Campylobacteriosis: Difference between revisions
No edit summary |
|||
| (17 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Conway}} | ||
==Etiology/Bacteriology== | ==Etiology/Bacteriology== | ||
===Taxonomy=== | ===Taxonomy=== | ||
| Line 10: | Line 8: | ||
| Family = [[Campylobacteraceae]] | | Family = [[Campylobacteraceae]] | ||
| Genus = [[Campylobacter]] | | Genus = [[Campylobacter]] | ||
| Species = [[ | | Species = [[C. jejuni]] | ||
{| | {| | ||
| Line 16: | Line 14: | ||
'''NCBI: [http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=197&lvl=3&lin=f&keep=1&srchmode=1&unlock Taxonomy] Genome: <font size="2">[http://www.ncbi.nlm.nih.gov/genome/?term=Campylobacter+jejuni Genome]</font>''' | '''NCBI: [http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=197&lvl=3&lin=f&keep=1&srchmode=1&unlock Taxonomy] Genome: <font size="2">[http://www.ncbi.nlm.nih.gov/genome/?term=Campylobacter+jejuni Genome]</font>''' | ||
|} | |} | ||
===Description=== | ===Description=== | ||
Camplobacteriosis, caused by a bacterial infection of <i>Campylobacter jejuni</i> in the human body, is the most common cause of diarrhea in the United States. <i>Campylobacter</i> is a Gram-negative spiral bacterium which damages the small intestine and colon. This pathogenic bacterium causes bloody diarrhea, vomiting, abdominal pain, and fever. [[#References|[1]]] <i>Campylobacter</i> is microaerophilic as well as a thermophile and takes 2-5 days to begin showing symptoms. Diarrhea and related symptoms are self-limiting, and typically last 5-7 days. This non-spore forming prokaryote was isolated in 1972 and genome sequenced in 2000. [[#References|[2]]] | [[Image:Campylobacter.jpg|thumb|300px|right|''Spiral rod Campylobacter jejunum. From: Wikipedia.org [http://upload.wikimedia.org/wikipedia/commons/b/ba/Campylobacter.jpg]]] | ||
Camplobacteriosis, caused by a bacterial infection of <i>Campylobacter jejuni</i> in the human body, is the most common cause of diarrhea in the United States. <i>Campylobacter</i> is a Gram-negative spiral bacterium which damages the small intestine and colon. This pathogenic bacterium causes bloody diarrhea, vomiting, abdominal pain, and fever. [[#References|[1]]] <i>Campylobacter</i> is microaerophilic as well as a thermophile and takes 2-5 days to begin showing symptoms. Diarrhea and related symptoms are self-limiting, and typically last 5-7 days. This non-spore forming prokaryote was isolated in 1972 and genome sequenced in 2000. [[#References|[2]]] Approximately 14 cases for each 100,000 persons in the population every year are infected. However, there are over 1.3 million persons per year with unreported cases. About 76 infected persons die each year.[[#References|[1]]] <i>C. jejuni</i> has a very low infectious dose with only 500 organisms being required for symptoms to present in an infected host. <i>C. jejuni</i> are motile pathogens that causes disease by producing cytolethal distending toxin, which stops the cell from dividing and activating the immune system. This helps <i>C. jejuni</i> to evade the small intestine and colon. <i>Campylobacter</i> is transmitted by raw or uncooked poultry, unpasteurized dairy, contaminated water, produce, and stool from animals or humans. It is rarely passed from human to human, but rather through consumption of infected food. Prevention is possible by simply cooking meat thoroughly; washing hands, and not using contaminated cooking utensils for uncooked goods. While some antibiotics such as Azithromycin are used to treat the illness, most people abstain from medicine allowing the pathogen to run its course while replenishing the body with water and electrolytes. In rare incidents <i>Camplobacter</i> can cause long term consequences such as arthritis, Guillian-Barre syndrome, and gastrointestinal perforation. <i>C. jejuni</i> is estimated to kill 76 people a year, mostly infants and children. To diagnose <i>Camplobacter</i> as the infectious agent of the disease, fecal matter must be cultured. | |||
==Pathogenesis== | ==Pathogenesis== | ||
| Line 38: | Line 38: | ||
==Clinical features== | ==Clinical features== | ||
===Symptoms=== | ===Symptoms=== | ||
