Peat Bogs: Difference between revisions
No edit summary |
|||
| (5 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
=Introduction= | =Introduction= | ||
Peat bogs are among the most widespread of [http://en.wikipedia.org/wiki/Wetland wetland] types today, characterized by acidic soil and peat mosses[[#References|[15]]]. They are often ombrotrophic, receiving nutrients only from dry and wet deposition such as rainfall[[#References|[13]]]. Due to constant water-saturation resulting in anoxia, the rate of net primary production by plants exceeds microbial decomposition[[#References|[13]]]. This long term accumulation of partially decomposed organic matter forms peat[[#References|[2]]]. With their unique characteristics, peat bogs are important ecosystems containing diverse organisms and are pertinent to global processes involving carbon and nitrogen, adding scientific value to the study of these bogs and their effects. | Peat bogs are among the most widespread of [http://en.wikipedia.org/wiki/Wetland wetland] types today, characterized by acidic soil and peat mosses<sup>[[#References|[15]]]</sup>. They are often ombrotrophic, receiving nutrients only from dry and wet deposition such as rainfall<sup>[[#References|[13]]]</sup>. Due to constant water-saturation resulting in anoxia, the rate of net primary production by plants exceeds microbial decomposition<sup>[[#References|[13]]]</sup>. This long term accumulation of partially decomposed organic matter forms peat<sup>[[#References|[2]]]</sup>. With their unique characteristics, peat bogs are important ecosystems containing diverse organisms and are pertinent to global processes involving carbon and nitrogen, adding scientific value to the study of these bogs and their effects. | ||
=Physical Environment= | =Physical Environment= | ||
| Line 8: | Line 9: | ||
[[File:Peat_Bogs_1.png|300px|thumb|right| A Canadian peat bog. Photo credits to Peupleloup under Creative Commons Licence[[#References|[12]]].]] | [[File:Peat_Bogs_1.png|300px|thumb|right| A Canadian peat bog. Photo credits to Peupleloup under Creative Commons Licence[[#References|[12]]].]] | ||
The majority of peat bogs are dominated by [http://en.wikipedia.org/wiki/Sphagnum ''Sphagnum''] mosses, rootless bryophytes[[#References|[10]]] that contribute to [http://en.wikipedia.org/wiki/Peat peat] formation. ''Sphagnum'' litter has antimicrobial properties allowing them to resist decomposition[[#References|[1]]]. These mosses out-compete other plants by forming a cold, acidic, nutrient-poor, and anoxic environment hostile to other forms of vegetation. The ''Sphagnum'' structure consists of polysaccharides with glucose and galacturonic acid units as well as phenols that contribute to environmental acidity[[#References|[14]]]. These conditions favour the accumulation of peat and deter the growth of many vascular plant species that require oxygen to survive. As a result, stunted trees and shrubs are seen on less water-saturated raised areas and have developed shallower root systems to avoid anoxic soil layers[[#References|[14]]].Such vascular bog plants include [http://en.wikipedia.org/wiki/Eriophorum_vaginatum hare’s-tail cottongrass] and the [http://en.wikipedia.org/wiki/Calluna common heather][[#References|[8]]][[#References|[1]]]. One species, ''Sphagnum cuspidatum'', consumes methane via symbiosis with methanotrophs that are found on their stem leaves and in hyaline cells (dead cells containing water and large pores). There, methanotrophs oxidize methane to carbon dioxide (CO<sub>2</sub>) which is then fixed by the mosses, allowing methane to be recycled within the bog[[#References|[16]]]. ''Sphagnum'' interactions with microbes and other plants are integral to maintaining the peat bog environment. | The majority of peat bogs are dominated by [http://en.wikipedia.org/wiki/Sphagnum ''Sphagnum''] mosses, rootless bryophytes<sup>[[#References|[10]]]</sup> that contribute to [http://en.wikipedia.org/wiki/Peat peat] formation. ''Sphagnum'' litter has antimicrobial properties allowing them to resist