio. <i>D. acetiphilus</i> cells viewed by phase-contrast microscopy are motile by bipolar flagellation and exhibit a rapid corkscrew movement. Cells measure 0.5-0.7 µm by 1.7-2.0 µm and can be seen as singles, in pairs, or arranged in long chains (See Figure 3). C16 and C18 fatty acids are major components of the cell membrane. [2] | ||
<i>Denitrovibrio acetiphilus</i>(strain N2460<sup>T</sup>) reduces nitrate to ammonia by supplementation of acetate which acts as a substrate and electron donor. Nitrate | <i>Denitrovibrio acetiphilus</i> (strain N2460<sup>T</sup>) reduces nitrate to ammonia by supplementation of acetate which acts as a substrate and electron donor. Nitrate then acts as an electron acceptor and acetate reduction to nitrite (an intermediate during reduction to ammonia) acts as the primary energy source for many dissimilatory nitrate reducing bacteria. This source of energy has not yet been confirmed for the strain N2460<sup>T</sup> [3]. Further reduction of nitrite to ammonia may act as a detoxification system and is a possible electron source (electron sink) from which coenzymes are regenerated. Selective enrichment from an oil reservoir model column demonstrated the ability of this organism to grow at temperatures ranging from 4 to 40 degrees Celsius with optimal growth temperature between 35-37 degrees Celsius. Optimum growth also requires the presence of vitamins as well as a pH range of 6.5-8.6 in addition to supplementation of acetate (and nitrate) to the medium. <i>D.acetiphilus</i> is unable to grow in the presence of oxygen and this organism is therefore labeled a strict anaerobic acetate specialist. This organism is also able to fermentatively grow on fumarate. Activity of 2-oxoglutarate dehydrogenase suggests acetate is oxidized via the citric acid cycle. <i>Denitrovibrio acetiphilus</i> (N2460<sup>T</sup>) is considered the nitrate reducing metabolic analogue of sulfate reducing <i>Desulfobacter</i> (also able to oxidize acetate through the citric acid cycle). From this information, <i>D.acetiphilus</i> can be described as a marine, mesophilic, obligate anaerobe that respires and possibly gains energy by dissimilatory nitrate reduction.[2] | ||
==Ecology== | ==Ecology== | ||
<table><tr> | <table><tr> | ||
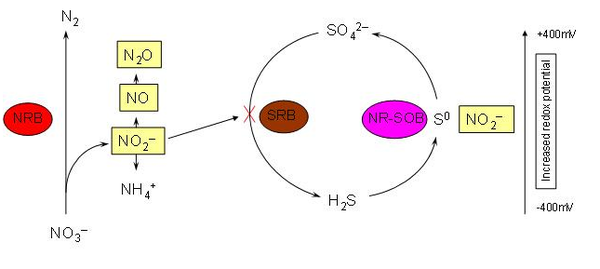
[[Image:Nitrogen_reduction.png|thumb|600px|left| FIGURE | [[Image:Nitrogen_reduction.png|thumb|600px|left| FIGURE 4. Schematic of potential inhibitory processes of sulfate-reducing bacteria by nitrate-reducing bacteria [4].]] | ||
As mentioned previously, this bacterium sheds insight into optimizing oil recovery in off-shore drilling. Presently, seawater is injected during oil recovery to maintain desired pressures and increase efficiency. In most cases the seawater contains high concentrations of sulfate which in turn results in a higher population of sulfate-reducing bacteria at these mine sites [3]. Through anaerobic respiration these sulfate-reducing bacteria produce hydrogen sulfide (H<sub>2</sub>S) that can potentially sour the oil reserves and lead to poor gas quality, corrosion and poor separation characteristics in further processing. Hydrogen sulfide may also harm the platform operators on oil rigs [4]. In many cases H<sub>2</sub>S inhibits the senses of the nose making it almost undetectable in the absence of air monitors. Experiments have shown that adding nitrate to these reservoirs may reduce the