Borrelia burgdorferi and Lyme Disease: Difference between revisions
Bartholomewa (talk | contribs) |
Bartholomewa (talk | contribs) |
||
| (40 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==History== | ==History== | ||
The first case of the Lyme disease was in Lyme, Connecticut in 1975. The disease presented arthritic-like symptoms, and was therefore referred to as Lyme arthritis. The deer tick, <i>Ixodes scapularis</i>, was associated with the transmission of the disease in 1977, but the cause of the disease remained unknown until Willy Burgdorferi discovered <i>Borrelia burgdorferi</i> in 1981. The disease is caused by three species of bacteria all belonging to <i>Borrelia</i>-<i>Borrelia burgdorferi</i>, <i>Borrelia afzelii</i>, and <i>Borrelia garinii</i>. <i>Borrelia burgdorferi</i> is the main cause of Lyme disease in North America, where the other two species affect Europe | The first case of the Lyme disease was in Lyme, Connecticut in 1975. The disease presented arthritic-like symptoms, and was therefore referred to as Lyme arthritis. The deer tick, <i>Ixodes scapularis</i>, was associated with the transmission of the disease in 1977, but the cause of the disease remained unknown until Willy Burgdorferi discovered <i>Borrelia burgdorferi</i> in 1981. The disease is caused by three species of bacteria all belonging to <i>Borrelia</i>-<i>Borrelia burgdorferi</i>, <i>Borrelia afzelii</i>, and <i>Borrelia garinii</i>. <i>Borrelia burgdorferi</i> is the main cause of Lyme disease in North America, where the other two species affect Europe[1, 2].<br> | ||
==<i>Borrelia burgdorferi</i> Description and Structure== | ==<i>Borrelia burgdorferi</i> Description and Structure== | ||
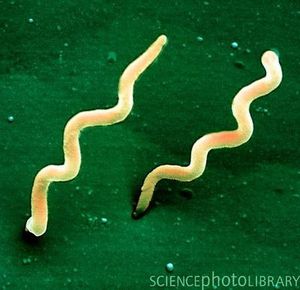
[[Image:Bburgdorferi.jpg|thumb|300px|right|<i>Borrelia burgdorferi</i> bacterium.]] | [[Image:Bburgdorferi.jpg|thumb|300px|right|Figure 1. <i>Borrelia burgdorferi</i> bacterium.[https://link http://www.niaid.nih.gov/topics/lymedisease/understanding/pages/intro.aspx]] | ||
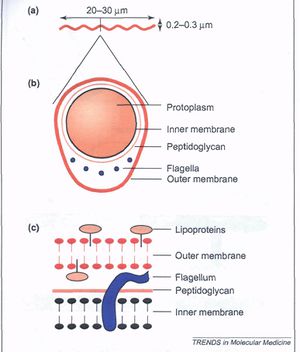
<i>Borrelia burgdorferi</i> is a Gram-negative bacterium belonging to the class Spirochaetes. This bacterium is helical and has both an inner and outer membrane as well as a flexible cell wall. The cell is usually | <i>Borrelia burgdorferi</i> is a Gram-negative bacterium belonging to the class Spirochaetes [Figure 1] . This bacterium is helical and has both an inner and outer membrane as well as a flexible cell wall. The cell is usually 1um wide, but can be up to 10-25 um long. Bacteria of the class spirochaetes have flagella located on the inside of the periplasm in between the inner and outer membranes. The flagellum along with the helical structure of the bacterium allows the bacterium to migrate through viscous fluids and burrow through various tissues. As a result, this cell is highly invasive. <i>B. burgdorferi</i> is also known for its outer surface proteins OspA and OspC have been studied extensively and have a role in transmission of the bacteria into the host cell. | ||
The metabolism of this cell is limited, therefore; <i>B. burgdorferi</i> relies on their host for energy precursors. Its genome encodes transport proteins such as ABC transporters. <i>B. burgdorferi</i> also codes for enzymes and proteins that are used in the phosphotransferase system. The host serum as well as the environment are targets for these transport systems. The genome of this bacterium is distinctive. It consists of one linear chromosome of 910,725 base pairs long with at least 17 linear and circular plasmids that combine to a size of more than 533,000 base pairs | The metabolism of this cell is limited, therefore; <i>B. burgdorferi</i> relies on their host for energy precursors. Its genome encodes transport proteins such as ABC transporters. <i>B. burgdorferi</i> also codes for enzymes and proteins that are used in the phosphotransferase system. The host serum as well as the environment are targets for these transport systems. The genome of this bacterium is distinctive. It consists of one linear chromosome of 910,725 base pairs long with at least 17 linear and circular plasmids that combine to a size of more than 533,000 base pairs [2] . As a result of its large number of plasmids, the genetic organization of <i>B. burgdorferi</i> is specialized. | ||
.<br> | .<br> | ||
==What is Lyme Disease?== | ==What is Lyme Disease?== | ||
[[Image:deertick.jpg|thumb|300px|right|The deer tick, <i>Ixodes scapularis</i>, carries <i>Borrelia burgdorferi</i> and an infected tick transmits the bacteria into a mammalian host through a tick bite.]] | [[Image:deertick.jpg|thumb|300px|right|Figure 2. The deer tick, <i>Ixodes scapularis</i>, carries <i>Borrelia burgdorferi</i> and an infected tick transmits the bacteria into a mammalian host through a tick bite.[https://link http://extension.entm.purdue.edu/publichealth/insects/tick.html]] | ||
Lyme disease is an infectious disease caused by the spirochete <i>Borrelia burgdorferi</i> that is carried in deer tick <i>Ixodes scapularis</i>. This disease is the most common vector borne disease in the United States, affecting far more individuals than West Nile virus. It has been estimates that there were nearly 40,000 cases of Lyme reported in 2009, a large underestimate. The Center for Disease Control estimates the true number of cases to be as much as 12 times higher, making the estimated total as high as 480,000. This estimate makes Lyme disease more prevalent than AIDS. Moreover, national surveillance began in 1982, which increased the number of cases reported nearly 25-fold | Lyme disease is an infectious disease caused by the spirochete <i>Borrelia burgdorferi</i> that is carried in deer tick <i>Ixodes scapularis</i>. This disease is the most common vector borne disease in the United States, affecting far more individuals than West Nile virus. It has been estimates that there were nearly 40,000 cases of Lyme reported in 2009, a large underestimate. The Center for Disease Control estimates the true number of cases to be as much as 12 times higher, making the estimated total as high as 480,000. This estimate makes Lyme disease more prevalent than AIDS. Moreover, national surveillance began in 1982, which increased the number of cases reported nearly 25-fold [3]. | ||
