Efficacy of vaccines against Streptococcus pneumoniae: Difference between revisions
Mharika5996 (talk | contribs) |
No edit summary |
||
| (32 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
=Introduction= | =Introduction= | ||
Streptococcus <i>pneumoniae</i> is a pathogenic, gram-positive, α-hemolytic, anaerobic bacterium | [http://en.wikipedia.org/wiki/Streptococcus_pneumoniae Streptococcus <i>pneumoniae</i>] is a pathogenic, gram-positive, α-hemolytic, anaerobic bacterium<sup>[20]</sup>. It causes pneumonia along with other pneumococcal infections some of which include bacterial meningitis, sinusitis, and otitis media<sup>[18][20]</sup>. S. <i>pneumoniae</i> has developed antibiotic resistance to traditional [http://en.wikipedia.org/wiki/Beta-lactam_antibiotic β-Lactam antibiotics] such as Penicillin (and its derivatives). Antibiotic resistance is acquired by alteration of the targeted penicillin binding proteins (PBPs) in the resistant strains that lower its affinity to bind to penicillin<sup>[20]</sup>. Genome sequencing of the penicillin resistant strains show that point mutations in one of its penicillin binding proteins,PBP2x, decreases its affinity to bind to penicillin<sup>[12]</sup>. | ||
Genome sequencing of the penicillin resistant strains show that point mutations in one of its penicillin binding proteins,PBP2x, decreases its affinity to bind to penicillin | |||
Due to the increasing rate of antibiotic resistance it is important to study newer ways to inhibit | Due to the increasing rate of antibiotic resistance it is important to study newer ways to inhibit S.<i>pneumoniae</i> growth. This is because antibiotics select for growth of rare microorganisms in a population that is otherwise susceptible to the drug<sup>[20]</sup>. Antibiotic resistance can be overcome by developing vaccines that target virulence factors on the surface of S.<i>pneumoniae</i> thereby disabling the bacteria<sup>[9]</sup>. Vaccines work by giving the organism immunity against infection by a particular pathogen. Vaccines contain a weakened or dead derivative of the pathogen and in its altered state, vaccine pathogens, are typically safe and unable to cause disease<sup>15</sup>. The non-virulent pathogens in the vaccine stimulate the organisms [http://en.wikipedia.org/wiki/B_cell B cells] to make antibodies for the particular antigens thereby developing immunity to that pathogen<sup>[15]</sup>. Subsequently, [http://en.wikipedia.org/wiki/Memory_T_cell Memory T cells] get stimulated upon re-exposure to cognate antigen and rapidly multiply thereby providing “memory” to the body’s immune system <sup>[15]</sup>. In addition to the weakened or dead form of bacteria or virus, the vaccine also contains antibiotics or preservatives to protect the body against any germs that might get into the vaccine<sup>[14]</sup>. | ||
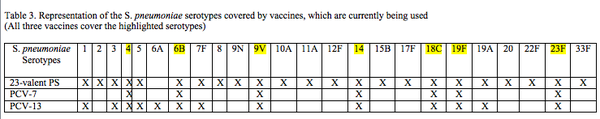
Current pneumococcal vaccines target the polysaccharide capsule | Current pneumococcal vaccines target the polysaccharide capsule because immunity after pneumococcal disease is directed against the capsular serotype of the S.<i>pneumoniae</i> bacteria involved<sup>[22]</sup>. [http://en.wikipedia.org/wiki/Serotype Serotype] is a characteristic set of antigens that help to distinguish a group of closely related microorganisms. 91 serotypes of S.<i>pneumoniae</i> have been identified based on the difference in composition of the polysaccharide capsule<sup>[18]</sup>. The polysaccharide capsule is a virulence factor however; only some of the 91 serotypes cause pneumococcal disease. Current pneumococcal vaccines, Polysaccharide (23-valent) PS vaccine and conjugate vaccine (PCV-7) cover the most common and virulent serotypes and give immunity against 23 and 7 serotypes respectively. However, given the large number of serotypes (~91 serotypes), serotype replacement, which is replacement of vaccine serotype (VTs) by non-vaccine serotypes (NVTs) in disease and in carriage has become a growing concern thereby making vaccination against current serotypes less effective<sup>[2][18]</sup>. | ||
=Current pneumococcal vaccines= | =Current pneumococcal vaccines= | ||
As an alternative to β-lactam antibiotics, vaccines have been created that target cell surface polysaccharide, which aim to develop immunity against the virulent serotypes of S. pneumoniae. The two vaccines widely used are polysaccharide vaccine (23-valent polysaccharide vaccine) and pneumococcal conjugate vaccine (heptavalent protein–polysaccharide conjugate vaccine, PCV-7) | |||
As an alternative to β-lactam antibiotics, vaccines have been created that target cell surface polysaccharide, which aim to develop immunity against the virulent serotypes of S. <i>pneumoniae</i>. The two vaccines widely used are polysaccharide vaccine (23-valent polysaccharide vaccine) and pneumococcal conjugate vaccine (heptavalent protein–polysaccharide conjugate vaccine, PCV-7) <sup>[2]</sup>. The polysaccharide vaccine provides immunity against 23 S.<i>pneumoniae</i> serotypes (Table 3.) and is effective in older immunocompetent patients <sup>[2][18]</sup>. However, this vaccine cannot be administered to infants below 2 years of age and to immunodeficient patients perhaps due to an immature or a sensitive immune system <sup>[18]</sup>. The newer conjugate vaccine, PCV-7 is able to provide immunity to this age group <sup>[2][18]</sup>. | |||
==23-valent polysaccharide (PS) vaccine== | ==23-valent polysaccharide (PS) vaccine== | ||
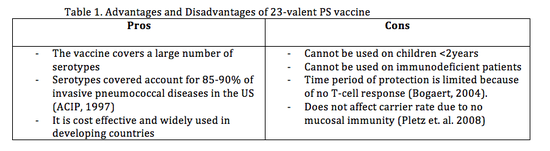