production of hydrogen sulfide by stimulating the growth of nitrate-reducing bacteria and inhibiting sulfate-reducing bacteria. This microbial shift may provide a more economical solution to minimizing H<sub>2</sub>S by | As mentioned previously, this bacterium sheds insight into optimizing oil recovery in off-shore drilling. Presently, seawater is injected into a reservoir during oil recovery to maintain desired pressures and increase efficiency. In most cases the seawater contains high concentrations of sulfate which in turn results in a higher population of sulfate-reducing bacteria at these mine sites [3]. Through anaerobic respiration these sulfate-reducing bacteria produce hydrogen sulfide (H<sub>2</sub>S) that can potentially sour the oil reserves and lead to poor gas quality, corrosion, and poor separation characteristics in further processing. Hydrogen sulfide may also harm the platform operators on oil rigs [4]. In many cases H<sub>2</sub>S inhibits the senses of the nose making it almost undetectable in the absence of air monitors. Experiments have shown that adding nitrate to these reservoirs may reduce the production of hydrogen sulfide by stimulating the growth of nitrate-reducing bacteria (such as <i>D. acetiphilus</i> ) and inhibiting sulfate-reducing bacteria. This microbial shift may provide a more economical solution to minimizing H<sub>2</sub>S by eliminating the need for expensive biocides that are currently used to treat oil reserves.[3] | ||
</tr></table> | </tr></table> | ||
Latest revision as of 22:31, 17 April 2012
Classification
Domain: Bacteria;
Phylum: Deferribacteres
Class: Deferribacteres
Order: Deferribacterales
Family: Deferribacteraceae
Genus: Denitrovibrio [1]
Species
Species: acetiphilus [2]
|
NCBI: Taxonomy |
Denitrovibrio acetiphilus
Description and Significance
Denitrovibrio acetiphilus is a Gram-negative, marine, mesophilic, obligately anerobic organism that respires through nitrate reduction.[3]
Denitrovibrio acetiphilus is of phylogenetic interest because there are currently only six genera described in the family Deferribacteraceae. The organism was found and isolated from a laboratory column reducing nitrate to ammonia. The column simulated off-shore oil drilling conditions, and the presence of this organism will prove beneficial to stopping the process of undesired hydrogen sulfide formation. Addition of nitrate to the system stimulates the replacement of sulfate reducing populations with nitrate reducing bacteria, therefore reducing hydrogen sulfide production. The ability of this organism to reduce nitrate is of economic significance by eliminating the need for expensive biocides currently used in the treatment of oil reserves. [3]
Genome Structure
Denitrovibrio acetiphilus has two known completed genome sequences, of the order Deferribacterales and the class Deferribacteres. This is the sole class in the phylum Deferribacteres. The genome is 3,222,077 base pairs in length and comprises one main circular chromosome. There are 3,085 total genes from which 3,034 are protein-coding and 51 are RNA. Research on the genome of Denitrovibrio acetiphilus is part of the Genomic Encyclopedia of Bacteria and Archaea project (GEBA) [3]. 16S rRNA gene analysis shows the strain N2460T D. acetiphilus is a bacterium, with the closest relatives being ‘Geovibrio ferrieducens’, ‘Deferribacter thermophilus’, and ‘Flexistipes sinusarabici’ [2] (See Figure 2).