Lyme disease is most commonly transmitted to humans through the bite of an infected tick as it bites. Without treatment, <i>B. burgdorferi</i> is able to migrate through the bloodstream and many connective tissues. In it’s early stages, Lyme disease affects the skin through skin rash and in later stages spreads to the joints, nervous system, and other organ systems in the later stages. If caught and treated early, Lyme disease is almost always cured. However, this disease has a high rate of progression and if it is not treated early, symptoms may last for months or even years. Unfortunately, thousands of patients go undiagnosed or misdiagnosed with doctors telling them their symptoms are in their head due to the difficulty in testing. Part of how this disease goes untreated and misdiagnosed is due to the characteristics of the burrowing bacterium, <i>Borrelia burgdorferi</i>. This bacterium has become an expert at hiding and surviving in human tissues, and its ability to take on different forms such as cysts, which allows the bacterium to escape the immune system, avoid antibiotics, and hide from detection by blood tests. The mechanism of transmission is poorly understood, but for many bacterial pathogens such as <i>B. burgdorferi</i>, the initial step in colonizing host tissues involves the expression of adhesive molecules that facilitate bacterial adherence to host cells or to the extracellular matrix. | Lyme disease is most commonly transmitted to humans through the bite of an infected tick as it bites [ Figure 2]. Without treatment, <i>B. burgdorferi</i> is able to migrate through the bloodstream and many connective tissues. In it’s early stages, Lyme disease affects the skin through skin rash and in later stages spreads to the joints, nervous system, and other organ systems in the later stages. If caught and treated early, Lyme disease is almost always cured. However, this disease has a high rate of progression and if it is not treated early, symptoms may last for months or even years. Unfortunately, thousands of patients go undiagnosed or misdiagnosed with doctors telling them their symptoms are in their head due to the difficulty in testing. Part of how this disease goes untreated and misdiagnosed is due to the characteristics of the burrowing bacterium, <i>Borrelia burgdorferi</i>. This bacterium has become an expert at hiding and surviving in human tissues, and its ability to take on different forms such as cysts, which allows the bacterium to escape the immune system, avoid antibiotics, and hide from detection by blood tests. The mechanism of transmission is poorly understood, but for many bacterial pathogens such as <i>B. burgdorferi</i>, the initial step in colonizing host tissues involves the expression of adhesive molecules that facilitate bacterial adherence to host cells or to the extracellular matrix. | ||
<br> | <br> | ||
==Symptoms== | ==Symptoms== | ||
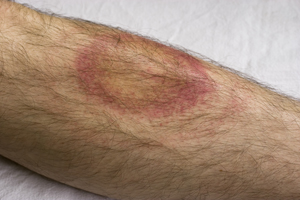
[[Image:lymeRash.jpg|thumb|300px|right|Erythema migrans, often the first symptom of Lyme disease.]] | [[Image:lymeRash.jpg|thumb|300px|right|Figure 3. Erythema migrans, often the first symptom of Lyme disease.]] | ||
<br>Lyme disease almost always first presents itself as a skin rash called erythema migrans. The rash most likely originates from the site of the tick bite and spreads. The rash can be a solid expanding red spot, or it can be a single red spot surrounded by lighter red skin resembling a bulls-eye. The skin rash is visible 1 to 2 weeks after the disease has been transmitted from the tick, and lasts for 3 to 5 weeks. Ticks are able to attach anywhere on the body, but-armpit, groin, back of the knee, nape-body creases are what is most common. Symptoms that can present themselves at the time of the rash include, joint pain, chills, fever, and fatigue. As the spirochete spreads throughout the body, more sever symptoms begin to surface. The more serious symptoms include tingling or numbness in the extremities, paralysis, severe fatigue and a stiff neck. Severe headaches, chronic arthritis, swelling of joints, cardiac abnormalities and mental disorders can appear weeks, months, and in some cases, years after being bitten. <br> | <br>Lyme disease almost always first presents itself as a skin rash called erythema migrans [Figure 3]. The rash most likely originates from the site of the tick bite and spreads. The rash can be a solid expanding red spot, or it can be a single red spot surrounded by lighter red skin resembling a bulls-eye. The skin rash is visible 1 to 2 weeks after the disease has been transmitted from the tick, and lasts for 3 to 5 weeks. Ticks are able to attach anywhere on the body, but-armpit, groin, back of the knee, nape-body creases are what is most common. Symptoms that can present themselves at the time of the rash include, joint pain, chills, fever, and fatigue. As the spirochete spreads throughout the body, more sever symptoms begin to surface. The more serious symptoms include tingling or numbness in the extremities, paralysis, severe fatigue and a stiff neck. Severe headaches, chronic arthritis, swelling of joints, cardiac abnormalities and mental disorders can appear weeks, months, and in some cases, years after being bitten. <br> | ||
==Treatment== | ==Treatment== | ||
| Line 22: | Line 22: | ||
==Adhesion Mechanisms== | ==Adhesion Mechanisms== | ||
[[Image:structure.jpg|thumb|300px|right|The Structure of <i>B. burgdorferi</i>. (A) illustrates the general shape of the bacterium. (B) represents a cross-sectional view. (C) shows in detail the inner and outer membranes separated by a peptidoglycan layer.]] | [[Image:structure.jpg|thumb|300px|right|Figure 4. The Structure of <i>B. burgdorferi</i>. (A) illustrates the general shape of the bacterium. (B) represents a cross-sectional view. (C) shows in detail the inner and outer membranes separated by a peptidoglycan layer. [https://link http://campother.blogspot.com/2012/02/memo-borrelia-are-not-gram-negative.html]] | ||
<br>The pathogenic bacterium <i>Borrelia burgdorferi</i> is the spirochete that causes Lyme disease. These spirochetes have distinctive characteristics that allow them to cause chronic infection and remain in an infected human. Many researchers have focused on the outer surface being proteins of <i>B. burgdorferi</i> as these proteins mediate the interaction between the bacteria and the human host. Adhesion to mammalian and tick substrates may have a role in the host response to the bacteria as well as evasion from the host immune system | <br>The pathogenic bacterium <i>Borrelia burgdorferi</i> is the spirochete that causes Lyme disease. These spirochetes have distinctive characteristics that allow them to cause chronic infection and remain in an infected human. Many researchers have focused on the outer surface being proteins of <i>B. burgdorferi</i> as these proteins mediate the interaction between the bacteria and the human host [Figure 5.] Adhesion to mammalian and tick substrates may have a role in the host response to the bacteria as well as evasion from the host immune system [4]. The following agents of <i>Borrelia burgdorferi</i> have been studied in hopes of understanding the mechanisms that allow these bacteria to cause infection, and in some cases disease. There are a few characteristics of this bacterium that caught the attention of many researchers. First of all, this bacterium has a large number of adhesion genes for its small (1.5 Mbp) genome. 7.8% of the genome encodes known or predicted lipoproteins, where some have been classified as adhesions [5]. Within the small genome, many proteins that bind to either mammalian or tick cells or extracellular matrix have been discovered and contribute to the regime of these bacteria.<br> | ||