The 23-valent PS vaccine contains capsular polysaccharide antigens for 23 serotypes and to gain immunity the polysaccharides induce B cell dependant response by releasing polysaccharide Immunoglobulin M (IgM), which are a type of antibodies produced by B cells | The 23-valent PS vaccine contains capsular polysaccharide antigens for 23 serotypes (Table 3.) and to gain immunity the polysaccharides induce B cell dependant response by releasing polysaccharide Immunoglobulin M (IgM), which are a type of antibodies produced by B cells <sup>[18]</sup>. This PS vaccine does not induce a T cell-dependant immune response. As mentioned above, T cells provide ‘memory’ to the body’s immune system <sup>[15]</sup> so antibodies can be made quickly upon re-exposure to [http://medical-dictionary.thefreedictionary.com/Cognates cognate] antigen. Also this vaccine cannot be administered to immunodeficient patients (Table 1.), HIV infected individuals have show an impaired antibody response to PS vaccination <sup>[11]</sup>. Further advantages and disadvantages are listed in (Table 1.) | ||
[[Image:Picture 4.png|thumb|550px|left|23 PS Vaccine]] | [[Image:Picture 4.png|thumb|550px|left|23 PS Vaccine]] | ||
A study done in Mexico evaluating the immune response of this vaccine in children under 5, for 6 serotypes, suggests that the pneumococcal | A study done in Mexico evaluating the immune response of this vaccine in children under 5, for 6 serotypes, suggests that the pneumococcal PS vaccine produced adequate immunogenicity in the given age groups <sup>[18]</sup>. However, this vaccine is not effective in children under 2 years of age <sup>[1][7]</sup>. Therefore, a more comprehensive vaccine is still needed. | ||
Unlike the 23-valent polysaccharide vaccine, which can only be used in children over 2 years, the conjugate vaccine can be administered to infants as young as 2 months old | |||
==Heptavalent protein–polysaccharide conjugate (PCV-7) vaccine == | |||
Unlike the 23-valent polysaccharide vaccine, which can only be used in children over 2 years, the conjugate vaccine can be administered to infants as young as 2 months old <sup>[2][13][18]</sup>. Polysaccharides in this vaccine are from seven serotypes that are most frequently involved in infant infections (Table 3.) <sup>[10][18]</sup>. As the name suggests this vaccine is conjugated to protein (CRM197), which is a non-toxic diphtheria toxoid protein<sup>[18]</sup>. The protein-specific [https://en.wikipedia.org/wiki/T_helper_cell type 2 helper T cells] associate with B cells, which are bound to the polysaccharide-protein complex via a polysaccharide specific IgM<sup>[18]</sup>. This association between T and B cells presents the processed protein (CRM197) along with class II MHC (Major Histocompatibility Complex) to the effector T cells<sup>[18]</sup>. Class II MHCs are cell-surface molecules that mediate interaction between immune cells and body cells. The antigens from Class II peptides come from extracellular proteins. This mechanism leads to adaptive immunity in infants, which is switching of antibody isotype and generation of memory B-cells <sup>[18]</sup>. | |||
[[Image:Picture_6.png|thumb|550px|left|PCV-7 vaccine]] | [[Image:Picture_6.png|thumb|550px|left|PCV-7 vaccine]] | ||
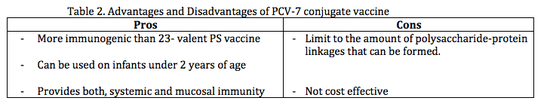
Advanatages and Disadvantages of Pcv-7 vaccine are listed in Table 2. Early maturation of antipolysacchrides antibodies allows for use in | Advanatages and Disadvantages of Pcv-7 vaccine are listed in Table 2. Early maturation of antipolysacchrides antibodies allows for use in infants under 2 years of age (Table2.). This vaccine is also more immunogenic that PS vaccine because capsular polysacchrides are bound covalently (or via reactive groups) to protein carriers<sup>[17]</sup>. The bound protein helps in eliciting a T cell dependent response, which induces memory of B cells <sup>[2]</sup>. The PCV-7 vaccine also affects carrier rate by decreasing nasopharyngeal carriage of vaccine serotype, which suggests that conjugate vaccine provides both, systemic (throughout the body) and mucosal immunity<sup>[2][19]</sup>. Obtaining specific mucosal immunity is important because it allows asymptomatic carriers to eliminate colonizing of vaccine serotypes thereby decreasing carrier rate<sup>[18]</sup>. However, this vaccine is not cost effective (Table 2.), it is expensive to develop and does not cover the major pneumococcal disease causing serotypes (1 and 5) in developing countries<sup>[10][13]</sup>. There is also a limit to the amount of polysaccharide-protein linkages that can be formed. Too many carrier antigens used can impair antibody response to the antigens<sup>[5]</sup>. | ||
==Combined use of 23-Valent PS and PCV-7== | ==Combined use of 23-Valent PS and PCV-7== | ||
After the newer PCV-7 vaccine was developed, the best form of immunity was seen in patients that took the conjugate vaccine followed by the PS vaccine<sup>[2]</sup>. A combined vaccination program is recommended for children 2-5 years by the Advisory Committee on Immunization Practices (ACIP). According to the ACIP, PCV-7 vaccine should be followed by the use of PS vaccine as a booster<sup>[2]</sup>. This is perhaps because PCV-7 confers immunity in children from the invasive serotypes and later the PS vaccine provides immunity against additional serotypes not covered by PCV-7. | |||
=Serotype Replacement= | =Serotype Replacement= | ||