<i>Campylobacteriosis</i> can often go undiagnosed or it is often underreported because it is one of many diarrheal diseases. The disease is characterized by bloody or mucosal diarrhea[[#References|[4]]]. The diarrhea is a result of the bacteria's colonization in the intestine and cell death due to the cytolethal toxin. Other symptoms may include muscle pain, headache, fever, and nausea which are due to dehydration from the diarrhea. The disease is self-limiting and most symptoms cease after five days. Reactive arthritis, Guillain-Barré syndrome, and bacteremia are known side effects of this disease, but they rarely occur. Guillain-Barré syndrome could occur several weeks after the diarrhea symptoms. This is due to the bacteria's role in mimicking the gangliosides of the neural system which can lead to the immune system attacking self cells. This may lead to paralysis, but is usually recoverable. Bacteremia may result | <i>Campylobacteriosis</i> can often go undiagnosed or it is often underreported because it is one of many diarrheal diseases. The disease is characterized by bloody or mucosal diarrhea[[#References|[4]]]. The diarrhea is a result of the bacteria's colonization in the intestine and cell death due to the cytolethal toxin. Other symptoms may include muscle pain, headache, fever, and nausea which are due to dehydration from the diarrhea. The disease is self-limiting and most symptoms cease after five days. Reactive arthritis, Guillain-Barré syndrome, and bacteremia are known side effects of this disease, but they rarely occur. Guillain-Barré syndrome could occur several weeks after the diarrhea symptoms. This is due to the bacteria's role in mimicking the gangliosides of the neural system which can lead to the immune system attacking self cells. This may lead to paralysis, but is usually recoverable. Bacteremia may be a result but mostly effects the immunocompromised or elderly. This can lead to death in the host. | ||
[[Image:Damageresponse framework for Campylobacter jejuni.jpeg|thumb|400px|right|''Campylobacter jejunum damage-response timeline[https://microbewiki.kenyon.edu/index.php/File:Damageresponse_framework_for_Campylobacter_jejuni.jpeg]]] | |||
===Morbidity and Mortality=== | ===Morbidity and Mortality=== | ||
| Line 44: | Line 46: | ||
==Diagnosis== | ==Diagnosis== | ||
Diagnosis of <i>Campylobacter</i> is done by confirming its presence in the patient’s stool. The two methods currently used in identification are growth on a selective medium such as Preston <i>Campylobacter</i> selective agar [[#References|[10]]] and Polymerization Chain Reaction (PCR) [[#References|[11]]] to determine the DNA presence of <i>Campylobacter jejuni</i> as well as other related species and subspecies. | Diagnosis of <i>Campylobacter</i> is done by confirming its presence in the patient’s stool. The two methods currently used in the identification of the pathogen are growth on a selective medium such as Preston <i>Campylobacter</i> selective agar [[#References|[10]]] and Polymerization Chain Reaction (PCR) [[#References|[11]]] to determine the DNA presence of <i>Campylobacter jejuni</i> as well as other related species and subspecies. | ||
==Treatment== | ==Treatment== | ||
| Line 76: | Line 78: | ||
==Host Immune Response== | ==Host Immune Response== | ||
The innate immune response from the host includes several things such as TLR's (toll like receptors), serum type, and sugars. <i>Campylobacter</i> has evolved to specifically overcome a few of the immune system's most basic responses including the TLR5 receptors that recognize the flagellum of the bacterium, and also the TLR9 receptors responsible for recognition of CpG di-nucleotides present in <i>Campylobacter</i>. Other experiments have also shown the importance of downstream TLR's in the immune response and their stimulation against the disease. Macrophage activation from NOD1 receptors and their mediation play a part in <i>Campylobacter's</i> plan to circumvent the innate immune response. Studies have also been conducted as to the role of fucosylated sugars in breast milk and how their presence prevents the attachment of <i>Campylobacter</i> to the intestinal mucosa. <i>C. jejuni</i> is found to be sensitive to specific types of serum, emphizising the importance of compliment mediated responses of the immune response. | |||
The Adaptive Immune response has developed several responses to many antigens present on the bacterium. Some tests using human volunteers have shown the earliest signs of antibody presence as approximately 6-7 days after inoculation. Of the antibodies that are detected there are several of isotypes. Almost unsurprisingly, many of the antigens recognized by antibodies come from <i>Campylobacter's</i> more prodominant virulence fators including the bacterial flagellum, many periplasmic proteins, capsular polysaccharides, and the CDT toxin. | |||
