Leptospira Interrogans: Difference between revisions
Egratke4272 (talk | contribs) |
Egratke4272 (talk | contribs) |
||
| Line 49: | Line 49: | ||
==References== | ==References== | ||
1. [http://www.cdc.gov/leptospirosis/symptoms/ "Leptospirosis Signs and Symptoms." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 17 June 2011. Web. 21 Mar. 2015.] | 1. [http://www.cdc.gov/leptospirosis/symptoms/ "Leptospirosis Signs and Symptoms." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 17 June 2011. Web. 21 Mar. 2015.] | ||
<br>2. [http://www.vetbact.org/vetbact/?artid=101 | <br>2. [http://www.vetbact.org/vetbact/?artid=101 "VetBact." VetBact. N.p., n.d. Web. 22 Mar. 2015.] | ||
<br>3. [http://www.vetmed.wisc.edu/pbs/zoonoses/leptospira/leptohuman.html | <br>3. [http://www.vetmed.wisc.edu/pbs/zoonoses/leptospira/leptohuman.html "Leptospirosis in Humans." VetMed. N.p., n.d. Web. 22 Mar. 2015.] | ||
<br>4. [http://jb.asm.org/content/186/7/2164.full | <br>4. [http://jb.asm.org/content/186/7/2164.full Nascimento, A. L. T. O. et al. “Comparative Genomics of Two Leptospira Interrogans Serovars Reveals Novel Insights into Physiology and Pathogenesis .” Journal of Bacteriology 186.7 (2004): 2164–2172. PMC. Web. 26 Feb. 2015.] | ||
<br>5. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1949541/pdf/amjpathol00329-0024.pdf | <br>5. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1949541/pdf/amjpathol00329-0024.pdf Arean, Victor M., M.D. "The Pathologic Anatomy and Pathogenesis of Fatal Human Leptospirosis (Weil's Disease)." The American Journal of Pathology 40.4 (1962): 393- 423. Web. 25 Feb. 2015.] | ||
<br>6. [http://femsle.oxfordjournals.org/content/244/2/305.abstract | <br>6. [http://femsle.oxfordjournals.org/content/244/2/305.abstract Gamberini, Marcia, Ricardo M. Gomez, Marina V. Atzingen, Elizabeth AL Martins, Silvio A. Vasconcellos, Eliete C. Romero, Luciana C.C. Leite, Paulo L. Ho, and Ana L.T.O Nascimento. "Whole-genome Analysis of Leptospira Interrogans to Identify Potential Vaccine Candidates against Leptospirosis." FEMS Microbiology Letters 244.2 (2005): 305-13. Web. 24 Feb. 2015.] | ||
<br>7. [http://www.ncbi.nlm.nih.gov/pubmed/14652202 | <br>7. [http://www.ncbi.nlm.nih.gov/pubmed/14652202 Bharti, Ajay R., Jarlath E. Nally, Jessica N. Ricaldi, Michael A. Matthias, Monica M. Diaz, Michael A. Lovett, Paul N. Levett, Robert H. Gilman, Michael R. Willig, Eduardo Gotuzzo, and Joseph M. Vinetz. "Leptospirosis: A Zoonotic Disease of Global Importance." The Lancet Infectious Diseases 3.12 (2003): 757-71. Web. 25 Feb. 2015.] | ||
<br>8. [http://www.nature.com/nature/journal/v422/n6934/full/nature01597.html | <br>8. [http://www.nature.com/nature/journal/v422/n6934/full/nature01597.html Ren, Shuang-Xi, Gang Fu, Xiu-Gao Jiang, Rong Zeng, You-Gang Miao, Hai Xu, Yi-Xuan Zhang, Hui Xiong, Gang Lu, Ling-Feng Lu, Hong-Quan Jiang, Jia Jia, Yue-Feng Tu, Ju-Xing Jiang, Wen-Yi Gu, Yue-Qing Zhang, Zhen Cai, Hai-Hui Sheng, Hai-Feng Yin, Yi Zhang, Gen-Feng Zhu, Ma Wan, Hong-Lei Huang, Zhen Qian, Sheng-Yue Wang, Wei Ma, Zhi-Jian Yao, Yan Shen, Bo-Qin Qiang, Qi-Chang Xia, Xiao-Kui Guo, Antoine Danchin, Isabelle Saint Girons, Ronald L. Somerville, Yu-Mei Wen, Man-Hua Shi, Zhu Chen, Jian-Guo Xu, and Guo-Ping Zhao. "Unique Physiological and Pathogenic Features of Leptospira Interrogans Revealed by Whole-genome Sequencing." Nature 422.6934 (2003): 888-93. Nature. Web. 23 Mar. 2015.] | ||