Although antibiotic resistance has been a growing concern, use of vaccines against the major virulent serotypes has giving rise to a new problem that is serotype replacement. Serotype replacement is the processes in which pre-existing clones of non-vaccine serotypes (NVTs) can multiply and essentially replace the serotypes for which there is vaccination | Although antibiotic resistance has been a growing concern, use of vaccines against the major virulent serotypes has giving rise to a new problem that is serotype replacement. Serotype replacement is the processes in which pre-existing clones of non-vaccine serotypes (NVTs) can multiply and essentially replace the serotypes for which there is vaccination <sup>[2]</sup>.Replacement occurs because vaccines eradicate particular serotypes, this creates a niche for non-vaccine serotypes, allowing them to proliferate <sup>[2][18]</sup>. | ||
==Increase in non-vaccine serotypes (NVTs)== | ==Increase in non-vaccine serotypes (NVTs)== | ||
Studies have shown that with the administration of conjugate vaccine combined with re-immunization by 23-valent | Studies have shown that with the administration of conjugate vaccine combined with re-immunization by 23-valent PS vaccine, reduces the prevalence of nasopharyngeal carriage of vaccine serotypes i.e. gives mucosal immunity<sup>[19]</sup>. However, there has not been any significant reduction in the frequency of pneumococcal carriage because of ‘near-complete’ replacement with pneumococci of NVTs<sup>[19][24]</sup>. Beall et. al. 2006<sup>[3]</sup> genotyped invasive isolates, which were collected from patients after the administration of vaccine and compared it to isolates collected from patients that had not been administered PCV7. They found that in the non-PCV7 serogroup emergence of new serotypes was uncommon however, there was a significant increase in the proliferation of the ‘pre-existing clones’ of non-vaccine serotypes<sup>[3]</sup>. These results suggests that increase in NVTs is not due to the emergence of new serotypes but instead is from the expansion of already established serotypes from which the vaccine gives no immunity. | ||
These results suggests that increase in NVTs is not due to emergence of new serotypes but instead is from the expansion of already established serotypes from which the vaccine gives no immunity. | |||
==Near-complete replacement in carriage Vs. Partial replacement for disease== | ==Near-complete replacement in carriage Vs. Partial replacement for disease== | ||
There has been no significant decrease in the carriage of S. pneumonia because of the increase in the prevalence of NVTs in asymptotic carriers | There has been no significant decrease in the carriage of S.<i>pneumonia</i> because of the increase in the prevalence of NVTs in asymptotic carriers<sup>[24]</sup>. As mentioned above, Beall et. al. 2006<sup>[3]</sup> found that vaccinated serogroups saw an increase of the preexisting NVTs. Randomized trials in South Africa, Netherlands and The Gambia show that NVTs have almost completely replaced vaccinated serotypes in carriage<sup>[3][19][24]</sup>. Moreover, the presence of NVTs, like serotype 19A, has led to an increase in the number of diseases caused by NVTs<sup>[21]</sup>. Study done by Vestrheim et. al. 2010<sup>[21]</sup> on the total Norwegian population from 1989-2008 suggests that once the conjugate vaccine was administered in 2006 the rate of invasive pneumococcal disease (IPD) caused by NVTs 19A, 9N, 33F and 35B in children under 5 increased significantly<sup>[21]</sup>. Moreover, IPD caused by 19A increased till 2007, after the conjugate vaccine was administered, suggesting that the increase in IPD by NVTs is due to serotype replacement and not due to an unrelated trend<sup>[21]</sup>. However, the increase in IPD by NVTs has been modest and the rate of NVT replacement in carriage far exceeds the replacement in pneumococcal diseases caused by NVTs<sup>[24]</sup>. | ||
The discrepancy in NVTs completely replacing carriage but only somewhat replacing for IPD is due to a combination of various factors: | |||
• | • Difference in microbiological properties such as varying capsular size, adhesion, toxins and proteins of serotypes play a role in influencing carriage and invasiveness<sup>[24]</sup>. Serotypes that persist in carriage (NVTs) after vaccination tend to be heavily capsulated<sup>[24]</sup>. | ||
• | • NVTs are less invasive than VTs therefore they are present in carriage form but do not cause pneumococcal disease<sup>[24]</sup>. | ||
• Effect of ‘unmasking’ of the NVTs due to the reduction in VTs post vaccination, it is easier to detect new serotypes post-vaccination than when vaccine serotypes were present<sup>[24]</sup>. | |||
== Current ways to prevent Serotype Replacement == | == Current ways to prevent Serotype Replacement == | ||
[[Image:Picture_8.png|thumb|600px|right]] | [[Image:Picture_8.png|thumb|600px|right]] | ||
<b> Regularly monitoring and developing new conjugate vaccine </b> | <b> 1. Regularly monitoring and developing new conjugate vaccine </b> | ||
13-valent conjugated pneumococcal vaccine (PCV 13) is the newer version of PCV-7 conjugate vaccine and has a broader coverage. It consists of thirteen pneumococcal capsular polysaccharides, which are individually conjugated to the diphtheria-derived protein carrier CRM (197)<sup>[10]</sup>. PCV-13 includes 13 serotypes (Table 3.) and will replace PCV-7 particularly because it includes serotype 19A, which was a virulent NVT. It is a more cost effective option for developing countries because unlike PCV-7, it also covers serotypes 1 and 5, which cause majority of pneumococcal diseases in developing countries<sup>[10]</sup>. Although PCV-13 is a better version of PCV-7 it is not antigenically covered across all serotypes, a newer form of this vaccine might be required in the future to address diseases caused by other NVT. | |||