==References== | ==References== | ||
1 Centers for Disease Control and Prevention (2010), Campylobacter <http://www.cdc.gov/nczved/divisions/dfbmd/diseases/campylobacter.> | 1 Centers for Disease Control and Prevention (2010), Campylobacter <http://www.cdc.gov/nczved/divisions/dfbmd/diseases/campylobacter.> | ||
| Line 105: | Line 111: | ||
13 Jin-lin Huang, Yan-Xin Yin, [...], and Xin-an Jiao, Intranasal Immunization with Chitosan/pCAGGS-flaA Nanoparticles Inhibits Campylobacter jejuni in a White Leghorn Model, Journal of Biomedicine and Biotechnology. 2010; 2010: 589476<http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2948919/> | 13 Jin-lin Huang, Yan-Xin Yin, [...], and Xin-an Jiao, Intranasal Immunization with Chitosan/pCAGGS-flaA Nanoparticles Inhibits Campylobacter jejuni in a White Leghorn Model, Journal of Biomedicine and Biotechnology. 2010; 2010: 589476<http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2948919/> | ||
Created by Halen Borron, Kelley Raines, and Evan Robinson, students of Tyrrell Conway at the University of Oklahoma. | Created by Halen Borron, Kelley Raines, and Evan Robinson, students of Tyrrell Conway at the University of Oklahoma. <p> | ||
Edited by Hae Cha and Derek Lehman, students of Tyrrell Conway at the University of Oklahoma. | |||
Latest revision as of 14:18, 11 February 2016

Etiology/Bacteriology
Taxonomy
| Domain = Bacteria | Phylum = Proteobacteria | Class = Epsilon Proteobacteria | Order = Campylobacterales | Family = Campylobacteraceae | Genus = Campylobacter | Species = C. jejuni
Description
Camplobacteriosis, caused by a bacterial infection of Campylobacter jejuni in the human body, is the most common cause of diarrhea in the United States. Campylobacter is a Gram-negative spiral bacterium which damages the small intestine and colon. This pathogenic bacterium causes bloody diarrhea, vomiting, abdominal pain, and fever. [1] Campylobacter is microaerophilic as well as a thermophile and takes 2-5 days to begin showing symptoms. Diarrhea and related symptoms are self-limiting, and typically last 5-7 days. This non-spore forming prokaryote was isolated in 1972 and genome sequenced in 2000. [2] Approximately 14 cases for each 100,000 persons in the population every year are infected. However, there are over 1.3 million persons per year with unreported cases. About 76 infected persons die each year.[1] C. jejuni has a very low infectious dose with only 500 organisms being required for symptoms to present in an infected host. C. jejuni are motile pathogens that causes disease by producing cytolethal distending toxin, which stops the cell from dividing and activating the immune system. This helps C. jejuni to evade the small intestine and colon. Campylobacter is transmitted by raw or uncooked poultry, unpasteurized dairy, contaminated water, produce, and stool from animals or humans. It is rarely passed from human to human, but rather through consumption of infected food. Prevention is possible by simply cooking meat thoroughly; washing hands, and not using contaminated cooking utensils for uncooked goods. While some antibiotics such as Azithromycin are used to treat the illness, most people abstain from medicine allowing the pathogen to run its course while replenishing the body with water and electrolytes. In rare incidents Camplobacter can cause long term consequences such as arthritis, Guillian-Barre syndrome, and gastrointestinal perforation. C. jejuni is estimated to kill 76 people a year, mostly infants and children. To diagnose Camplobacter as the infectious agent of the disease, fecal matter must be cultured.
Pathogenesis
Transmission
Campylobacter is transmitted by ingesting contaminated food and water as well as human to human contact. Roughly 57% of cases can be traced to chickens and 35% to cattle. Animal farms, as well as slaughterhouses, were found to have a high infestation of Campylobacter[3]. Transmission can be found in cross-contamination of animal farms and water supply such as contamination in ones own kitchen. Unpasteurized milk also transmits Campylobacter through utter infection and contact with milk. Campylobacter is most common in developing countries, thus 19% of diagnosed C. jejuni infections are associated with international travel.