decomposition<sup>[[#References|[1]]]</sup>. These mosses out-compete other plants by forming a cold, acidic, nutrient-poor, and anoxic environment hostile to other forms of vegetation. The ''Sphagnum'' structure consists of polysaccharides with glucose and galacturonic acid units as well as phenols that contribute to environmental acidity<sup>[[#References|[14]]]</sup>. These conditions favour the accumulation of peat and deter the growth of many vascular plant species that require oxygen to survive. As a result, stunted trees and shrubs are seen on less water-saturated raised areas and have developed shallower root systems to avoid anoxic soil layers<sup>[[#References|[14]]]</sup>.Such vascular bog plants include [http://en.wikipedia.org/wiki/Eriophorum_vaginatum hare’s-tail cottongrass] and the [http://en.wikipedia.org/wiki/Calluna common heather]<sup>[[#References|[8]]]</sup><sup>[[#References|[1]]]</sup>. One species, ''Sphagnum cuspidatum'', consumes methane via symbiosis with methanotrophs that are found on their stem leaves and in hyaline cells (dead cells containing water and large pores). There, methanotrophs oxidize methane to carbon dioxide (CO<sub>2</sub>) which is then fixed by the mosses, allowing methane to be recycled within the bog<sup>[[#References|[16]]]</sup>. ''Sphagnum'' interactions with microbes and other plants are integral to maintaining the peat bog environment. | ||
===Hydrology=== | ===Hydrology=== | ||
Bogs have varied surfaces often with alternating hummocks (raised areas) and hollows (depressed areas) as well as lawns and pools[[#References|[14]]]. Lawns predominantly contain species that grow above the water table while pools consist of species growing below[[#References|[16]]]. The surface layer or acrotelm, is fairly permeable with large amounts of lateral water movement while the deeper layer, the catotelm, is relatively impermeable[[#References|[14]]]. Trapped methane gas plays a role in restricting water flow in deeper layers of the bog[[#References|[4]]]. To accumulate peat, bog systems must be continually saturated with water. Water loss increases aeration and decomposition, that can lead to an irreversible increase in peat permeability, altering the ecosystem as a whole[[#References|[14]]]. | Bogs have varied surfaces often with alternating hummocks (raised areas) and hollows (depressed areas) as well as lawns and pools<sup>[[#References|[14]]]</sup>. Lawns predominantly contain species that grow above the water table while pools consist of species growing below<sup>[[#References|[16]]]</sup>. The surface layer or acrotelm, is fairly permeable with large amounts of lateral water movement while the deeper layer, the catotelm, is relatively impermeable<sup>[[#References|[14]]]</sup>. Trapped methane gas plays a role in restricting water flow in deeper layers of the bog<sup>[[#References|[4]]]</sup>. To accumulate peat, bog systems must be continually saturated with water. Water loss increases aeration and decomposition, that can lead to an irreversible increase in peat permeability, altering the ecosystem as a whole<sup>[[#References|[14]]]</sup>. | ||
=Microbial Processes= | =Microbial Processes= | ||
| Line 32: | Line 33: | ||
===Nitrogen Mineralization=== | ===Nitrogen Mineralization=== | ||
Nitrogen is often sequestered in peat soil along with the accumulating organic matter. The release of inorganic nitrogen is strongly associated with microbial decomposition. Decreased cellulose decomposition is correlated with increased nitrogen [http://en.wikipedia.org/wiki/Mineralization_(soil) mineralization] which also decreases the amount of [http://en.wikipedia.org/wiki/Immobilization_(soil_science) immobilization] or nitrogen uptake by microbes[[#References|[15]]]. Mineralization dominates in soils with ''Sphagnum'' litter due to the lack of microbial activity associated with the chemical composition and toxic products produced by ''Sphagnum''. Instead, microbes decompose easily degradable compounds containing nitrogen so that inorganic nitrogen is released and not incorporated into microbial