<b> 2. Identification of serotype independent antigens </b> | |||
< | Pneumococcal surface proteins, which have serotype-independence, can be used as an alternative to vaccine candidates<sup>[2]</sup>. Current pneumococcal vaccines fail to cover all polysaccharide types and infections in adults particularly, is caused by strains that have a variety of capsular types<sup>[13]</sup>. Therefore it is necessary to develop serotype independent vaccines. | ||
Various cell surface proteins have been tested for as potential vaccine candidates however the most probable vaccine candidates are Pneumococcal surface protein A (PspA), Pneumococcal surface adhesin A (PsaA) and Pneumolysin. PspA is a protein virulence factor whose N-terminal domain is surface exposed and it is a member of choline-binding surface proteins<sup>[2][9]</sup>. PspA seems to be specific for bacteria producing choline residues on their surfaces<sup>[9]</sup>. PsaA is a metal binding lipoprotein and part of the ABC transporter complex, which is involved in transport of manganese into pneumococci<sup>[6]</sup>. PsaA has shown to be highly effective in mice models and a combination of PsaA and PspA also gave promising results in mice because PsaA and PspA have different functions in virulence<sup>[2]</sup>. Pneumolysin is also a virulence factor from S.<i>pneumoniae</i>; it is part of the thiol-activated cytolysin family<sup>[8]</sup>. Pneumolysin has a choline-binding domain and one way by which it seems to interfere with host immunity and inflammatory responses is by inhibiting phagocyte function<sup>[2]</sup>. | |||
More recent research has used whole genome approach to detect protein-based pneumococcal vaccines<sup[13]</sup>. In a study done, Morsczeck et.al. (2007)<sup>[13]</sup> used proteomics to identify cell-wall associated proteins, which were screened for vaccine candidates. Further immunization experiments on the 5 selected protein candidates; expressed in E.Coli revealed proteins that were detected in 40 or more different serotypes of S.<i>pneumoniae</i>. This study has selected 2 of the 5 candidates that are lipoate protein ligase (Lpl) and the ClpP protease that have shown a decrease in CFU count and should be used for further investigation. | |||
=Conclusion= | |||
Developing alternative protein vaccines seems like a likely next step to reduce the likelihood of growth of S. pneumoniae. Although the problem of antibiotic resistance and replacement is growing, targeting common denominators across different serotypes will help in eliminating | Developing alternative protein vaccines seems like a likely next step to reduce the likelihood of growth of S. <i>pneumoniae</i>. Although the problem of antibiotic resistance and serotype replacement is growing, targeting common denominators across different serotypes will help in eliminating different strains of S. <i>pneumoniae</i>. However, the disadvantage with using immunogenic proteins is that in vivo they might not work as well. This is because antibodies might not be able to bind to targeted proteins in the cell wall that lie under the capsule polysaccharides<sup>[18]</sup>. A lot of research has yet to be done to prove the efficacy of serotype independent antigens. | ||
=References= | =References= | ||
[http://www.ncbi.nlm.nih.gov/pubmed/10749457 Black. S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen J.R, Elvin L, Ensor K.M, Hackell J, Siber G, Malinoski F, Madore D, Chang I, Kohberger R, Watson W, Austrian R, Edwards K. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern CA Kaiser Permanente Vaccine Study Center Group. The Pediatric Infectious Disease Journal] | 1. [http://www.ncbi.nlm.nih.gov/pubmed/10749457 Black. S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen J.R, Elvin L, Ensor K.M, Hackell J, Siber G, Malinoski F, Madore D, Chang I, Kohberger R, Watson W, Austrian R, Edwards K. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern CA Kaiser Permanente Vaccine Study Center Group. The Pediatric Infectious Disease Journal] | ||
[http://www.swissrhythm.org/2006/escmidschool2006/pdf/edu_mat_2006_16.pdf Bogaert D, Hermans P.W.M, Adrian P.V, Rümke H.C and Groot R. de. 2004. Pneumococcal vaccines: an update on current strategies. Vaccine] | 2. [http://www.swissrhythm.org/2006/escmidschool2006/pdf/edu_mat_2006_16.pdf Bogaert D, Hermans P.W.M, Adrian P.V, Rümke H.C and Groot R. de. 2004. Pneumococcal vaccines: an update on current strategies. Vaccine] | ||
[http://www.ncbi.nlm.nih.gov/pubmed/16517889Beall B, McEllistrem MC, Gertz Jr RE, Wedel S, Boxrud DJ, Gonzalez AL, Medina MJ, Pair R, Thompson TA, Harrison LH, McGee L, Whitney CG and the Active Bacterial Core Surveillance Team. 2006. Pre- and postvaccination clonal compositions of invasive pneumococcal serotypes for isolates collected in the United States in 1999, 2001, and 2002. J Clin Microbiol] | 3. [http://www.ncbi.nlm.nih.gov/pubmed/16517889Beall B, McEllistrem MC, Gertz Jr RE, Wedel S, Boxrud DJ, Gonzalez AL, Medina MJ, Pair R, Thompson TA, Harrison LH, McGee L, Whitney CG and the Active Bacterial Core Surveillance Team. 2006. Pre- and postvaccination clonal compositions of invasive pneumococcal serotypes for isolates collected in the United States in 1999, 2001, and 2002. J Clin Microbiol] | ||