Infectious Dose, Incubation, Colonization
The infectious dose of C. jejuni is around 500 organisms [4]. This is relatively low compared to some of the other gastrointestinal pathogens. The incubation period for this organism is one-eleven days with the average being two-five days. C. jejuni typically colonizes the small intestine and colon through the use of virulence factors such as motility, chemotaxis, adhesion, and invasion. Flagellin are very important in this process. Colonization typically occurs in children under 5 or in young adults.
Epidemiology
Campylobacter jejuni is prevalent in the United States and other developed countries. The first isolation of Campylobacteriosis was in 1972 [5] . The majority of campylobacteriosis cases are sporadic with only 3% being associated with households and 2.3% being in a cluster[6]. Campylobacteriosis is often underreported so numbers of infected persons per year are often low. It is estimated that around 70% of poultry are infected with Campylobacter depending on the region [7]. The incidence of disease has remained stable in the past few years [8].
Virulence Factors
C. jejuni has many virulence factors that attribute to its pathogenicity in humans. Flagellin contribute to the bacteria's motility which allows it to travel throughout the host [9]. Another portion of the flagella's virulence is chemotaxis, which includes sensing the environment and rotating the flagella accordingly to benefit the bacteria. Mucin has been found to be a positive chemotaxin for C. jejuni which is compatible with the bacteria’s colonization of the intestine where there is abundant mucus. This virulence factor would serve as a guide towards colonization for the bacteria. Bile and L-fucose are also positive chemotaxins for C. jejuni. Motility and chemotaxis help lead the bacteria to its colonization site. It has been found that motility is a necessary component for the bacteria to be virulent[10].
Adhesion and invasion are important virulence factors for colonizing the host's intestinal cells. Adhesion is a necessary part of virulence for this bacteria because it allows C. jejuni to stay on the host cell long enough to cross into it. Adhesion is possible through various proteins, flagella, and lipopolysaccharide. Once adhered, the bacteria can be taken up by a cytoplasmic vacuole. The bacteria invade the host cell barriers through the use of flagellin.
C. jejuni also contains virulence factors based on its cell wall. Gram-negative bacteria contain lipopolysaccharides (LPS) in their outer membrane. This LPS plays a role in adherence as well as evading the immune system. The bacteria have the ability to shift its LPS antigen composition which makes it harder for the immune system to detect the pathogen. An important factor in contracting Guillians Barre syndrome from this pathogen is the sialic acid that is contained in its core oligosaccharide. This compound can resemble gangliosides which can cause this neurological disease.
The bacteria’s core toxicity pathway is cytolethal distending toxin. This toxin stops the cell’s growth cycle in G2 and the cell eventually dies. The cell death that accompanies this toxin is a reason for blood in the host’s diarrhea. This toxin is also thought to cause immunosuppression. There are three portions of this toxin, CdtA, CdtB, and CdtC. Little is known about the exact mechanism the three portions have for carrying out the disease. But CdtB is a known nuclease, which can disrupt DNA in the cell.
Iron acquisition is important for sustaining nutrients within the host. C. jejuni accomplishes this through using heme compounds, siderophores, and ferric iron. Iron is important for electron transport, anaerobic respiration, and energy metabolism. Superoxide dismutase is also one of the virulence factors for C. jejuni because it gets rid of the reactive oxygen species superoxide which could harm the cell's DNA or membrane factors. Antibiotic resistance to tetracycline, erythromycin, ciprofloxacin, kanamycin, nalidixic acid, and chloramphenicol is important for staying within the host to carry out its pathogenesis.
Clinical features
Symptoms
Campylobacteriosis can often go undiagnosed or it is often underreported because it is one of many diarrheal diseases. The disease is characterized by bloody or mucosal diarrhea[4]. The diarrhea is a result of the bacteria's colonization in the intestine and cell death due to the cytolethal toxin. Other symptoms may include muscle pain, headache, fever, and nausea which are due to dehydration from the diarrhea. The disease is self-limiting and most symptoms cease after five days. Reactive arthritis, Guillain-Barré syndrome, and bacteremia are known side effects of this disease, but they rarely occur. Guillain-Barré syndrome could occur several weeks after the diarrhea symptoms. This is due to the bacteria's role in mimicking the gangliosides of the neural system which can lead to the immune system attacking self cells. This may lead to paralysis, but is usually recoverable. Bacteremia may be a result but mostly effects the immunocompromised or elderly. This can lead to death in the host.