biomass[[#References|[15]]]. | Nitrogen is often sequestered in peat soil along with the accumulating organic matter. The release of inorganic nitrogen is strongly associated with microbial decomposition. Decreased cellulose decomposition is correlated with increased nitrogen [http://en.wikipedia.org/wiki/Mineralization_(soil) mineralization] which also decreases the amount of [http://en.wikipedia.org/wiki/Immobilization_(soil_science) immobilization] or nitrogen uptake by microbes<sup>[[#References|[15]]]</sup>. Mineralization dominates in soils with ''Sphagnum'' litter due to the lack of microbial activity associated with the chemical composition and toxic products produced by ''Sphagnum''. Instead, microbes decompose easily degradable compounds containing nitrogen so that inorganic nitrogen is released and not incorporated into microbial biomass<sup>[[#References|[15]]]</sup>. | ||
=Key Microorganisms= | =Key Microorganisms= | ||
| Line 41: | Line 42: | ||
[http://en.wikipedia.org/wiki/Alphaproteobacteria ''Alphaproteobacteria''] have been found to be the most abundant class of bacteria in peat bogs[[#References|[7]]], encompassing a large proportion of methanotrophs[[#References|[16]]]. Methanotrophs found in alpine peat bog ''Sphagnum mosses'', were similar to those of ''Methylocystis'', ''Methylomonas'', ''Methylocella'', and ''Methylosinus'' genera. Moss-associated methanotrophs can provide CO<SUB>2</SUB> to their host plant, though peat mosses obtain most of their carbon from autotrophic fixation of CO<SUB>2</SUB>[[#References|[3]]]. Besides methane oxidation, some of these methanotrophs such as Methylocapsa acidiphila are capable of atmospheric nitrogen fixation[[#References|[5]]], providing nitrogen essential for plant growth[[#References|[3]]]. | [http://en.wikipedia.org/wiki/Alphaproteobacteria ''Alphaproteobacteria''] have been found to be the most abundant class of bacteria in peat bogs<sup>[[#References|[7]]]</sup>, encompassing a large proportion of methanotrophs<sup>[[#References|[16]]]</sup>. Methanotrophs found in alpine peat bog ''Sphagnum mosses'', were similar to those of ''Methylocystis'', ''Methylomonas'', ''Methylocella'', and ''Methylosinus'' genera. Moss-associated methanotrophs can provide CO<SUB>2</SUB> to their host plant, though peat mosses obtain most of their carbon from autotrophic fixation of CO<SUB>2</SUB><sup>[[#References|[3]]]</sup>. Besides methane oxidation, some of these methanotrophs such as Methylocapsa acidiphila are capable of atmospheric nitrogen fixation<sup>[[#References|[5]]]</sup>, providing nitrogen essential for plant growth<sup>[[#References|[3]]]</sup>. | ||
===''Actinobacteria''=== | ===''Actinobacteria''=== | ||
[http://en.wikipedia.org/wiki/Actinobacteria ''Actinobacteria''] are present in large amounts in the peat layers, decomposing cellulose and other plant polymers[[#References|[11]]]. While most known species are aerobic, they are tolerant of anaerobic conditions, allowing them to survive deeper in peat bogs[[#References|[9]]]. Examples of ''Actinobacteria'' include ''Conexibacter woesei'', an aerobic bacterium widely distributed in peat soils and the acidophilic autotroph, ''Acidimicrobium ferooxidans''[[#References|[7]]]. | [http://en.wikipedia.org/wiki/Actinobacteria ''Actinobacteria''] are present in large amounts in the peat layers, decomposing cellulose and other plant polymers<sup>[[#References|[11]]]</sup>. While most known species are aerobic, they are tolerant of anaerobic conditions, allowing them to survive deeper in peat bogs<sup>[[#References|[9]]]</sup>. Examples of ''Actinobacteria'' include ''Conexibacter woesei'', an aerobic bacterium widely distributed in peat soils and the acidophilic autotroph, ''Acidimicrobium ferooxidans''<sup>[[#References|[7]]]</sup>. | ||
===''Acidobacteria''=== | ===''Acidobacteria''=== | ||