[http://jid.oxfordjournals.org/content/179/Supplement_2/S353.full Chambers H.F. 1999. Penicillin-Binding Protein–Mediated Resistance in Pneumococci and Staphylococci. Journal of Infectious Diseases] | 4. [http://jid.oxfordjournals.org/content/179/Supplement_2/S353.full Chambers H.F. 1999. Penicillin-Binding Protein–Mediated Resistance in Pneumococci and Staphylococci. Journal of Infectious Diseases] | ||
[http://www.ncbi.nlm.nih.gov/pubmed/2480499 Di J D, Wasserman SS, Torres JR, Cortesia MJ, Losonsky GA, Herrington DA, Sturcher D and Levine M. 1989. Effect of priming with carrier on response to conjugate vaccine. Lancet] | 5. [http://www.ncbi.nlm.nih.gov/pubmed/2480499 Di J D, Wasserman SS, Torres JR, Cortesia MJ, Losonsky GA, Herrington DA, Sturcher D and Levine M. 1989. Effect of priming with carrier on response to conjugate vaccine. Lancet] | ||
[[http://www.ncbi.nlm.nih.gov/pubmed/9379902 Dintilhac A, Alloing G, Granadel C and Claverys JP. 1997. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol] | 6. [[http://www.ncbi.nlm.nih.gov/pubmed/9379902 Dintilhac A, Alloing G, Granadel C and Claverys JP. 1997. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol] | ||
[http://www.ncbi.nlm.nih.gov/pubmed/22868187 Espinosa-Padilla SE, Murata C, Estrada-Parra S, Santos-Argumedo L, Mascarenas C, Franco-Paredes C and Espinosa-Rosales F.J. 2012. Immunogenicity of A 23-Valent Pneumococcal Polysaccharide Vaccine Among Mexican Children. Archives of Medical Research] | 7. [http://www.ncbi.nlm.nih.gov/pubmed/22868187 Espinosa-Padilla SE, Murata C, Estrada-Parra S, Santos-Argumedo L, Mascarenas C, Franco-Paredes C and Espinosa-Rosales F.J. 2012. Immunogenicity of A 23-Valent Pneumococcal Polysaccharide Vaccine Among Mexican Children. Archives of Medical Research] | ||
[http://www.sciencedirect.com/science/article/pii/S0022283698921672 Jamie R, Robert JCG, Dennis C, Peter JM, Timothy JM, Arthur JR, Peter WA, James CP, Rodney KT and Michael WP. 1998. The molecular Mechanism of Pneumolysin, a Virulence Factor from Streptococcus pneumoniae. Journal of Molecular Biology] | 8. [http://www.sciencedirect.com/science/article/pii/S0022283698921672 Jamie R, Robert JCG, Dennis C, Peter JM, Timothy JM, Arthur JR, Peter WA, James CP, Rodney KT and Michael WP. 1998. The molecular Mechanism of Pneumolysin, a Virulence Factor from Streptococcus pneumoniae. Journal of Molecular Biology] | ||
[http://www.ncbi.nlm.nih.gov/pubmed/17687514 Jedrzejas M.J. 2007. Unveiling molecular mechanisms of bacterial surface proteins: Streptococcus pneumoniae as a model organism for structural studies. Cell. Mol. Life Sci] | 9. [http://www.ncbi.nlm.nih.gov/pubmed/17687514 Jedrzejas M.J. 2007. Unveiling molecular mechanisms of bacterial surface proteins: Streptococcus pneumoniae as a model organism for structural studies. Cell. Mol. Life Sci] | ||
[http://www.ncbi.nlm.nih.gov/pubmed/21941097 Jefferies JM, Macdonald E, Faust SN and Clarke SC. 2011. 13-valent pneumococcal conjugate vaccine (PCV13). Human Vaccine] | 10. [http://www.ncbi.nlm.nih.gov/pubmed/21941097 Jefferies JM, Macdonald E, Faust SN and Clarke SC. 2011. 13-valent pneumococcal conjugate vaccine (PCV13). Human Vaccine] | ||
[http://www.ncbi.nlm.nih.gov/pubmed/10519943 Kroon FP, van Dissel JT, Ravensbergen E, Nibbering PH, van Furth R. 1999. Antibodies against pneumococcal polysaccharides after vaccination in HIV-infected individuals: 5-year follow-up of antibody concentrations. Vaccine] | 11. [http://www.ncbi.nlm.nih.gov/pubmed/10519943 Kroon FP, van Dissel JT, Ravensbergen E, Nibbering PH, van Furth R. 1999. Antibodies against pneumococcal polysaccharides after vaccination in HIV-infected individuals: 5-year follow-up of antibody concentrations. Vaccine] | ||
[http://jb.asm.org/content/173/21/6986 Laible.G and Hakenbeck. R. 1991. Five Independent Combinations of Mutations Can Result in Low Affinity Penicillin-Binding Protein 2x of Streptococcus pneumoniae. Journal of Bacteriology] | 12. [http://jb.asm.org/content/173/21/6986 Laible.G and Hakenbeck. R. 1991. Five Independent Combinations of Mutations Can Result in Low Affinity Penicillin-Binding Protein 2x of Streptococcus pneumoniae. Journal of Bacteriology] | ||
[http://onlinelibrary.wiley.com/doi/10.1111/j.1469-0691.2007.01878.x/full Morsczeck C, Prokhorova T, Sigh J, Pfeiffer M, Bille-Nielsen M, Petersen J, Boysen A,Kofoed T, Frimodt-Møller N, Nyborg-Nielsen P and Schrotz-King P. 2007. Streptococcus pneumoniae: proteomics of surface proteins for vaccine development. European Society of Clinical Microbiology and Infectious Diseases] | 13. [http://onlinelibrary.wiley.com/doi/10.1111/j.1469-0691.2007.01878.x/full Morsczeck C, Prokhorova T, Sigh J, Pfeiffer M, Bille-Nielsen M, Petersen J, Boysen A,Kofoed T, Frimodt-Møller N, Nyborg-Nielsen P and Schrotz-King P. 2007. Streptococcus pneumoniae: proteomics of surface proteins for vaccine development. European Society of Clinical Microbiology and Infectious Diseases] | ||
[http://www.niaid.nih.gov/topics/vaccines/documents/undvacc.pdf National Institute of Allergy and Infectious Diseases. 2008. Understanding Vaccines: What they are and how they work. National Institutes of Health] | 14. [http://www.niaid.nih.gov/topics/vaccines/documents/undvacc.pdf National Institute of Allergy and Infectious Diseases. 2008. Understanding Vaccines: What they are and how they work. National Institutes of Health] | ||
[http://www.niaid.nih.gov/topics/vaccines/understanding/pages/howwork.aspx National Institute of Allergy and Infectious Diseases. 2011. How do vaccines work? National Institutes of Health] | 15. [http://www.niaid.nih.gov/topics/vaccines/understanding/pages/howwork.aspx National Institute of Allergy and Infectious Diseases. 2011. How do vaccines work? National Institutes of Health] | ||