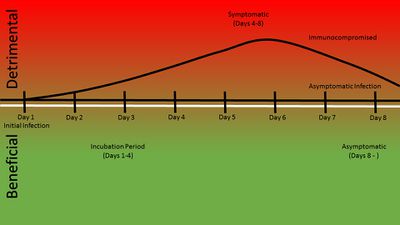
Morbidity and Mortality
Campylobacter has a high infection rate of 1.3 million a year and a low mortality rate of 76 a year. Mortality is usually due to rare complications such as Guillain-Barre syndrome and impoverished environments dehydration. For the most part Campylobacter passes through ones system without any residual effects.
Diagnosis
Diagnosis of Campylobacter is done by confirming its presence in the patient’s stool. The two methods currently used in the identification of the pathogen are growth on a selective medium such as Preston Campylobacter selective agar [10] and Polymerization Chain Reaction (PCR) [11] to determine the DNA presence of Campylobacter jejuni as well as other related species and subspecies.
Treatment
Treatment of Campylobacteriosis is done by managing the symptoms and any complications until the symptoms subside. Symptoms mainly include diarrhea leading to dehydration, and vomiting. Antibiotics can be used but are not usually administered unless serious complications arise. A majority of people recover from the symptoms within a week; however, some cases have known to take up to approximately 10 days.
Replacement of fluids and electrolytes lost during diarrhea and vomiting are keys to recovery and preventing symptoms from being prolonged. Water or rehydration drinks are recommended. Drinks such as soda and fruit juices contain too much sugar and too few electrolytes to be considered effective treatments for dehydration.
Maintaining a normal diet as much as possible will help in recovering faster. Avoid foods that have high fat and sugar content as well as spicy foods, alcohol, and coffee until approximately 2 days after symptoms subside.
Prevention
Several leading organizations including WHO, CDC, FDA, USDA, and state health departments have ongoing studies, investigations, and monitoring of Campylobacter across the world. Although the bacteria spreads through fecal oral transmission, a majority of infections occur from food born contamination, especially unpasteurized milk and poultry products.
Risk Avoidance
Proper food handling and hand washing skills are key practices to prevent the spread of Campylobacter jejuni.
• Make sure that the meat is cooked throughout (no longer pink in the center). All poultry should be cooked to at least an internal temperature of 165°F.
• Wash hands with soap before and after preparing food, especially raw meats.
• Prevent cross-contamination while preparing foods by using separate cutting boards for raw meats and other foods
• Cleaning all cutting boards, kitchen countertops, and silverware with soap and hot water.
• Do not drink unpasteurized milk or untreated surface water.
• Be sure that persons with diarrhea wash their hands carefully and frequently with soap to help reduce the risk of spreading the infection.
• Washing hands with soap after coming in contact with pet feces.
Immunization
Studies are still ongoing as to human vaccinations and immunizations against Campylobacter jejuni. Currently there are Immunizations available to chickens. Conventional methods of using heat or chemically killed vaccines have not completely protected against infection occurring. Using nanoparticales and constructing a DNA vaccine that targets the flagellum of the bacteria, which is a key mechanism of attachment in the gastrointestinal tract, have resulted in a significant decrease in the campylobacter’s ability to colonize the host.[12]
Host Immune Response
The innate immune response from the host includes several things such as TLR's (toll like receptors), serum type, and sugars. Campylobacter has evolved to specifically overcome a few of the immune system's most basic responses including the TLR5 receptors that recognize the flagellum of the bacterium, and also the TLR9 receptors responsible for recognition of CpG di-nucleotides present in Campylobacter. Other experiments have also shown the importance of downstream TLR's in the immune response and their stimulation against the disease. Macrophage activation from NOD1 receptors and their mediation play a part in Campylobacter's plan to circumvent the innate immune response. Studies have also been conducted as to the role of fucosylated sugars in breast milk and how their presence prevents the attachment of Campylobacter to the intestinal mucosa. C. jejuni is found to be sensitive to specific types of serum, emphizising the importance of compliment mediated responses of the immune response.