Cell Structure, Metabolism and Life Cycle
D. acetiphilus is a gram negative bacterium that possesses a curved rod shaped structure (See Figure 1) similar to those in the genus, Vibrio. D. acetiphilus cells viewed by phase-contrast microscopy are motile by bipolar flagellation and exhibit a rapid corkscrew movement. Cells measure 0.5-0.7 µm by 1.7-2.0 µm and can be seen as singles, in pairs, or arranged in long chains (See Figure 3). C16 and C18 fatty acids are major components of the cell membrane. [2]
Denitrovibrio acetiphilus (strain N2460T) reduces nitrate to ammonia by supplementation of acetate which acts as a substrate and electron donor. Nitrate then acts as an electron acceptor and acetate reduction to nitrite (an intermediate during reduction to ammonia) acts as the primary energy source for many dissimilatory nitrate reducing bacteria. This source of energy has not yet been confirmed for the strain N2460T [3]. Further reduction of nitrite to ammonia may act as a detoxification system and is a possible electron source (electron sink) from which coenzymes are regenerated. Selective enrichment from an oil reservoir model column demonstrated the ability of this organism to grow at temperatures ranging from 4 to 40 degrees Celsius with optimal growth temperature between 35-37 degrees Celsius. Optimum growth also requires the presence of vitamins as well as a pH range of 6.5-8.6 in addition to supplementation of acetate (and nitrate) to the medium. D.acetiphilus is unable to grow in the presence of oxygen and this organism is therefore labeled a strict anaerobic acetate specialist. This organism is also able to fermentatively grow on fumarate. Activity of 2-oxoglutarate dehydrogenase suggests acetate is oxidized via the citric acid cycle. Denitrovibrio acetiphilus (N2460T) is considered the nitrate reducing metabolic analogue of sulfate reducing Desulfobacter (also able to oxidize acetate through the citric acid cycle). From this information, D.acetiphilus can be described as a marine, mesophilic, obligate anaerobe that respires and possibly gains energy by dissimilatory nitrate reduction.[2]
Ecology
As mentioned previously, this bacterium sheds insight into optimizing oil recovery in off-shore drilling. Presently, seawater is injected into a reservoir during oil recovery to maintain desired pressures and increase efficiency. In most cases the seawater contains high concentrations of sulfate which in turn results in a higher population of sulfate-reducing bacteria at these mine sites [3]. Through anaerobic respiration these sulfate-reducing bacteria produce hydrogen sulfide (H2S) that can potentially sour the oil reserves and lead to poor gas quality, corrosion, and poor separation characteristics in further processing. Hydrogen sulfide may also harm the platform operators on oil rigs [4]. In many cases H2S inhibits the senses of the nose making it almost undetectable in the absence of air monitors. Experiments have shown that adding nitrate to these reservoirs may reduce the production of hydrogen sulfide by stimulating the growth of nitrate-reducing bacteria (such as D. acetiphilus ) and inhibiting sulfate-reducing bacteria. This microbial shift may provide a more economical solution to minimizing H2S by eliminating the need for expensive biocides that are currently used to treat oil reserves.[3]
References
[1] Denitrovibrio acetiphilus taxonomy - (http://www.catalogueoflife.org/details/species/id/4257206)
[2] Myhr, S., and Torsvik, T. "Denitrovibrio acetiphilus, a novel genus and species of dissimilatory nitrate-reducing bacterium isolated from an oil reservoir model column". International Journal of Systematic and Evolutionary Microbiology". 2000. Volume 50. pgs. 1611–1619 (http://ijs.sgmjournals.org/content/50/4/1611.full.pdf).
[3] Kiss, H., Lang, E., Lapidus, A., Copeland, A., Nolan, M., Glavina Del Rio, T., Chen, F., Lucas, S., Tice, H., Cheng, J., Han, C., Goodwin, L., Pitluck, S., Liolios, K., Pati, A., Ivanova, N., Mavromatis, K., Chen, A., Palaniappan, K., Land, M., Hauser, L., Chang, Y., Jeffries, C., Detter, J., Brettin, T., Spring, S., Rohde, M., GöKer, M., Woyke, T., Bristow, J., Eisen, J., Markowitz, V., Hugenholtz, P., Kyrpides, N., Klenk, H.. "Complete genome sequence of Denitrovibrio acetiphilus type strain (N2460T)". Standards in Genomic Sciences, North America, 2, jun. 2010. Available at: (http://www.standardsingenomics.org/index.php/sigen/article/view/sigs.892105) . Date accessed: 21 Mar. 2012.
[4] "Nitrate: A Cost Efficient Alternative to Biocides." Norwegian Centre of Excellence. Web. 11 Apr. 2012. <http://www.cipr.uni.no/contentitem.aspx?ci=1546>.
Authors
Page authored by Rodney Tocco, Minh Tran, Samantha Wengert, and Eric Werner, students of Prof. Jay Lennon at Michigan State University.
<-- Do not remove this line-->