<br><i>Borrelia burgdorferi</i> has numerous proteins that support an interaction with the extracellular components of the host cell. Different adhesions of <i>Borrelia burgdorferi</i> will be reviewed here to understand the characteristics of this bacterium and the disease it causes.<br> | <br><i>Borrelia burgdorferi</i> has numerous proteins that support an interaction with the extracellular components of the host cell. Different adhesions of <i>Borrelia burgdorferi</i> will be reviewed here to understand the characteristics of this bacterium and the disease it causes.<br> | ||
[[Image:adhesion.jpg|thumb|300px|right| The interaction of <i>Borrelia burgdorferi</i> with a host cell.]] | [[Image:adhesion.jpg|thumb|300px|right|Figure 5. The interaction of <i>Borrelia burgdorferi</i> with a host cell.[https://link http://neurowiki2012.wikispaces.com/Lyme+Disease+and+Neuroborreliosis]] | ||
<br><u>Interaction with Extracellular Matrix</u><br> | <br><u>Interaction with Extracellular Matrix</u><br> | ||
<br>Fibronectin (Fn) is a large, dimeric glycoprotein and is produced by numerous cell types. It can exist as a soluble molecule as well as an insoluble molecule, and can therefore reside in body fluids as well as in the extracellular matrix. Pertaining to its structure, fibronectin is made up of three types of protein modules that are arranged in different domains. Through these multiple domains, Fn is able to bind to many trans-membrane receptors as well as other extracellular components including, collagen, fibrinogen, and proteoglycans. However, Fn specifically encourages cell adhesion, growth, migration, and differentiation. Moreover, Fn plays an important role in wound healing and embryonic development. <i>Borrelia burgdorferi</i> synthesizes multiple Fn-binding adhesions, with BBK32 being the binding protein best characterized. BBK32 gene has been isolated from <i>B. burgdorferi</i> and has an Fn-binding region that has been localized to 32 amino acids and was found in <i>B. burgdorferi</i> sense stricto, B. garinii, and B. afzelii, which are strains known to cause disease in humans. In the isolate, BBK32 remained on the outer surface and the adhesion specifically interacted with the collagen-binding domain of fibronectin. The binding of BBK32 to Fn relates <i>B. burgdorferi</i> to Fn-binding adhesions of gram-positive pathogens Staphylococcus aureus and Staphylococcus pyogenes. In relation to Lyme disease, in <i>B. burgdorferi</i> BBK32 is expressed as the tick feeds and while the bacteria are in the mammalian host | <br>Fibronectin (Fn) is a large, dimeric glycoprotein and is produced by numerous cell types. It can exist as a soluble molecule as well as an insoluble molecule, and can therefore reside in body fluids as well as in the extracellular matrix. Pertaining to its structure, fibronectin is made up of three types of protein modules that are arranged in different domains. Through these multiple domains, Fn is able to bind to many trans-membrane receptors as well as other extracellular components including, collagen, fibrinogen, and proteoglycans. However, Fn specifically encourages cell adhesion, growth, migration, and differentiation. Moreover, Fn plays an important role in wound healing and embryonic development. <i>Borrelia burgdorferi</i> synthesizes multiple Fn-binding adhesions, with BBK32 being the binding protein best characterized. BBK32 gene has been isolated from <i>B. burgdorferi</i> and has an Fn-binding region that has been localized to 32 amino acids and was found in <i>B. burgdorferi</i> sense stricto, B. garinii, and B. afzelii, which are strains known to cause disease in humans. In the isolate, BBK32 remained on the outer surface and the adhesion specifically interacted with the collagen-binding domain of fibronectin. The binding of BBK32 to Fn relates <i>B. burgdorferi</i> to Fn-binding adhesions of gram-positive pathogens Staphylococcus aureus and Staphylococcus pyogenes. In relation to Lyme disease, in <i>B. burgdorferi</i> BBK32 is expressed as the tick feeds and while the bacteria are in the mammalian host [6,2].<br> | ||
<br><u>Adhesion to Host cell</u><br> | <br><u>Adhesion to Host cell</u><br> | ||
<br>Decorin is a proteoglycan with a protein core that consists primarily of leucine rich repeats and a glycosaminoglycan protein. These proteins function as structural components of the extracellular matrix as well as signaling mediators. The borrelial decorin-binding proteins, DbpA and DbpB, have been discovered and identified as targets for host cell decorin. These surface lipoproteins are coded by the dbpB/A operon, Decorin binds collagen, which is found in many tissues as a part of the connective tissue. This relationship allows <i>B. burgdorferi</i> to interact with connective tissues. Studies have shown that the expression of both proteins is increased in response to a reduced pH and by a shift in temperature from 23 to 37 ° C, which illustrates their role in the mammalian environment. When DbpA and DbpB are expressed in <i>B. burgdorferi</i> strain lacking the gene that codes for the dbpB/A operon, lp54, the strain was able to bind mammalian epithelial cells. However, when the proteins are not expressed, no binding can occur | <br>Decorin is a proteoglycan with a protein core that consists primarily of leucine rich repeats and a glycosaminoglycan protein. These proteins function as structural components of the extracellular matrix as well as signaling mediators. The borrelial decorin-binding proteins, DbpA and DbpB, have been discovered and identified as targets for host cell decorin. These surface lipoproteins are coded by the dbpB/A operon, Decorin binds collagen, which is found in many tissues as a part of the connective tissue. This relationship allows <i>B. burgdorferi</i> to interact with connective tissues. Studies have shown that the expression of both proteins is increased in response to a reduced pH and by a shift in temperature from 23 to 37 ° C, which illustrates their role in the mammalian environment. When DbpA and DbpB are expressed in <i>B. burgdorferi</i> strain lacking the gene that codes for the dbpB/A operon, lp54, the strain was able to bind mammalian epithelial cells. However, when the proteins are not expressed, no binding can occur [2].<br> | ||
<br>Many studies have researched the role of DbpA and DbpB in the life cycle of <i>B. burgdorferi</i> and their contribution to Lyme disease. Brown et. Al. reported the multiplication of bacterial organisms to be significantly lower in decorin-deficient mice compared to wild-type mice | <br>Many studies have researched the role of DbpA and DbpB in the life cycle of <i>B. burgdorferi</i> and their contribution to Lyme disease. Brown et. Al. reported the multiplication of bacterial organisms to be significantly lower in decorin-deficient mice compared to wild-type mice [7]. Moreover, Shi et al. found that these proteins are not necessary for causing an infection, but are critical for the overall virulence of the bacteria [8]. In regards to Lyme disease, decorin binding proteins play a strong role in the later stages of disease-during dissemination and while establishing chronic infections in decorin rich tissues.<br> | ||