<br>9. [http://www.labbies.com/lepto.htm | <br>9. [http://www.labbies.com/lepto.htm Davol, Pamela A. "Canine Leptospirosis." Wing-N-Wave Labradors. N.p., n.d. Web. 24 Mar. 2015.] | ||
<br>10. [http://onlinelibrary.wiley.com/doi/10.1111/j.1748-5827.1998.tb03640.x/abstract | <br>10. [http://onlinelibrary.wiley.com/doi/10.1111/j.1748-5827.1998.tb03640.x/abstract Birnbaum, N., S. C. Barr, S. A. Center, T. Schermerhorn, J. F. Randolph, and K. W. Simpson. "Naturally Acquired Leptospirosis in 36 Dogs: Serological and Clinicopathological Features." Journal of Small Animal Practice 39.5 (1998): 231-36. Web. 24 Feb. 2015.] | ||
<!--Do not remove this line--> | <!--Do not remove this line--> | ||
Revision as of 02:43, 14 April 2015
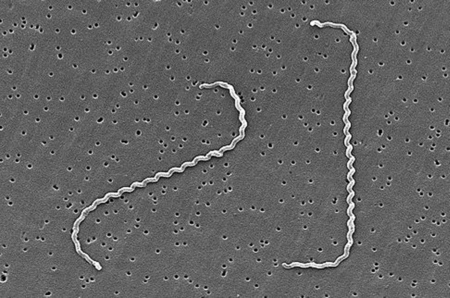
Leptospira interrogans is a thin, helical shaped bacteria that causes the zoonotic disease known as Leptospirosis. Leptospirosis is considered the most widespread zoonotic disease and can be deadly in severe, untreated cases. The spread of the disease can be attributed to the ability of L. interrogans to thrive in soil and water without a host for extended periods of time. The disease is difficult to prevent in humans due to the high amount of variety in serovars of L. interrogans, but current research is moving toward the creation of a human vaccination against Leptospirosis and a more indepth understanding of the genome and infectious mechanisms of the species.
Classification
Higher Order Taxa
Bacteria; Spirochaetes; Spirochaetia; Spirochaetales; Leptospiraceae; Leptospira
Species
Leptospira interrogans
Cell Structure and Metabolism
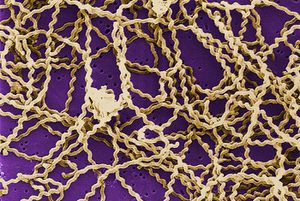
Leptospira interrogans is a gram negative bacteria. The cell is thin and spiral shaped with a hook on each end. It is motile and possesses two periplasmic flagella. The L. interrogans species can be broken down into roughly 250 serovars [2]. Leptospiral serovar diversity results from structural differences in carbohydrate component of lipopolysaccharides. Many serovars are adapted for specific mammalian reservoir hosts [4]. Leptospira interrogans is an aerobic bacteria and possesses both catalase and oxidase enzymes and does not ferment carbohydrates [2]. Leptospira species have only one glucose uptake system. This system is a glucose-sodium symporter that is dependent on a sodium gradient across the bacterial membrane. This limited system results in an inability to utilize glucose as an energy source in many environments. Consequently, Leptospira species utilize beta-oxidation of long-chain fatty acids as the major energy and carbon source instead of the more common sugar oxidative pathways [4].