The [http://en.wikipedia.org/wiki/Acidobacteria ''Acidobacteria''] group contains the greatest diversity of bacteria in acidic ombrotrophic peat bogs. One example is an unnamed species closely related to ''Geothrix fermentans''. As an anaerobic chemoorganotroph, ''G. fermentans'' uses organic acids as electron donors and typically ferric iron as an electron acceptor, though it may also use humic acids such as those found in peat bogs as electron acceptors[[#References|[7]]]. | The [http://en.wikipedia.org/wiki/Acidobacteria ''Acidobacteria''] group contains the greatest diversity of bacteria in acidic ombrotrophic peat bogs. One example is an unnamed species closely related to ''Geothrix fermentans''. As an anaerobic chemoorganotroph, ''G. fermentans'' uses organic acids as electron donors and typically ferric iron as an electron acceptor, though it may also use humic acids such as those found in peat bogs as electron acceptors<sup>[[#References|[7]]]</sup>. | ||
=References= | =References= | ||
1 | <sup>1</sup> Bragazza, L., Buttler, A., Habermacher, J., Brancaleoni, L., Gerdol, R., Fritze, H., Hanajík, P., Laiho, R. and Johnson, D."High Nitrogen Deposition Alters the Decomposition of Bog Plant Litter and Reduces Carbon Accumulation." Global Change Biology, 2012, DOI: 10.1111/j.1365-2486.2011.02585.x | ||
2 | <sup>2</sup> Bragazza, L., Hájek, T., Iacumin, P., Kutnar, L., Tahvanainen, T., Toberman, H., Freeman, C., Jones, T., Rydin, H., Limpens, J., Fenner, N., Ellis, T., Gerdol, R., and Hájek, M. "Atmospheric Nitrogen Deposition Promotes Carbon Loss from Peat Bogs." Proceedings of the National Academy of Sciences of the United States of America, 2006, DOI: 10.1073/pnas.0606629104 | ||
3 | <sup>3</sup> Bragina, A., Berg, C., Müller, H., Moser, D. and Berg, G. "Insights into Functional Bacterial Diversity and its Effects on Alpine Bog Ecosystem Functioning." Scientific Reports, 2013, DOI: 10.1038/srep01955 | ||
4 | <sup>4</sup> Brown, A., Mathur, S. P. and Kushner, D. J. "An Ombrotrophic Bog as a Methane Reservoir." Global Biogeochemical Cycles, 1989, DOI: 10.1029/GB003i003p00205 | ||
5 | <sup>5</sup> Dedysh, S. N., Khmelenina, V. N., Suzina, N., Trotsenko, Y. A., Semrau, J. D., Liesack, W. and Tiedje, J. M. “Methylocapsa Acidiphila Gen. Nov., Sp. Nov., a Novel Methane-Oxidizing and Dinitrogen-Fixing Acidophilic Bacterium from Sphagnum Bog." International Journal of Systematic and Evolutionary Microbiology, 2002. | ||
6 | <sup>6</sup> Dedysh, S. N., Panikov, N. S. and Tiedje, J. M. "Acidophilic Methanotrophic Communities from Sphagnum Peat Bogs." Applied and Environmental Microbiology, 1998. | ||
7 | <sup>7</sup> Dedysh, S. N., Pankratov, T. A., Belova, S. E., Kulichevskaya, I. S. and Liesack, W. "Phylogenetic Analysis and in Situ Identification of Bacteria Community Composition in an Acidic Sphagnum Peat Bog." Applied and Environmental Microbiology, 2006, DOI: 10.1128/AEM.72.3.2110- | ||
2117.2006 | 2117.2006 | ||
8 | <sup>8</sup> Freitag, T. E., Toet, S., Ineson, P. and Prosser, J. I. "Links between Methane Flux and Transcriptional Activities of Methanogens and Methane Oxidizers in a Blanket Peat Bog." FEMS Microbiology Ecology, 2010, DOI: 10.1111/j.1574-6941.2010.00871.x | ||
9 | <sup>9</sup> Kotiaho, M., Fritze, H., Merilä, P., Tuomivirta, T., Väliranta, M., Korhola, A., Karofeld, E. and Tuittila, E. "Actinobacteria Community Structure in the Peat Profile of Boreal Bogs Follows a Variation in the Microtopographical Gradient Similar to Vegetation." Plant and Soil, 2013, DOI: 10.1007/s11104-012-1546-3 | ||
10 | <sup>10</sup> Lloyd, D., Thomas, K. L., Benstead, J., Davies, K. L., Lloyd, S. H., Arah, J. R. M. and Stephen, K. D. "Methanogenesis and CO<SUB>2</SUB> Exchange in an Ombrotrophic Peat Bog." Atmospheric Environment, 1998, DOI: 10.1016/S1352-2310(97)00481-0 | ||
11 | <sup>11</sup> Pankratov, T. A. "Acidobacteria in Microbial Communities of the Bog and Tundra Lichens." Microbiology, 2012, DOI: 10.1134/S0026261711060166 | ||
12 | <sup>12</sup> Peupleloup. “Tourbière/ Peat bog.” Photo. Flickr, 2008. 19 Nov. 2013. | ||
<http://www.flickr.com/photos/10601432@N08/3030857688/in/photolist-5BPWp3> | <http://www.flickr.com/photos/10601432@N08/3030857688/in/photolist-5BPWp3> | ||