[http://www.cdc.gov/mmwr/pdf/rr/rr4608.pdf Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). 1997. MMWR Morb Mortal Wkly Rep] | 16. [http://www.cdc.gov/mmwr/pdf/rr/rr4608.pdf Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). 1997. MMWR Morb Mortal Wkly Rep] | ||
[http://www.ncbi.nlm.nih.gov/pubmed/10194822 Poland GA. 1999. The burden of pneumococcal disease: the role of conjugate vaccines. Vaccine] | 17. [http://www.ncbi.nlm.nih.gov/pubmed/10194822 Poland GA. 1999. The burden of pneumococcal disease: the role of conjugate vaccines. Vaccine] | ||
[http://www2.sph.unc.edu/images/stories/academic_programs/epid/documents/Qualifying_Exams/Infectious_Disease/Readings/pletz_int_j_antimicrobial_agents_2008.pdf Pletz. MW, Maus U, Krug N, Welte T and Lode H. 2008 Pneumococcal vaccines: mechanism of action, impact on epidemiology and adaption of the species. International Journal of Antimicrobial Agents] | 18. [http://www2.sph.unc.edu/images/stories/academic_programs/epid/documents/Qualifying_Exams/Infectious_Disease/Readings/pletz_int_j_antimicrobial_agents_2008.pdf Pletz. MW, Maus U, Krug N, Welte T and Lode H. 2008 Pneumococcal vaccines: mechanism of action, impact on epidemiology and adaption of the species. International Journal of Antimicrobial Agents] | ||
[http://www.ncbi.nlm.nih.gov/pubmed/11072934 Spratt BG, Greenwood BM. 2000. Prevention of pneumococcal disease by vaccination: does serotype replacement matter? Lancet] | 19. [http://www.ncbi.nlm.nih.gov/pubmed/11072934 Spratt BG, Greenwood BM. 2000. Prevention of pneumococcal disease by vaccination: does serotype replacement matter? Lancet] | ||
Slonczewski, J.L and Foster J. 2009. Microbiology. 2nd. W.W.Norton and Company. | 20. Slonczewski, J.L and Foster J. 2009. Microbiology. 2nd. W.W.Norton and Company. | ||
[http://www.sciencedirect.com/science/article/pii/S0264410X09019719 Vestrheim DF, Høiby EA, Bergsaker MR, Rønning K, Aaberge IS and Caugant DA. 2010. Indirect effect of conjugate pneumococcal vaccination in a 2 + 1 dose schedule. Vaccine] | 21. [http://www.sciencedirect.com/science/article/pii/S0264410X09019719 Vestrheim DF, Høiby EA, Bergsaker MR, Rønning K, Aaberge IS and Caugant DA. 2010. Indirect effect of conjugate pneumococcal vaccination in a 2 + 1 dose schedule. Vaccine] | ||
[http://www.who.int/wer/pdf/1999/wer7423.pdf World Health Organization. 1999. Pneumococcal vaccines. Weekly Epidemiological Record] | 22. [http://www.who.int/wer/pdf/1999/wer7423.pdf World Health Organization. 1999. Pneumococcal vaccines. Weekly Epidemiological Record] | ||
[http://www.plospathogens.org/article/info%3Adoi%2F10.1371%2Fjournal.ppat.1000476 Weinberger DM, Trzcinski K, Lu Y-J, Bogaert D, Brandes A, Galagan J, Anderson PW, Malley R and Lipsitch M. 2009. Pneumococcal Capsular Polysaccharide Structure Predicts Serotype Prevalence. PLoS Pathog] | 23. [http://www.plospathogens.org/article/info%3Adoi%2F10.1371%2Fjournal.ppat.1000476 Weinberger DM, Trzcinski K, Lu Y-J, Bogaert D, Brandes A, Galagan J, Anderson PW, Malley R and Lipsitch M. 2009. Pneumococcal Capsular Polysaccharide Structure Predicts Serotype Prevalence. PLoS Pathog] | ||
[http://www.ncbi.nlm.nih.gov/pubmed/21492929 Weinberger DM, Malley R and Lipsitch M. 2012. Serotype replacement in disease following pneumococcal vaccination: A discussion of the evidence. The Lancet] | 24. [http://www.ncbi.nlm.nih.gov/pubmed/21492929 Weinberger DM, Malley R and Lipsitch M. 2012. Serotype replacement in disease following pneumococcal vaccination: A discussion of the evidence. The Lancet] | ||
Latest revision as of 18:52, 29 September 2015
Introduction
Streptococcus pneumoniae is a pathogenic, gram-positive, α-hemolytic, anaerobic bacterium[20]. It causes pneumonia along with other pneumococcal infections some of which include bacterial meningitis, sinusitis, and otitis media[18][20]. S. pneumoniae has developed antibiotic resistance to traditional β-Lactam antibiotics such as Penicillin (and its derivatives). Antibiotic resistance is acquired by alteration of the targeted penicillin binding proteins (PBPs) in the resistant strains that lower its affinity to bind to penicillin[20]. Genome sequencing of the penicillin resistant strains show that point mutations in one of its penicillin binding proteins,PBP2x, decreases its affinity to bind to penicillin[12].
Due to the increasing rate of antibiotic resistance it is important to study newer ways to inhibit S.pneumoniae growth. This is because antibiotics select for growth of rare microorganisms in a population that is otherwise susceptible to the drug[20]. Antibiotic resistance can be overcome by developing vaccines that target virulence factors on the surface of S.pneumoniae thereby disabling the bacteria[9]. Vaccines work by giving the organism immunity against infection by a particular pathogen. Vaccines contain a weakened or dead derivative of the pathogen and in its altered state, vaccine pathogens, are typically safe and unable to cause disease15. The non-virulent pathogens in the vaccine stimulate the organisms B cells to make antibodies for the particular antigens thereby developing immunity to that pathogen[15]. Subsequently, Memory T cells get stimulated upon re-exposure to cognate antigen and rapidly multiply thereby providing “memory” to the body’s immune system [15]. In addition to the weakened or dead form of bacteria or virus, the vaccine also contains antibiotics or preservatives to protect the body against any germs that might get into the vaccine[14].