<br>In addition to decorin, there has been evidence of binding activity with more generalized proteoglycans (PGs). Even though PG binding accounts for some of <i>B. burgdorferi</i> binding activity, it does not account for all. With PG binding, recognizing glycosaminoglycan (GAG) chains are essential. Parveen et al. discovered a borrelia glycosaminoglycan binding protein as it presented itself on the surface of the bacteria | <br>In addition to decorin, there has been evidence of binding activity with more generalized proteoglycans (PGs). Even though PG binding accounts for some of <i>B. burgdorferi</i> binding activity, it does not account for all. With PG binding, recognizing glycosaminoglycan (GAG) chains are essential. Parveen et al. discovered a borrelia glycosaminoglycan binding protein as it presented itself on the surface of the bacteria [9]. The different strains of <i>Borrelia</i> have different cell and GAG binding preferences, which allows a variety in the cell types the bacteria can bind as well as the GAG proteins expressed.<br> | ||
<br><u>Persistence in ticks</u><br> | <br><u>Persistence in ticks</u><br> | ||
<br>Outer surface proteins (Osp) A and OspB are two lipoproteins that have a role in the persistence of <i>B. burgdorferi</i> in ticks. Interestingly, these two proteins were discovered when antibodies specific to recognize OspA were reactive with isolates from Lyme disease patients. The antigenic characteristic of this protein was also discovered. These proteins are transcribed together from a single promoter and are encoded on the linear plasmid of <i>B. burgdorferi</i> | <br>Outer surface proteins (Osp) A and OspB are two lipoproteins that have a role in the persistence of <i>B. burgdorferi</i> in ticks. Interestingly, these two proteins were discovered when antibodies specific to recognize OspA were reactive with isolates from Lyme disease patients. The antigenic characteristic of this protein was also discovered. These proteins are transcribed together from a single promoter and are encoded on the linear plasmid of <i>B. burgdorferi</i> [10]. As a result, these two proteins have a high percentage of similarity in sequence and structure [11]. OspA and OspB are expressed in the gut of starving ticks and their expression is down regulated once they begin feeding. OspA specifically facilitates the binding of <i>B. burgdorferi</i> to the gut of the tick through the binding receptor TROSPA. Moreover, the expression of OspA is down regulated in order to allow the spirochete to travel from the gut into the salivary glands. On the other hand, OspB has its role in the bacterium’s ability to colonize and live in the gut of the tick. When working together, these proteins allow for persistence of <i>B. burgdorferi</i> in the gut of a tick soon to be transferred to the human host.<br> | ||
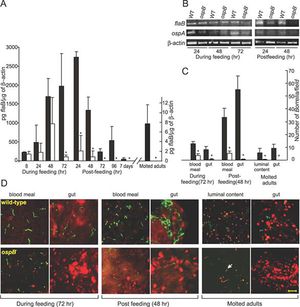
[[Image:researcj.jpg|thumb|300px|right|Figure 6. (A): Q-RT-PCR results from wild-type and ospB deficient represented by black and white bars, respectively. The amount of <i>fla</i>Bugof tick b-actin in each DNA sample measured in pg. * indicated a significant difference between wild-type and ospB deficient mice. (B): RT-PCR results of RNA samples extracted from wild-type and ospB deficient mice using b-actin as the internal control. These results were used to determine the presence of spirochetes. (C): Quantitative results from RT-PCR of RNA samples. Illustrates the measurement of spirochetes per microscopic field in wild-type vs. ospB deficient mice. Again, wild-type and ospB deficient mice are represented by black and white bars, respectively. (D): Images taken from identical microscope focus of wild-type and osp deficient mice. Using FITC-labeling anti-<i>Borrelia</i> antibody is viewed in green, and labeled cell nucleic acid stained with propidium iodide is viewed in red. Results show that only in ospB deficient mice is the spirochete present consistently in 15 random microscope fields of the mice. [https://link http://www.plospathogens.org/article/info%3Adoi%2F10.1371%2Fjournal.ppat.0030033]] | |||
[[Image:tick.jpg|thumb|300px|right| | [[Image:tick.jpg|thumb|300px|right|Figure 7. A summary of the interactions between an infected tick and a mammalian host. ospA expression increases during feeding and ospC is made and binds the salivary protein, Salp15. [https://link http://www.sciencedirect.com/science/article/pii/S1471492207001699]] | ||
<br><u>Transmission from Tick to Host</u><br> | <br><u>Transmission from Tick to Host</u><br> | ||
<br>OspC, like the previous outer surface proteins, is a lipoprotein that lies on the surface of the bacterium and is encoded by a circular plasmid. As discussed earlier, the expression of OspA and OspB is down regulated soon after the tick is done feeding. While, the expression of these proteins is down regulated, the expression of OspC is increased | <br>OspC, like the previous outer surface proteins, is a lipoprotein that lies on the surface of the bacterium and is encoded by a circular plasmid. As discussed earlier, the expression of OspA and OspB is down regulated soon after the tick is done feeding. While, the expression of these proteins is down regulated, the expression of OspC is increased [12,13]. Osp binds a salivary protein in ticks, Salp15, which accounts for its role in transmission from the tick to the host [14]. Grimm et al. showed through their research that OspC is an essential component of <i>B. burgdorferi</i> to cause infection [15]. OspC deletion mutants remain a virulent when infected through needle and tick [16]. Overall, OspC is required for transmission and infection in the host cell.<br> | ||
<br><u>Avoiding host immune response</u><br> | <br><u>Avoiding host immune response</u><br> | ||
<br>As mentioned in the introduction, <i>B. burgdorferi</i> is able to remain in patients for long periods of time and establish chronic infection in the host tissues. This bacterium has developed strategies in order to escape death by the host immune system. An example of this type of mechanism is antigenic variation. “Antigenic variation has been defined as changes in the structure or expression of antigenic proteins that occur during infection at a frequency greater than the usual mutation rate” | <br>As mentioned in the introduction, <i>B. burgdorferi</i> is able to remain in patients for long periods of time and establish chronic infection in the host tissues. This bacterium has developed strategies in order to escape death by the host immune system. An example of this type of mechanism is antigenic variation. “Antigenic variation has been defined as changes in the structure or expression of antigenic proteins that occur during infection at a frequency greater than the usual mutation rate” [Seifert, 1988], [17]. In their study, Zhang and collogues identified the vmp-like sequence (vls) in <i>B. burgdorferi</i>. A single vls expression site (vlsE) along with 15 additional silent, or non-expressed, vls cassettes were discovered on a linear plasmid. Genetic variation at the vls locus leads to antigenic variation. The mechanism of evading the host immune system includes parts of multiple silent vls cassette sequences recombining into the main vlsE cassette, which causes antigenic variation of the lipoprotein being expressed and results in many vlsE sequence products [18]. Overall, vlsE is significant to the bacterium to cause infection, through colonization, dissemination, adherence, and evasion of host immune systems [19]. Antigenic variation allows for the expression of vlsE without the protein being eliminated by the host cell immune response. As a result, the bacterium is able to reside in the host tissues and cause chronic illness.<br> | ||