13 | <sup>13</sup> Preston, M. D., Smemo, K. A., McLaughlin, J. W. and Basiliko, N. "Peatland Microbial Communities and Decomposition Processes in the James Bay Lowlands, Canada." Frontiers in Microbiology, 2012, DOI: 10.3389/fmicb.2012.00070 | ||
14 | <sup>14</sup> Van Breemen, N. "How Sphagnum Bogs Down Other Plants." Trends in Ecology & Evolution, 1995, DOI: 10.1016/0169-5347(95)90007-1 | ||
15 | <sup>15</sup> Verhoeven, J. T. A., Maltby, E. and Schmitz, M. B. "Nitrogen and Phosphorus Mineralization in Fens and Bogs." Journal of Ecology, 1990, DOI: 10.2307/2260894 | ||
16 | <sup>16</sup> Wolters-Arts, M., Derksen, J., Jetten, M. S. M., Smolders, A. J. P., Lamers, L. P. M., Sinninghe Damsté, J. S., Strous, M., Schouten, S., Schmid, M. C., Raghoebarsing, A. A, Roelofs, J. G. M., Rijpstra, W. I. C. and Op den Camp, H. J. M. "Methanotrophic Symbionts Provide Carbon for Photosynthesis in Peat Bogs." Nature, 2005, DOI: 10.1038/nature03802 | ||
Latest revision as of 20:21, 29 September 2015
Introduction
Peat bogs are among the most widespread of wetland types today, characterized by acidic soil and peat mosses[15]. They are often ombrotrophic, receiving nutrients only from dry and wet deposition such as rainfall[13]. Due to constant water-saturation resulting in anoxia, the rate of net primary production by plants exceeds microbial decomposition[13]. This long term accumulation of partially decomposed organic matter forms peat[2]. With their unique characteristics, peat bogs are important ecosystems containing diverse organisms and are pertinent to global processes involving carbon and nitrogen, adding scientific value to the study of these bogs and their effects.
Physical Environment
Vegetation

The majority of peat bogs are dominated by Sphagnum mosses, rootless bryophytes[10] that contribute to peat formation. Sphagnum litter has antimicrobial properties allowing them to resist decomposition[1]. These mosses out-compete other plants by forming a cold, acidic, nutrient-poor, and anoxic environment hostile to other forms of vegetation. The Sphagnum structure consists of polysaccharides with glucose and galacturonic acid units as well as phenols that contribute to environmental acidity[14]. These conditions favour the accumulation of peat and deter the growth of many vascular plant species that require oxygen to survive. As a result, stunted trees and shrubs are seen on less water-saturated raised areas and have developed shallower root systems to avoid anoxic soil layers[14].Such vascular bog plants include hare’s-tail cottongrass and the common heather[8][1]. One species, Sphagnum cuspidatum, consumes methane via symbiosis with methanotrophs that are found on their stem leaves and in hyaline cells (dead cells containing water and large pores). There, methanotrophs oxidize methane to carbon dioxide (CO2) which is then fixed by the mosses, allowing methane to be recycled within the bog[16]. Sphagnum interactions with microbes and other plants are integral to maintaining the peat bog environment.
Hydrology
Bogs have varied surfaces often with alternating hummocks (raised areas) and hollows (depressed areas) as well as lawns and pools[14]. Lawns predominantly contain species that grow above the water table while pools consist of species growing below[16]. The surface layer or acrotelm, is fairly permeable with large amounts of lateral water movement while the deeper layer, the catotelm, is relatively impermeable[14]. Trapped methane gas plays a role in restricting water flow in deeper layers of the bog[4]. To accumulate peat, bog systems must be continually saturated with water. Water loss increases aeration and decomposition, that can lead to an irreversible increase in peat permeability, altering the ecosystem as a whole[14].
Microbial Processes
Carbon Cycle
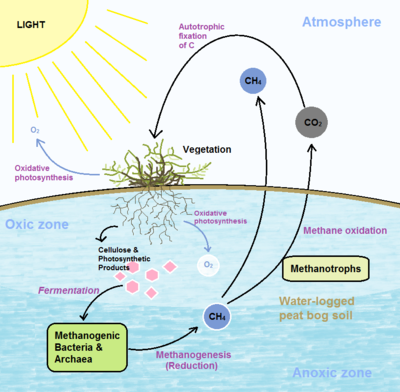
Peat bogs are significant contributors to the global carbon cycle, acting as large repositories of atmospheric carbon[13] and containing over one third of the organic carbon in global soils[16]. Emissions of methane and CO2 greenhouse gases occur primarily by two processes: Methanogenesis and methane oxidation.