The Adaptive Immune response has developed several responses to many antigens present on the bacterium. Some tests using human volunteers have shown the earliest signs of antibody presence as approximately 6-7 days after inoculation. Of the antibodies that are detected there are several of isotypes. Almost unsurprisingly, many of the antigens recognized by antibodies come from Campylobacter's more prodominant virulence fators including the bacterial flagellum, many periplasmic proteins, capsular polysaccharides, and the CDT toxin.
References
1 Centers for Disease Control and Prevention (2010), Campylobacter <http://www.cdc.gov/nczved/divisions/dfbmd/diseases/campylobacter.>
2 “Campylobacter.” Wikipedia: The Free Encyclopedia. Wikimedia Foundation, Inc., (15 July 2013). Web. (14 July 2013). <http://en.wikipedia.org/wiki/Campylobacter>
3 Nordqvist, Christian. "Transmission Routes For The Bacterium Campylobacter." Medical News Today. MediLexicon, Intl., 28 Dec. 2006. Web. 16 Jul. 2013. <http://www.medicalnewstoday.com/releases/59254.php>
4 Curtis, Laurie. "Campylobacter." Food Safety Watch. Nov. 2007. Web. 13 July 2013. <http://www.foodsafetywatch.com/public/498.cfm>.
5 Vauxe, Robert. Centers for Disease Control and Prevention. 1 June 1988. Web. 16 July 2013. <http://www.cdc.gov/mmwr/preview/mmwrhtml/00001764.htm>.
6"Factors Associated with Geographic and Temporal Variation in Campylobacteriosis in Humans." Food Standards Agency. 5 Oct. 2011. Web. 13 July 2013. <http://food.gov.uk/science/research/devolvedadmins/scotlandresearch/scotlandresearch/ScotlandProjectList/s14004/#.UeLVY-At9bo>.
7 "Causes of Foodborne Illness: Bad Bug Book." Food and Drug Administration. 15 Mar. 2013. Web. 16 July 2013. <http://www.fda.gov/Food/FoodborneIllnessContaminants/CausesOfIllnessBadBugBook/ucm070024.htm>.
8 "Preliminary FoodNet Data on the Incidence of Infection with Pathogens Transmitted Commonly Through Food." Centers for Disease Control and Prevention. 10 Apr. 2009. Web. 16 July 2013. <http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5813a2.htm>.
9 S.P. Bhavsar, B.P. Kapadnis: Virulence factors of Campylobacter. The Internet Journal of Microbiology. 2007 Volume 3 Number 2. DOI: 10.5580/62b - See more at: http://archive.ispub.com/journal/the-internet-journal-of-microbiology/volume-3-number-2/virulence-factors-of-campylobacter.html#sthash.8GNLneuR.bzVE2MRq.dpuf
10 Grant, Christopher C. R., Michael. E. Konkel, Wietold Cieplak, Lucy Tompkins, "Role of Flagella in Adherence, Internalization, and Translocation of Campylobacter jejuni in Nonpolarized and Polarized Epithelial Cell Cultures" American Society for Microbiology. May 1993. <http://iai.asm.org/content/61/5/1764.long>.
11 Bolton F.J. and Robertson L., 1982, Journal of Clinical Pathology 35, pg. 462-467
12 Mao-Jun Zhang, Bo Qiao, Xue-Bin Xu, and Jian-Zhong Zhang, World J Gastroenterol. May 28,2013, 19(20): 3090–3095. Published online 2013 May 28. doi: 10.3748/wjg.v19.i20.3090, PMC3662949 <http://www.wjgnet.com/1007-9327/journal/v19/i20/>
13 Jin-lin Huang, Yan-Xin Yin, [...], and Xin-an Jiao, Intranasal Immunization with Chitosan/pCAGGS-flaA Nanoparticles Inhibits Campylobacter jejuni in a White Leghorn Model, Journal of Biomedicine and Biotechnology. 2010; 2010: 589476<http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2948919/>
Created by Halen Borron, Kelley Raines, and Evan Robinson, students of Tyrrell Conway at the University of Oklahoma.
Edited by Hae Cha and Derek Lehman, students of Tyrrell Conway at the University of Oklahoma.