==Future Work== | ==Future Work== | ||
<br>With the increasing research of surface proteins, there has been talk of generating a new Lyme disease vaccine. There was a Lyme disease vaccine made based on the outer surface lipoprotein A (OspA) that was approved, but then taken off the market about ten years ago and is no longer used | <br>With the increasing research of surface proteins, there has been talk of generating a new Lyme disease vaccine. There was a Lyme disease vaccine made based on the outer surface lipoprotein A (OspA) that was approved, but then taken off the market about ten years ago and is no longer used [20]. As more outer surface proteins of <i>Borrelia burgdorferi</i> increases, we can make advancements towards a new generation vaccine for Lyme disease. Being such a dangerous disease that can evade the host immune system and avoid detection, a vaccine for Lyme disease would be highly beneficial.<br> | ||
==Concluding Remarks== | ==Concluding Remarks== | ||
| Line 56: | Line 57: | ||
==References== | ==References== | ||
<br> | <br> | ||
<br>1. | <br>1. [http://www.niaid.nih.gov/topics/lymedisease/understanding/pages/intro.aspx A history of Lyme Disease, Symptoms, Diagnosis, and Treatment” National Institute of Allergy and Infectious Diseases. N.P. 2012. Web 22 Apr. 2013.]<br> | ||
<br>2. Fraser, CM et al (1997). Genomic Sequence of a Lyme Disease Spirochaete, Borrelia Burgdorferi. Nature. 580-86.<br>. | <br>2. [http://www.ncbi.nlm.nih.gov/pubmed/9403685 Fraser, CM et al (1997). Genomic Sequence of a Lyme Disease Spirochaete, Borrelia Burgdorferi. Nature. 580-86.]<br>. | ||
<br>3. "What Is Lyme Disease?" American Lyme Disease Foundation. N.p., 2006. Web. 22 Apr. 2013.<br> | <br>3. [http://www.aldf.com/lyme.shtml "What Is Lyme Disease?" American Lyme Disease Foundation. N.p., 2006. Web. 22 Apr. 2013.]<br> | ||
<br>4. Coburn, J., Laura Ristow, and Styliani Antonara (2011). Adhesion Mechanisms of Borrelia Burgdorferi. Advances in Experimental Medicine and Biology 35-49.<br> | <br>4. [http://www.ingentaconnect.com/content/ben/cdtid/2001/00000001/00000002/art00008 Coburn, J., Laura Ristow, and Styliani Antonara (2011). Adhesion Mechanisms of Borrelia Burgdorferi. Advances in Experimental Medicine and Biology 35-49.]<br> | ||
<br>5. Setubal JC, Reis M, Matsunaga J, Haake DA (2006). Lipoprotein computational prediction in spirochaetal genomes. Microbiology 152: 113-121.<br> | <br>5. [http://www.ncbi.nlm.nih.gov/pubmed/16385121 Setubal JC, Reis M, Matsunaga J, Haake DA (2006). Lipoprotein computational prediction in spirochaetal genomes. Microbiology 152: 113-121.]<br> | ||
<br>6. Probert WS, Kim JH, Hook M, Johnson BJB (2001). Mapping the ligand-binding region of the Borrelia burgdorferi fibronectin-binding protein BBK32. Infect Immun 69:4129-4133.<br> | <br>6. [http://iai.asm.org/content/69/6/4129.full Probert WS, Kim JH, Hook M, Johnson BJB (2001). Mapping the ligand-binding region of the Borrelia burgdorferi fibronectin-binding protein BBK32. Infect Immun 69:4129-4133.]<br> | ||
<br>7. Brown EL, Wooten RM, Johnson BJ, Iozzo RV, Smith A, Dolan MC, Guo BP, Weis JJ, Hook K (2001). Resistance to Lyme disease in decorin-deficient mice. J Clin Invest 107: 845-852.<br> | <br>7. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC199574/ Brown EL, Wooten RM, Johnson BJ, Iozzo RV, Smith A, Dolan MC, Guo BP, Weis JJ, Hook K (2001). Resistance to Lyme disease in decorin-deficient mice. J Clin Invest 107: 845-852.]<br> | ||
<br>8. Shi Y, Xu Q, McShan K, Liang FT (2008). Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect Immun 76:1239-1246.<br> | <br>8. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2258843/ Shi Y, Xu Q, McShan K, Liang FT (2008). Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect Immun 76:1239-1246.]<br> | ||
<br>9. Parveen N, Leong JM (2000). Identification of a candidate glycosaminoglycan-binding adhesion of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol 35: 1220-1234.<br> | <br>9. [http://www.ncbi.nlm.nih.gov/pubmed/10712702 Parveen N, Leong JM (2000). Identification of a candidate glycosaminoglycan-binding adhesion of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol 35: 1220-1234.]<br> | ||
<br>10. Howe TR, Mayer LW, Barbour AG (1985). A single recombinant plasmid expressing two major outer surface proteins of the Lyme disease spirochete. Science 227: 645-646.<br> | <br>10. [http://www.ncbi.nlm.nih.gov/pubmed/3969554 Howe TR, Mayer LW, Barbour AG (1985). A single recombinant plasmid expressing two major outer surface proteins of the Lyme disease spirochete. Science 227: 645-646.]<br> | ||
<br>11. Bergstrom S, Bundoc VG, Barbour AG (1989). Molecular analysis of linear pladmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol 3: 479-486.<br> | <br>11. [http://www.ncbi.nlm.nih.gov/pubmed/2761388 Bergstrom S, Bundoc VG, Barbour AG (1989). Molecular analysis of linear pladmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol 3: 479-486.]<br> | ||
<br>12. Schwan TG (2003). Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi. Biochem Soc Trans 31: 108-112.<br> | <br>12. [http://www.ncbi.nlm.nih.gov/pubmed/12546665 Schwan TG (2003). Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi. Biochem Soc Trans 31: 108-112.]<br> | ||
<br>13. Schwan TG, Piesman J (2000). Temporal changes in outer surface proteins A and C of the Lyme desase-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol 38: 382-388.<br> | <br>13. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC88728/ Schwan TG, Piesman J (2000). Temporal changes in outer surface proteins A and C of the Lyme desase-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol 38: 382-388.]<br> | ||
<br>14. Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, Schwan TG, Policastro PF, Elias AF, Rosa PA (2004). Outer-surface protein C of the Lyme disease spirochete: a protein induced n ticks for infection of mammals. P Natl Acad Sci USA 101:3142-3147.<br> | <br>14. [http://www.ncbi.nlm.nih.gov/pubmed/14970347 Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, Schwan TG, Policastro PF, Elias AF, Rosa PA (2004). Outer-surface protein C of the Lyme disease spirochete: a protein induced n ticks for infection of mammals. P Natl Acad Sci USA 101:3142-3147.]<br> | ||