Methanogenesis
Methanogenesis results in the production of methane gas that can then be oxidized by methanotrophs or released into the atmosphere. Bog methane is largely produced by methanogens performing anaerobic fermentation of carbohydrate substrates, such as cellulose[4] and photosynthetic products from the roots of vascular plants[10]. Degradation in peat bogs is slow and accomplished by anaerobic bacteria that degrade cellulose into 1 or 2 carbon compounds that can then be reduced to methane by methanogens[4] in waterlogged peat soils[8]. Peat bog anaerobes degrade approximately half the organic carbon that is eventually converted into methane which can negatively impact global warming[4].
Methane Oxidation
Methanotrophic bacteria are largely responsible for the oxidation of methane to CO2 which plays an important role in the peat bog ecosystem as a whole. Methane oxidation tends to occur at oxic-anoxic interfaces since methane-oxidizing bacteria such as Alphaproteobacteria and Gammaproteobacteria are restricted to soil depths and areas in which oxygen is present[8]. Vascular plants expand the oxic zone just beneath the peat surface by producing oxygen from oxygenic photosynthesis, which increases rates of bacterial respiration and methane oxidation[10].Methanotrophs are crucial for efficient recycling of both methane from decaying plants and oxygen from photosynthesis[16]. Nitrogen deposition also influences methane oxidation by increasing bacterial biomass along with hydrolytic and oxidative enzyme activity in surface peat, decreasing accumulation of litter carbon[1]. This loss of ability to act as a carbon sink has important implications for the global carbon cycle.
Nitrogen Mineralization
Nitrogen is often sequestered in peat soil along with the accumulating organic matter. The release of inorganic nitrogen is strongly associated with microbial decomposition. Decreased cellulose decomposition is correlated with increased nitrogen mineralization which also decreases the amount of immobilization or nitrogen uptake by microbes[15]. Mineralization dominates in soils with Sphagnum litter due to the lack of microbial activity associated with the chemical composition and toxic products produced by Sphagnum. Instead, microbes decompose easily degradable compounds containing nitrogen so that inorganic nitrogen is released and not incorporated into microbial biomass[15].
Key Microorganisms
Alphaproteobacteria
Alphaproteobacteria have been found to be the most abundant class of bacteria in peat bogs[7], encompassing a large proportion of methanotrophs[16]. Methanotrophs found in alpine peat bog Sphagnum mosses, were similar to those of Methylocystis, Methylomonas, Methylocella, and Methylosinus genera. Moss-associated methanotrophs can provide CO2 to their host plant, though peat mosses obtain most of their carbon from autotrophic fixation of CO2[3]. Besides methane oxidation, some of these methanotrophs such as Methylocapsa acidiphila are capable of atmospheric nitrogen fixation[5], providing nitrogen essential for plant growth[3].
Actinobacteria
Actinobacteria are present in large amounts in the peat layers, decomposing cellulose and other plant polymers[11]. While most known species are aerobic, they are tolerant of anaerobic conditions, allowing them to survive deeper in peat bogs[9]. Examples of Actinobacteria include Conexibacter woesei, an aerobic bacterium widely distributed in peat soils and the acidophilic autotroph, Acidimicrobium ferooxidans[7].
Acidobacteria
The Acidobacteria group contains the greatest diversity of bacteria in acidic ombrotrophic peat bogs. One example is an unnamed species closely related to Geothrix fermentans. As an anaerobic chemoorganotroph, G. fermentans uses organic acids as electron donors and typically ferric iron as an electron acceptor, though it may also use humic acids such as those found in peat bogs as electron acceptors[7].