Genomic Structure
The L. interrogans genome consists of 4,691,184 base pairs (bp). The genome consists of two circular chromosomes: a larger one of 4,332,241 bp (CI) and a smaller one of 358,943 bp [8]. Unlike most other bacteria, rRNA genes in L. interrogans are not organized in operons; they are scattered over the CI chromosome. Signal transduction mechanisms are regulated by at least 79 genes encoding two-component sensor histidine kinase-response regulator proteins. This allows L. interrogans to respond to a wide variety of environmental factors. Two copies of genes encoding BolA-like proteins can be identified in the L. interrogans genomes but not in the genomes of two other spirochetes, T. pallidum and B. burgdorferi. BolA has recently been linked to maintenance of cell shape in extreme conditions, suggesting that L. interrogans has this capability. The L. interrogans genome contains at least 263 genes that encode potential surface-exposed integral membrane protein [4]. At least 50 of the 4768 predicted genes identified in the genome sequence are related to motility [7].
Mechanisms of Infection
L. interrogans can be spread through the bodily fluids, excluding the saliva, of infected animals. The bacteria can enter the body through skin or mucous membranes and via consumption of contaminated water. Infected wild and domestic animals may continue to excrete the bacteria into the environment for up to several years and the bacteria can remain in soil and water for months at a time [1]. Virulence mechanisms of pathogenic leptospires, such as motility and chemotaxis responses, enable them to penetrate the host tissues during infection. These leptospires contain almost twice the number ofm ethyl-accepting chemotaxis proteins as compared to other spirochetes, indicating that the chemotaxis mechanism of L. interrogans is much more complex. Leptospira interrogans cells are also thought to secrete enzymes that degrade host cell matricies, allowing for faster invasion and infection [4]. Motility contributes greatly to the decimating effect of the disease from the site of entry to sites of end-organ damage in the lungs, liver, kidney, eyes, and brain [7].
Symptoms and Impact
Humans
Human infection by L. interrogans is classified as either icteric or anicteric. The anticteric form makes up 90% of infections and is less severe. The icteric form makes up 10% of infections and is very severe. The icteric form is also known as Weil’s Disease. Weil’s Disease is most often due to infection with serovar L. icterohaemorrhagiae, but humans are susceptible to infection via a variety of serovars [3]. Typically, symptoms begin to appear within two to four weeks of exposure. The infection can be divided into two phases: phase one which represents the anticteric infection, and phase two which represents the icteric infection. In phase one, symptoms include fever, chills, headache, muscle aches, vomiting, or diarrhea. These symptoms can often be mistaken for other infections, and in some cases, no symptoms appear [1]. If the infection progresses into phase two (Weil’s disease), symptoms include petechiae, hepatomegaly, jaundice, renal tubular damage and subsequent renal insufficiency. The disease can progress and affect almost all internal organs, producing inflammation and hepatic lesions that lead to organ failure [4]. If the infection and liver/renal failure are not promptly diagnosed and treated, recovery can take months, and mortality can reach 20% [3].
Other Mammals
During the first 4-12 days following infection, symptoms include fever, depression, vomiting, loss of appetite, conjunctivitis, and generalized pain. Within 2 days of the onset of these primary symptoms, body temperature may drop suddenly and there may be a noticeable increase in thirst [9]. Color intensity of the urine may vary from lemon to deep orange. This color change combined with jaundice can be the only diagnostic indicators of Leptospirosis. Additionally, frequent urination and subsequent dehydration (uremia) are consistent with invasion of the kidney tubule cells by L. interrogans [10]. In advanced cases of infection, difficulty breathing, muscular tremors, and bloody excrement are often observed. These symptoms appear as the infection progresses to include the liver, gastrointestinal system and other organs. Course and severity of the disease is often dependent upon the serovar responsible for the infection [9].
Prevention of Leptospirosis
Due to the common transmission of L. interrogans via rodents, one of the most effective methods of prevention in animals is minimizing rodent populations near animal residences. Pets can also be vaccinated against Leptospirosis, but the vaccine only inhibits a handful of serovars. There is no formal method of Leptospirosis prevention in humans. Chance of contraction can be reduced by minimizing or avoiding direct contact with animal excrement or water contaminated with animal waste [1].