Current pneumococcal vaccines target the polysaccharide capsule because immunity after pneumococcal disease is directed against the capsular serotype of the S.pneumoniae bacteria involved[22]. Serotype is a characteristic set of antigens that help to distinguish a group of closely related microorganisms. 91 serotypes of S.pneumoniae have been identified based on the difference in composition of the polysaccharide capsule[18]. The polysaccharide capsule is a virulence factor however; only some of the 91 serotypes cause pneumococcal disease. Current pneumococcal vaccines, Polysaccharide (23-valent) PS vaccine and conjugate vaccine (PCV-7) cover the most common and virulent serotypes and give immunity against 23 and 7 serotypes respectively. However, given the large number of serotypes (~91 serotypes), serotype replacement, which is replacement of vaccine serotype (VTs) by non-vaccine serotypes (NVTs) in disease and in carriage has become a growing concern thereby making vaccination against current serotypes less effective[2][18].
Current pneumococcal vaccines
As an alternative to β-lactam antibiotics, vaccines have been created that target cell surface polysaccharide, which aim to develop immunity against the virulent serotypes of S. pneumoniae. The two vaccines widely used are polysaccharide vaccine (23-valent polysaccharide vaccine) and pneumococcal conjugate vaccine (heptavalent protein–polysaccharide conjugate vaccine, PCV-7) [2]. The polysaccharide vaccine provides immunity against 23 S.pneumoniae serotypes (Table 3.) and is effective in older immunocompetent patients [2][18]. However, this vaccine cannot be administered to infants below 2 years of age and to immunodeficient patients perhaps due to an immature or a sensitive immune system [18]. The newer conjugate vaccine, PCV-7 is able to provide immunity to this age group [2][18].
23-valent polysaccharide (PS) vaccine
The 23-valent PS vaccine contains capsular polysaccharide antigens for 23 serotypes (Table 3.) and to gain immunity the polysaccharides induce B cell dependant response by releasing polysaccharide Immunoglobulin M (IgM), which are a type of antibodies produced by B cells [18]. This PS vaccine does not induce a T cell-dependant immune response. As mentioned above, T cells provide ‘memory’ to the body’s immune system [15] so antibodies can be made quickly upon re-exposure to cognate antigen. Also this vaccine cannot be administered to immunodeficient patients (Table 1.), HIV infected individuals have show an impaired antibody response to PS vaccination [11]. Further advantages and disadvantages are listed in (Table 1.)
A study done in Mexico evaluating the immune response of this vaccine in children under 5, for 6 serotypes, suggests that the pneumococcal PS vaccine produced adequate immunogenicity in the given age groups [18]. However, this vaccine is not effective in children under 2 years of age [1][7]. Therefore, a more comprehensive vaccine is still needed.
Heptavalent protein–polysaccharide conjugate (PCV-7) vaccine
Unlike the 23-valent polysaccharide vaccine, which can only be used in children over 2 years, the conjugate vaccine can be administered to infants as young as 2 months old [2][13][18]. Polysaccharides in this vaccine are from seven serotypes that are most frequently involved in infant infections (Table 3.) [10][18]. As the name suggests this vaccine is conjugated to protein (CRM197), which is a non-toxic diphtheria toxoid protein[18]. The protein-specific type 2 helper T cells associate with B cells, which are bound to the polysaccharide-protein complex via a polysaccharide specific IgM[18]. This association between T and B cells presents the processed protein (CRM197) along with class II MHC (Major Histocompatibility Complex) to the effector T cells[18]. Class II MHCs are cell-surface molecules that mediate interaction between immune cells and body cells. The antigens from Class II peptides come from extracellular proteins. This mechanism leads to adaptive immunity in infants, which is switching of antibody isotype and generation of memory B-cells [18].
Advanatages and Disadvantages of Pcv-7 vaccine are listed in Table 2. Early maturation of antipolysacchrides antibodies allows for use in infants under 2 years of age (Table2.). This vaccine is also more immunogenic that PS vaccine because capsular polysacchrides are bound covalently (or via reactive groups) to protein carriers[17]. The bound protein helps in eliciting a T cell dependent response, which induces memory of B cells [2]. The PCV-7 vaccine also affects carrier rate by decreasing nasopharyngeal carriage of vaccine serotype, which suggests that conjugate vaccine provides both, systemic (throughout the body) and mucosal immunity[2][19]. Obtaining specific mucosal immunity is important because it allows asymptomatic carriers to eliminate colonizing of vaccine serotypes thereby decreasing carrier rate[18]. However, this vaccine is not cost effective (Table 2.), it is expensive to develop and does not cover the major pneumococcal disease causing serotypes (1 and 5) in developing countries[10][13]. There is also a limit to the amount of polysaccharide-protein linkages that can be formed. Too many carrier antigens used can impair antibody response to the antigens[5].
Combined use of 23-Valent PS and PCV-7
After the newer PCV-7 vaccine was developed, the best form of immunity was seen in patients that took the conjugate vaccine followed by the PS vaccine[2]. A combined vaccination program is recommended for children 2-5 years by the Advisory Committee on Immunization Practices (ACIP). According to the ACIP, PCV-7 vaccine should be followed by the use of PS vaccine as a booster[2]. This is perhaps because PCV-7 confers immunity in children from the invasive serotypes and later the PS vaccine provides immunity against additional serotypes not covered by PCV-7.
Serotype Replacement
Although antibiotic resistance has been a growing concern, use of vaccines against the major virulent serotypes has giving rise to a new problem that is serotype replacement. Serotype replacement is the processes in which pre-existing clones of non-vaccine serotypes (NVTs) can multiply and essentially replace the serotypes for which there is vaccination [2].Replacement occurs because vaccines eradicate particular serotypes, this creates a niche for non-vaccine serotypes, allowing them to proliferate [2][18].