References
1 Bragazza, L., Buttler, A., Habermacher, J., Brancaleoni, L., Gerdol, R., Fritze, H., Hanajík, P., Laiho, R. and Johnson, D."High Nitrogen Deposition Alters the Decomposition of Bog Plant Litter and Reduces Carbon Accumulation." Global Change Biology, 2012, DOI: 10.1111/j.1365-2486.2011.02585.x
2 Bragazza, L., Hájek, T., Iacumin, P., Kutnar, L., Tahvanainen, T., Toberman, H., Freeman, C., Jones, T., Rydin, H., Limpens, J., Fenner, N., Ellis, T., Gerdol, R., and Hájek, M. "Atmospheric Nitrogen Deposition Promotes Carbon Loss from Peat Bogs." Proceedings of the National Academy of Sciences of the United States of America, 2006, DOI: 10.1073/pnas.0606629104
3 Bragina, A., Berg, C., Müller, H., Moser, D. and Berg, G. "Insights into Functional Bacterial Diversity and its Effects on Alpine Bog Ecosystem Functioning." Scientific Reports, 2013, DOI: 10.1038/srep01955
4 Brown, A., Mathur, S. P. and Kushner, D. J. "An Ombrotrophic Bog as a Methane Reservoir." Global Biogeochemical Cycles, 1989, DOI: 10.1029/GB003i003p00205
5 Dedysh, S. N., Khmelenina, V. N., Suzina, N., Trotsenko, Y. A., Semrau, J. D., Liesack, W. and Tiedje, J. M. “Methylocapsa Acidiphila Gen. Nov., Sp. Nov., a Novel Methane-Oxidizing and Dinitrogen-Fixing Acidophilic Bacterium from Sphagnum Bog." International Journal of Systematic and Evolutionary Microbiology, 2002.
6 Dedysh, S. N., Panikov, N. S. and Tiedje, J. M. "Acidophilic Methanotrophic Communities from Sphagnum Peat Bogs." Applied and Environmental Microbiology, 1998.
7 Dedysh, S. N., Pankratov, T. A., Belova, S. E., Kulichevskaya, I. S. and Liesack, W. "Phylogenetic Analysis and in Situ Identification of Bacteria Community Composition in an Acidic Sphagnum Peat Bog." Applied and Environmental Microbiology, 2006, DOI: 10.1128/AEM.72.3.2110-
2117.2006
8 Freitag, T. E., Toet, S., Ineson, P. and Prosser, J. I. "Links between Methane Flux and Transcriptional Activities of Methanogens and Methane Oxidizers in a Blanket Peat Bog." FEMS Microbiology Ecology, 2010, DOI: 10.1111/j.1574-6941.2010.00871.x
9 Kotiaho, M., Fritze, H., Merilä, P., Tuomivirta, T., Väliranta, M., Korhola, A., Karofeld, E. and Tuittila, E. "Actinobacteria Community Structure in the Peat Profile of Boreal Bogs Follows a Variation in the Microtopographical Gradient Similar to Vegetation." Plant and Soil, 2013, DOI: 10.1007/s11104-012-1546-3
10 Lloyd, D., Thomas, K. L., Benstead, J., Davies, K. L., Lloyd, S. H., Arah, J. R. M. and Stephen, K. D. "Methanogenesis and CO2 Exchange in an Ombrotrophic Peat Bog." Atmospheric Environment, 1998, DOI: 10.1016/S1352-2310(97)00481-0
11 Pankratov, T. A. "Acidobacteria in Microbial Communities of the Bog and Tundra Lichens." Microbiology, 2012, DOI: 10.1134/S0026261711060166
12 Peupleloup. “Tourbière/ Peat bog.” Photo. Flickr, 2008. 19 Nov. 2013.
<http://www.flickr.com/photos/10601432@N08/3030857688/in/photolist-5BPWp3>
13 Preston, M. D., Smemo, K. A., McLaughlin, J. W. and Basiliko, N. "Peatland Microbial Communities and Decomposition Processes in the James Bay Lowlands, Canada." Frontiers in Microbiology, 2012, DOI: 10.3389/fmicb.2012.00070
14 Van Breemen, N. "How Sphagnum Bogs Down Other Plants." Trends in Ecology & Evolution, 1995, DOI: 10.1016/0169-5347(95)90007-1
15 Verhoeven, J. T. A., Maltby, E. and Schmitz, M. B. "Nitrogen and Phosphorus Mineralization in Fens and Bogs." Journal of Ecology, 1990, DOI: 10.2307/2260894
16 Wolters-Arts, M., Derksen, J., Jetten, M. S. M., Smolders, A. J. P., Lamers, L. P. M., Sinninghe Damsté, J. S., Strous, M., Schouten, S., Schmid, M. C., Raghoebarsing, A. A, Roelofs, J. G. M., Rijpstra, W. I. C. and Op den Camp, H. J. M. "Methanotrophic Symbionts Provide Carbon for Photosynthesis in Peat Bogs." Nature, 2005, DOI: 10.1038/nature03802