Current Research
The vaccines currently available to target L. interrogans have low efficacy, are serovar-specific, and do not induce long-term protection against infection. Major limitations to improvements include the large number of pathogenic serovars and the cost of producing a multiserovar vaccine. It is anticpated that examination of candidate protective immunogens, such as conserved outer membrane proteins among serovars will provide new approaches for vaccine development [4]. In another study conducted in 2005, this approach was taken, and sixteen proteins, out of a hundred tested, were recognized by antibodies present in human sera. Four of these proteins were conserved among eight serovars of L. interrogans. These proteins could be used to develop a more effective vaccination [5]. Further sequencing and analysis of serovar genomes has the capacity to lead to a greater understanding and more accurate diagnosis of Leptospirosis caused by L. interrogans.
Further Reading
[1]—Genomic Center for Infectious Diseases, Leptospira Genomics and Human Health
References
1. "Leptospirosis Signs and Symptoms." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 17 June 2011. Web. 21 Mar. 2015.
2. "VetBact." VetBact. N.p., n.d. Web. 22 Mar. 2015.
3. "Leptospirosis in Humans." VetMed. N.p., n.d. Web. 22 Mar. 2015.
4. Nascimento, A. L. T. O. et al. “Comparative Genomics of Two Leptospira Interrogans Serovars Reveals Novel Insights into Physiology and Pathogenesis .” Journal of Bacteriology 186.7 (2004): 2164–2172. PMC. Web. 26 Feb. 2015.
5. Arean, Victor M., M.D. "The Pathologic Anatomy and Pathogenesis of Fatal Human Leptospirosis (Weil's Disease)." The American Journal of Pathology 40.4 (1962): 393- 423. Web. 25 Feb. 2015.
6. Gamberini, Marcia, Ricardo M. Gomez, Marina V. Atzingen, Elizabeth AL Martins, Silvio A. Vasconcellos, Eliete C. Romero, Luciana C.C. Leite, Paulo L. Ho, and Ana L.T.O Nascimento. "Whole-genome Analysis of Leptospira Interrogans to Identify Potential Vaccine Candidates against Leptospirosis." FEMS Microbiology Letters 244.2 (2005): 305-13. Web. 24 Feb. 2015.
7. Bharti, Ajay R., Jarlath E. Nally, Jessica N. Ricaldi, Michael A. Matthias, Monica M. Diaz, Michael A. Lovett, Paul N. Levett, Robert H. Gilman, Michael R. Willig, Eduardo Gotuzzo, and Joseph M. Vinetz. "Leptospirosis: A Zoonotic Disease of Global Importance." The Lancet Infectious Diseases 3.12 (2003): 757-71. Web. 25 Feb. 2015.
8. Ren, Shuang-Xi, Gang Fu, Xiu-Gao Jiang, Rong Zeng, You-Gang Miao, Hai Xu, Yi-Xuan Zhang, Hui Xiong, Gang Lu, Ling-Feng Lu, Hong-Quan Jiang, Jia Jia, Yue-Feng Tu, Ju-Xing Jiang, Wen-Yi Gu, Yue-Qing Zhang, Zhen Cai, Hai-Hui Sheng, Hai-Feng Yin, Yi Zhang, Gen-Feng Zhu, Ma Wan, Hong-Lei Huang, Zhen Qian, Sheng-Yue Wang, Wei Ma, Zhi-Jian Yao, Yan Shen, Bo-Qin Qiang, Qi-Chang Xia, Xiao-Kui Guo, Antoine Danchin, Isabelle Saint Girons, Ronald L. Somerville, Yu-Mei Wen, Man-Hua Shi, Zhu Chen, Jian-Guo Xu, and Guo-Ping Zhao. "Unique Physiological and Pathogenic Features of Leptospira Interrogans Revealed by Whole-genome Sequencing." Nature 422.6934 (2003): 888-93. Nature. Web. 23 Mar. 2015.
9. Davol, Pamela A. "Canine Leptospirosis." Wing-N-Wave Labradors. N.p., n.d. Web. 24 Mar. 2015.
10. Birnbaum, N., S. C. Barr, S. A. Center, T. Schermerhorn, J. F. Randolph, and K. W. Simpson. "Naturally Acquired Leptospirosis in 36 Dogs: Serological and Clinicopathological Features." Journal of Small Animal Practice 39.5 (1998): 231-36. Web. 24 Feb. 2015.
Edited by Emily Gratke, a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2015.