<br>15. Ramamoorthi N, Narasimhan S, Pal U (2005). The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436: 573-577.<br> | <br>15. [http://www.ncbi.nlm.nih.gov/pubmed/16049492 Ramamoorthi N, Narasimhan S, Pal U (2005). The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436: 573-577.]<br> | ||
<br>16. Tilly K, Krum JG, Bestor A (2006). Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun 74: 3554-3564.<br> | <br>16. [http://www.ncbi.nlm.nih.gov/pubmed/16714588 Tilly K, Krum JG, Bestor A (2006). Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun 74: 3554-3564.]<br> | ||
<br>17. Seifert HS, So H (1988). Genetic mechanisms of bacterial antigenic variation. Microbiol. Rev. 52: 327-336.<br> | <br>17. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC373148/ Seifert HS, So H (1988). Genetic mechanisms of bacterial antigenic variation. Microbiol. Rev. 52: 327-336.]<br> | ||
<br>18. Zhang JR, Norris SJ (1998). Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect Immun 66: 3689-3697.<br> | <br>18. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC108403/ Zhang JR, Norris SJ (1998). Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect Immun 66: 3689-3697.]<br> | ||
<br>19. Zhang JR, Hardham JM, Barbour AG, Norris SJ (1997). Antigenic variation in Lyme disease Borreliae by promiscuous recombination of Vmp-like sequence cassettes. Cell 89: 275-285.<br> | <br>19. [http://www.ncbi.nlm.nih.gov/pubmed/9108482 Zhang JR, Hardham JM, Barbour AG, Norris SJ (1997). Antigenic variation in Lyme disease Borreliae by promiscuous recombination of Vmp-like sequence cassettes. Cell 89: 275-285.]<br> | ||
<br>20. Kenedy, Melisha R., Tiffany R. Lenhart, and Darrin R. Akins (2012). The Role of Borrelia Burgdorferi Outer Surface Proteins. Immunol Med Microbiol 66: 1-19.<br> | <br>20. [http://www.ncbi.nlm.nih.gov/pubmed/22540535 Kenedy, Melisha R., Tiffany R. Lenhart, and Darrin R. Akins (2012). The Role of Borrelia Burgdorferi Outer Surface Proteins. Immunol Med Microbiol 66: 1-19.]<br> | ||
<br> | <br> | ||
Edited by Ariel Bartholomew of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol238/biol238syl09.html BIOL 238 Microbiology], 2013, [http://www.kenyon.edu/index.xml Kenyon College]. | Edited by Ariel Bartholomew of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol238/biol238syl09.html BIOL 238 Microbiology], 2013, [http://www.kenyon.edu/index.xml Kenyon College]. | ||
<!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | <!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | ||
Latest revision as of 21:50, 8 May 2013
History
The first case of the Lyme disease was in Lyme, Connecticut in 1975. The disease presented arthritic-like symptoms, and was therefore referred to as Lyme arthritis. The deer tick, Ixodes scapularis, was associated with the transmission of the disease in 1977, but the cause of the disease remained unknown until Willy Burgdorferi discovered Borrelia burgdorferi in 1981. The disease is caused by three species of bacteria all belonging to Borrelia-Borrelia burgdorferi, Borrelia afzelii, and Borrelia garinii. Borrelia burgdorferi is the main cause of Lyme disease in North America, where the other two species affect Europe[1, 2].
Borrelia burgdorferi Description and Structure

Borrelia burgdorferi is a Gram-negative bacterium belonging to the class Spirochaetes [Figure 1] . This bacterium is helical and has both an inner and outer membrane as well as a flexible cell wall. The cell is usually 1um wide, but can be up to 10-25 um long. Bacteria of the class spirochaetes have flagella located on the inside of the periplasm in between the inner and outer membranes. The flagellum along with the helical structure of the bacterium allows the bacterium to migrate through viscous fluids and burrow through various tissues. As a result, this cell is highly invasive. B. burgdorferi is also known for its outer surface proteins OspA and OspC have been studied extensively and have a role in transmission of the bacteria into the host cell.
The metabolism of this cell is limited, therefore; B. burgdorferi relies on their host for energy precursors. Its genome encodes transport proteins such as ABC transporters. B. burgdorferi also codes for enzymes and proteins that are used in the phosphotransferase system. The host serum as well as the environment are targets for these transport systems. The genome of this bacterium is distinctive. It consists of one linear chromosome of 910,725 base pairs long with at least 17 linear and circular plasmids that combine to a size of more than 533,000 base pairs [2] . As a result of its large number of plasmids, the genetic organization of B. burgdorferi is specialized.
.
What is Lyme Disease?

Lyme disease is an infectious disease caused by the spirochete Borrelia burgdorferi that is carried in deer tick Ixodes scapularis. This disease is the most common vector borne disease in the United States, affecting far more individuals than West Nile virus. It has been estimates that there were nearly 40,000 cases of Lyme reported in 2009, a large underestimate. The Center for Disease Control estimates the true number of cases to be as much as 12 times higher, making the estimated total as high as 480,000. This estimate makes Lyme disease more prevalent than AIDS. Moreover, national surveillance began in 1982, which increased the number of cases reported nearly 25-fold [3].
Lyme disease is most commonly transmitted to humans through the bite of an infected tick as it bites [ Figure 2]. Without treatment, B. burgdorferi is able to migrate through the bloodstream and many connective tissues. In it’s early stages, Lyme disease affects the skin through skin rash and in later stages spreads to the joints, nervous system, and other organ systems in the later stages. If caught and treated early, Lyme disease is almost always cured. However, this disease has a high rate of progression and if it is not treated early, symptoms may last for months or even years. Unfortunately, thousands of patients go undiagnosed or misdiagnosed with doctors telling them their symptoms are in their head due to the difficulty in testing. Part of how this disease goes untreated and misdiagnosed is due to the characteristics of the burrowing bacterium, Borrelia burgdorferi. This bacterium has become an expert at hiding and surviving in human tissues, and its ability to take on different forms such as cysts, which allows the bacterium to escape the immune system, avoid antibiotics, and hide from detection by blood tests. The mechanism of transmission is poorly understood, but for many bacterial pathogens such as B. burgdorferi, the initial step in colonizing host tissues involves the expression of adhesive molecules that facilitate bacterial adherence to host cells or to the extracellular matrix.