Increase in non-vaccine serotypes (NVTs)
Studies have shown that with the administration of conjugate vaccine combined with re-immunization by 23-valent PS vaccine, reduces the prevalence of nasopharyngeal carriage of vaccine serotypes i.e. gives mucosal immunity[19]. However, there has not been any significant reduction in the frequency of pneumococcal carriage because of ‘near-complete’ replacement with pneumococci of NVTs[19][24]. Beall et. al. 2006[3] genotyped invasive isolates, which were collected from patients after the administration of vaccine and compared it to isolates collected from patients that had not been administered PCV7. They found that in the non-PCV7 serogroup emergence of new serotypes was uncommon however, there was a significant increase in the proliferation of the ‘pre-existing clones’ of non-vaccine serotypes[3]. These results suggests that increase in NVTs is not due to the emergence of new serotypes but instead is from the expansion of already established serotypes from which the vaccine gives no immunity.
Near-complete replacement in carriage Vs. Partial replacement for disease
There has been no significant decrease in the carriage of S.pneumonia because of the increase in the prevalence of NVTs in asymptotic carriers[24]. As mentioned above, Beall et. al. 2006[3] found that vaccinated serogroups saw an increase of the preexisting NVTs. Randomized trials in South Africa, Netherlands and The Gambia show that NVTs have almost completely replaced vaccinated serotypes in carriage[3][19][24]. Moreover, the presence of NVTs, like serotype 19A, has led to an increase in the number of diseases caused by NVTs[21]. Study done by Vestrheim et. al. 2010[21] on the total Norwegian population from 1989-2008 suggests that once the conjugate vaccine was administered in 2006 the rate of invasive pneumococcal disease (IPD) caused by NVTs 19A, 9N, 33F and 35B in children under 5 increased significantly[21]. Moreover, IPD caused by 19A increased till 2007, after the conjugate vaccine was administered, suggesting that the increase in IPD by NVTs is due to serotype replacement and not due to an unrelated trend[21]. However, the increase in IPD by NVTs has been modest and the rate of NVT replacement in carriage far exceeds the replacement in pneumococcal diseases caused by NVTs[24].
The discrepancy in NVTs completely replacing carriage but only somewhat replacing for IPD is due to a combination of various factors:
• Difference in microbiological properties such as varying capsular size, adhesion, toxins and proteins of serotypes play a role in influencing carriage and invasiveness[24]. Serotypes that persist in carriage (NVTs) after vaccination tend to be heavily capsulated[24].
• NVTs are less invasive than VTs therefore they are present in carriage form but do not cause pneumococcal disease[24].
• Effect of ‘unmasking’ of the NVTs due to the reduction in VTs post vaccination, it is easier to detect new serotypes post-vaccination than when vaccine serotypes were present[24].
Current ways to prevent Serotype Replacement
1. Regularly monitoring and developing new conjugate vaccine
13-valent conjugated pneumococcal vaccine (PCV 13) is the newer version of PCV-7 conjugate vaccine and has a broader coverage. It consists of thirteen pneumococcal capsular polysaccharides, which are individually conjugated to the diphtheria-derived protein carrier CRM (197)[10]. PCV-13 includes 13 serotypes (Table 3.) and will replace PCV-7 particularly because it includes serotype 19A, which was a virulent NVT. It is a more cost effective option for developing countries because unlike PCV-7, it also covers serotypes 1 and 5, which cause majority of pneumococcal diseases in developing countries[10]. Although PCV-13 is a better version of PCV-7 it is not antigenically covered across all serotypes, a newer form of this vaccine might be required in the future to address diseases caused by other NVT.
2. Identification of serotype independent antigens
Pneumococcal surface proteins, which have serotype-independence, can be used as an alternative to vaccine candidates[2]. Current pneumococcal vaccines fail to cover all polysaccharide types and infections in adults particularly, is caused by strains that have a variety of capsular types[13]. Therefore it is necessary to develop serotype independent vaccines.
Various cell surface proteins have been tested for as potential vaccine candidates however the most probable vaccine candidates are Pneumococcal surface protein A (PspA), Pneumococcal surface adhesin A (PsaA) and Pneumolysin. PspA is a protein virulence factor whose N-terminal domain is surface exposed and it is a member of choline-binding surface proteins[2][9]. PspA seems to be specific for bacteria producing choline residues on their surfaces[9]. PsaA is a metal binding lipoprotein and part of the ABC transporter complex, which is involved in transport of manganese into pneumococci[6]. PsaA has shown to be highly effective in mice models and a combination of PsaA and PspA also gave promising results in mice because PsaA and PspA have different functions in virulence[2]. Pneumolysin is also a virulence factor from S.pneumoniae; it is part of the thiol-activated cytolysin family[8]. Pneumolysin has a choline-binding domain and one way by which it seems to interfere with host immunity and inflammatory responses is by inhibiting phagocyte function[2].
More recent research has used whole genome approach to detect protein-based pneumococcal vaccines<sup[13]. In a study done, Morsczeck et.al. (2007)[13] used proteomics to identify cell-wall associated proteins, which were screened for vaccine candidates. Further immunization experiments on the 5 selected protein candidates; expressed in E.Coli revealed proteins that were detected in 40 or more different serotypes of S.pneumoniae. This study has selected 2 of the 5 candidates that are lipoate protein ligase (Lpl) and the ClpP protease that have shown a decrease in CFU count and should be used for further investigation.
Conclusion
Developing alternative protein vaccines seems like a likely next step to reduce the likelihood of growth of S. pneumoniae. Although the problem of antibiotic resistance and serotype replacement is growing, targeting common denominators across different serotypes will help in eliminating different strains of S. pneumoniae. However, the disadvantage with using immunogenic proteins is that in vivo they might not work as well. This is because antibodies might not be able to bind to targeted proteins in the cell wall that lie under the capsule polysaccharides[18]. A lot of research has yet to be done to prove the efficacy of serotype independent antigens.
References
17. Poland GA. 1999. The burden of pneumococcal disease: the role of conjugate vaccines. Vaccine
20. Slonczewski, J.L and Foster J. 2009. Microbiology. 2nd. W.W.Norton and Company.
22. World Health Organization. 1999. Pneumococcal vaccines. Weekly Epidemiological Record