Symptoms
Lyme disease almost always first presents itself as a skin rash called erythema migrans [Figure 3]. The rash most likely originates from the site of the tick bite and spreads. The rash can be a solid expanding red spot, or it can be a single red spot surrounded by lighter red skin resembling a bulls-eye. The skin rash is visible 1 to 2 weeks after the disease has been transmitted from the tick, and lasts for 3 to 5 weeks. Ticks are able to attach anywhere on the body, but-armpit, groin, back of the knee, nape-body creases are what is most common. Symptoms that can present themselves at the time of the rash include, joint pain, chills, fever, and fatigue. As the spirochete spreads throughout the body, more sever symptoms begin to surface. The more serious symptoms include tingling or numbness in the extremities, paralysis, severe fatigue and a stiff neck. Severe headaches, chronic arthritis, swelling of joints, cardiac abnormalities and mental disorders can appear weeks, months, and in some cases, years after being bitten.
Treatment
When Lyme disease is caught early, within the first few weeks of initial infection, the treatment is straightforward and it is highly likely that treatment will end in a cure. However, once it has been longer than three weeks since being infected, the likelihood of finding a cure decreases as long as the patient goes untreated. According to the American Lyme Disease Foundation, the most common antibiotics recommended for most symptoms of Lyme disease are Doxycycline, amoxicillin, and ceftin. Treatment for Lyme disease can be frustrating and be seen as inconclusive due to the sporadic progression of the disease. Some patients may experience symptoms of Lyme disease for years . The documentary, Under Our Skin, tells the story of several patients whose lives have been destroyed by this disease. After being misdiagnosed, undiagnosed, and untreated for so long, these patients experience extreme symptoms preventing them from walking and carrying out a normal lifestyle.
Adhesion Mechanisms
The pathogenic bacterium Borrelia burgdorferi is the spirochete that causes Lyme disease. These spirochetes have distinctive characteristics that allow them to cause chronic infection and remain in an infected human. Many researchers have focused on the outer surface being proteins of B. burgdorferi as these proteins mediate the interaction between the bacteria and the human host [Figure 5.] Adhesion to mammalian and tick substrates may have a role in the host response to the bacteria as well as evasion from the host immune system [4]. The following agents of Borrelia burgdorferi have been studied in hopes of understanding the mechanisms that allow these bacteria to cause infection, and in some cases disease. There are a few characteristics of this bacterium that caught the attention of many researchers. First of all, this bacterium has a large number of adhesion genes for its small (1.5 Mbp) genome. 7.8% of the genome encodes known or predicted lipoproteins, where some have been classified as adhesions [5]. Within the small genome, many proteins that bind to either mammalian or tick cells or extracellular matrix have been discovered and contribute to the regime of these bacteria.
Borrelia burgdorferi has numerous proteins that support an interaction with the extracellular components of the host cell. Different adhesions of Borrelia burgdorferi will be reviewed here to understand the characteristics of this bacterium and the disease it causes.
Interaction with Extracellular Matrix
Fibronectin (Fn) is a large, dimeric glycoprotein and is produced by numerous cell types. It can exist as a soluble molecule as well as an insoluble molecule, and can therefore reside in body fluids as well as in the extracellular matrix. Pertaining to its structure, fibronectin is made up of three types of protein modules that are arranged in different domains. Through these multiple domains, Fn is able to bind to many trans-membrane receptors as well as other extracellular components including, collagen, fibrinogen, and proteoglycans. However, Fn specifically encourages cell adhesion, growth, migration, and differentiation. Moreover, Fn plays an important role in wound healing and embryonic development. Borrelia burgdorferi synthesizes multiple Fn-binding adhesions, with BBK32 being the binding protein best characterized. BBK32 gene has been isolated from B. burgdorferi and has an Fn-binding region that has been localized to 32 amino acids and was found in B. burgdorferi sense stricto, B. garinii, and B. afzelii, which are strains known to cause disease in humans. In the isolate, BBK32 remained on the outer surface and the adhesion specifically interacted with the collagen-binding domain of fibronectin. The binding of BBK32 to Fn relates B. burgdorferi to Fn-binding adhesions of gram-positive pathogens Staphylococcus aureus and Staphylococcus pyogenes. In relation to Lyme disease, in B. burgdorferi BBK32 is expressed as the tick feeds and while the bacteria are in the mammalian host [6,2].
Adhesion to Host cell
Decorin is a proteoglycan with a protein core that consists primarily of leucine rich repeats and a glycosaminoglycan protein. These proteins function as structural components of the extracellular matrix as well as signaling mediators. The borrelial decorin-binding proteins, DbpA and DbpB, have been discovered and identified as targets for host cell decorin. These surface lipoproteins are coded by the dbpB/A operon, Decorin binds collagen, which is found in many tissues as a part of the connective tissue. This relationship allows B. burgdorferi to interact with connective tissues. Studies have shown that the expression of both proteins is increased in response to a reduced pH and by a shift in temperature from 23 to 37 ° C, which illustrates their role in the mammalian environment. When DbpA and DbpB are expressed in B. burgdorferi strain lacking the gene that codes for the dbpB/A operon, lp54, the strain was able to bind mammalian epithelial cells. However, when the proteins are not expressed, no binding can occur [2].
Many studies have researched the role of DbpA and DbpB in the life cycle of B. burgdorferi and their contribution to Lyme disease. Brown et. Al. reported the multiplication of bacterial organisms to be significantly lower in decorin-deficient mice compared to wild-type mice [7]. Moreover, Shi et al. found that these proteins are not necessary for causing an infection, but are critical for the overall virulence of the bacteria [8]. In regards to Lyme disease, decorin binding proteins play a strong role in the later stages of disease-during dissemination and while establishing chronic infections in decorin rich tissues.
In addition to decorin, there has been evidence of binding activity with more generalized proteoglycans (PGs). Even though PG binding accounts for some of B. burgdorferi binding activity, it does not account for all. With PG binding, recognizing glycosaminoglycan (GAG) chains are essential. Parveen et al. discovered a borrelia glycosaminoglycan binding protein as it presented itself on the surface of the bacteria [9]. The different strains of Borrelia have different cell and GAG binding preferences, which allows a variety in the cell types the bacteria can bind as well as the GAG proteins expressed.
Persistence in ticks
Outer surface proteins (Osp) A and OspB are two lipoproteins that have a role in the persistence of B. burgdorferi in ticks. Interestingly, these two proteins were discovered when antibodies specific to recognize OspA were reactive with isolates from Lyme disease patients. The antigenic characteristic of this protein was also discovered. These proteins are transcribed together from a single promoter and are encoded on the linear plasmid of B. burgdorferi [10]. As a result, these two proteins have a high percentage of similarity in sequence and structure [11]. OspA and OspB are expressed in the gut of starving ticks and their expression is down regulated once they begin feeding. OspA specifically facilitates the binding of B. burgdorferi to the gut of the tick through the binding receptor TROSPA. Moreover, the expression of OspA is down regulated in order to allow the spirochete to travel from the gut into the salivary glands. On the other hand, OspB has its role in the bacterium’s ability to colonize and live in the gut of the tick. When working together, these proteins allow for persistence of B. burgdorferi in the gut of a tick soon to be transferred to the human host.
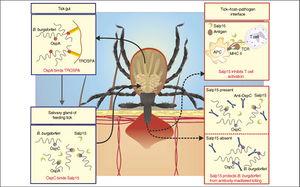
Transmission from Tick to Host
OspC, like the previous outer surface proteins, is a lipoprotein that lies on the surface of the bacterium and is encoded by a circular plasmid. As discussed earlier, the expression of OspA and OspB is down regulated soon after the tick is done feeding. While, the expression of these proteins is down regulated, the expression of OspC is increased [12,13]. Osp binds a salivary protein in ticks, Salp15, which accounts for its role in transmission from the tick to the host [14]. Grimm et al. showed through their research that OspC is an essential component of B. burgdorferi to cause infection [15]. OspC deletion mutants remain a virulent when infected through needle and tick [16]. Overall, OspC is required for transmission and infection in the host cell.
Avoiding host immune response
As mentioned in the introduction, B. burgdorferi is able to remain in patients for long periods of time and establish chronic infection in the host tissues. This bacterium has developed strategies in order to escape death by the host immune system. An example of this type of mechanism is antigenic variation. “Antigenic variation has been defined as changes in the structure or expression of antigenic proteins that occur during infection at a frequency greater than the usual mutation rate” [Seifert, 1988], [17]. In their study, Zhang and collogues identified the vmp-like sequence (vls) in B. burgdorferi. A single vls expression site (vlsE) along with 15 additional silent, or non-expressed, vls cassettes were discovered on a linear plasmid. Genetic variation at the vls locus leads to antigenic variation. The mechanism of evading the host immune system includes parts of multiple silent vls cassette sequences recombining into the main vlsE cassette, which causes antigenic variation of the lipoprotein being expressed and results in many vlsE sequence products [18]. Overall, vlsE is significant to the bacterium to cause infection, through colonization, dissemination, adherence, and evasion of host immune systems [19]. Antigenic variation allows for the expression of vlsE without the protein being eliminated by the host cell immune response. As a result, the bacterium is able to reside in the host tissues and cause chronic illness.
Future Work
With the increasing research of surface proteins, there has been talk of generating a new Lyme disease vaccine. There was a Lyme disease vaccine made based on the outer surface lipoprotein A (OspA) that was approved, but then taken off the market about ten years ago and is no longer used [20]. As more outer surface proteins of Borrelia burgdorferi increases, we can make advancements towards a new generation vaccine for Lyme disease. Being such a dangerous disease that can evade the host immune system and avoid detection, a vaccine for Lyme disease would be highly beneficial.
Concluding Remarks
Lyme disease is a tick borne disease caused by the spirochete Borrelia burgdorferi. Since it’s discovery in 1975, there have been extensive research on the mechanisms of this bacterium. The last two decades have identified a number of outer surface lipoproteins that are needed for the transmission of the disease in mammalian tissues. The diversity in surface proteins allows for the bacterium to adapt to different environments, which is beneficial in infecting different mammalian hosts. However, many of the adhesions of the bacterium overlap in function, which suggests that these functions are necessary in order for the bacterium to cause infection in its mammalian host. Further research on the interaction between B. burgdorferi and its mammalian host will benefit those who are infected and can work towards prevention though vaccines. Also, looking at the interaction of B. burgdorferi adhesions with host substrates at the molecular level will be interesting and may lead to additional antibiotics for treatment.
References
1. A history of Lyme Disease, Symptoms, Diagnosis, and Treatment” National Institute of Allergy and Infectious Diseases. N.P. 2012. Web 22 Apr. 2013.
2. Fraser, CM et al (1997). Genomic Sequence of a Lyme Disease Spirochaete, Borrelia Burgdorferi. Nature. 580-86.
.
3. "What Is Lyme Disease?" American Lyme Disease Foundation. N.p., 2006. Web. 22 Apr. 2013.
4. Coburn, J., Laura Ristow, and Styliani Antonara (2011). Adhesion Mechanisms of Borrelia Burgdorferi. Advances in Experimental Medicine and Biology 35-49.
5. Setubal JC, Reis M, Matsunaga J, Haake DA (2006). Lipoprotein computational prediction in spirochaetal genomes. Microbiology 152: 113-121.
6. Probert WS, Kim JH, Hook M, Johnson BJB (2001). Mapping the ligand-binding region of the Borrelia burgdorferi fibronectin-binding protein BBK32. Infect Immun 69:4129-4133.
7. Brown EL, Wooten RM, Johnson BJ, Iozzo RV, Smith A, Dolan MC, Guo BP, Weis JJ, Hook K (2001). Resistance to Lyme disease in decorin-deficient mice. J Clin Invest 107: 845-852.
8. Shi Y, Xu Q, McShan K, Liang FT (2008). Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect Immun 76:1239-1246.
9. Parveen N, Leong JM (2000). Identification of a candidate glycosaminoglycan-binding adhesion of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol 35: 1220-1234.
10. Howe TR, Mayer LW, Barbour AG (1985). A single recombinant plasmid expressing two major outer surface proteins of the Lyme disease spirochete. Science 227: 645-646.
11. Bergstrom S, Bundoc VG, Barbour AG (1989). Molecular analysis of linear pladmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol 3: 479-486.
12. Schwan TG (2003). Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi. Biochem Soc Trans 31: 108-112.
13. Schwan TG, Piesman J (2000). Temporal changes in outer surface proteins A and C of the Lyme desase-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol 38: 382-388.
14. Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, Schwan TG, Policastro PF, Elias AF, Rosa PA (2004). Outer-surface protein C of the Lyme disease spirochete: a protein induced n ticks for infection of mammals. P Natl Acad Sci USA 101:3142-3147.
15. Ramamoorthi N, Narasimhan S, Pal U (2005). The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436: 573-577.
16. Tilly K, Krum JG, Bestor A (2006). Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun 74: 3554-3564.
17. Seifert HS, So H (1988). Genetic mechanisms of bacterial antigenic variation. Microbiol. Rev. 52: 327-336.
18. Zhang JR, Norris SJ (1998). Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect Immun 66: 3689-3697.
19. Zhang JR, Hardham JM, Barbour AG, Norris SJ (1997). Antigenic variation in Lyme disease Borreliae by promiscuous recombination of Vmp-like sequence cassettes. Cell 89: 275-285.
20. Kenedy, Melisha R., Tiffany R. Lenhart, and Darrin R. Akins (2012). The Role of Borrelia Burgdorferi Outer Surface Proteins. Immunol Med Microbiol 66: 1-19.
Edited by Ariel Bartholomew of Joan Slonczewski for BIOL 238 Microbiology, 2013, Kenyon College.