Utilization of Bacillus thuringiensis in Genetically Modified Crops: Difference between revisions
| Line 15: | Line 15: | ||
==Life Cycle== | ==Life Cycle== | ||
[[Image:Bt_Life_Cycle.png|thumb|300px|right|Figure 2. Transition electron micrograph of <i>Bacillus thuringiensis</i> at the final stage of sporulation. The parasporal crystals are indicated using white arrows. These crystals are produced in the fourth through sixth stages of sporulation and are toxic to insects if consumed | [[Image:Bt_Life_Cycle.png|thumb|300px|right|Figure 2. Transition electron micrograph of <i>Bacillus thuringiensis</i> at the final stage of sporulation. The parasporal crystals are indicated using white arrows. These crystals are produced in the fourth through sixth stages of sporulation and are toxic to insects if consumed. http://www.tandfonline.com/doi/full/10.4161/bbug.1.1.10519]] | ||
The life cycle of <i>Bacillus thuringiensis</i> occurs in two cycles: vegetative cell division and sporulation. During cell division, a septum forms midway along the plasma membrane. The original vegetative cell then divides into two identical daughter cells. Sporulation of <i>Bacillus thuringiensis</i> is a far more complicated process that involves asymmetric cell division and is completed after a total of seven stages. Stage 1 is when the axial filament forms and stage 2 is when the forespore septum begins to form. Next, during stage 3, the parasporal crystals and the forespore begin to form. Stages 4 through 6 involve the development of the spore nucleoid as well as the formation of the spore cortex and coat. In the final stage, the spore becomes fully mature. Figure 2 shows an endospore at the end of the seventh stage of sporulation; it has a fully developed spore and several parasporal crystals within the endospore. [[#References|[9]]]. While sporulation is a common process for many microbes, the production of the parasporal crystals is unique to <i>Bacillus thuringiensis</i>. These toxic crystalline proteins are created due to the expression of <i>Cry</i> genes and are produced only during sporulation. These proteins constitute 20-30% of the cell dry weight. Once the spores are released into a soil environment, they are able to survive for between 6 months and a year. The germination speed of the spores greatly depends on the surrounding environment. There is little bacterial competition in an insect larvae gut, which is when there are slow rates of <i>Bacillus thuringiensis</i> spore germination. However, in the soil, the spores have a faster germination speed, which may enhance the microbe’s ability to survive. The type of soil, whether it is used for agricultural purposes or not, does not have an effect on the survival of <i>Bacillus thuringiensis</i> spores. Only intense heat-shock or extremely acidic soils are shown to kill off <i>Bacillus thuringiensis</i> spores [[#References|[11]]]. <br> | The life cycle of <i>Bacillus thuringiensis</i> occurs in two cycles: vegetative cell division and sporulation. During cell division, a septum forms midway along the plasma membrane. The original vegetative cell then divides into two identical daughter cells. Sporulation of <i>Bacillus thuringiensis</i> is a far more complicated process that involves asymmetric cell division and is completed after a total of seven stages. Stage 1 is when the axial filament forms and stage 2 is when the forespore septum begins to form. Next, during stage 3, the parasporal crystals and the forespore begin to form. Stages 4 through 6 involve the development of the spore nucleoid as well as the formation of the spore cortex and coat. In the final stage, the spore becomes fully mature. Figure 2 shows an endospore at the end of the seventh stage of sporulation; it has a fully developed spore and several parasporal crystals within the endospore. [[#References|[9]]]. While sporulation is a common process for many microbes, the production of the parasporal crystals is unique to <i>Bacillus thuringiensis</i>. These toxic crystalline proteins are created due to the expression of <i>Cry</i> genes and are produced only during sporulation. These proteins constitute 20-30% of the cell dry weight. Once the spores are released into a soil environment, they are able to survive for between 6 months and a year. The germination speed of the spores greatly depends on the surrounding environment. There is little bacterial competition in an insect larvae gut, which is when there are slow rates of <i>Bacillus thuringiensis</i> spore germination. However, in the soil, the spores have a faster germination speed, which may enhance the microbe’s ability to survive. The type of soil, whether it is used for agricultural purposes or not, does not have an effect on the survival of <i>Bacillus thuringiensis</i> spores. Only intense heat-shock or extremely acidic soils are shown to kill off <i>Bacillus thuringiensis</i> spores [[#References|[11]]]. <br> | ||
Revision as of 00:07, 5 May 2015
Introduction
By Zoë Frazier
The bacterium Bacillus thuringiensis is a gram-positive and rod-shaped microbe that is 2–5 µm in length and 1.0 µm wide [9]. It is found in soil ecosystems, moist environments, and within caterpillar guts [5]. Bacillus thuringiensis is non-pathogenic for most organisms expect for a variety of insect species. Bacillus thuringiensis contains a gene that is known to code for Cry proteins. These proteins are produced during the sporulation phase of the Bacillus thuringiensis lifecycle. The Cry proteins are crystalline in structure and incredibly toxin to insects if consumed. The insect species that are killed by a variety of different Bacillus thuringiensis strains include vegetable insects (tomato and tobacco hornworms), field crop insects (European corn borers, alfalfa caterpillars and webworms), fruit crop insects (Leaf roller and Achemon sphinx), and tree insects (tent caterpillars, fall webworm, pine butterfly, Western spruce budworm). Additionally, mosquitoes, black flies, fungus gnats, cottonwood leaf beetles, elm leaf beetles, and Colorado potato beetles can also be affected if they consume the toxic Cry proteins [4].
Historically the Cry proteins produced by Bacillus thuringiensis have been used as a topical pesticide. However, due to advances in biotechnology, scientists have developed a mechanism to extract the Cry gene and insert it into another organism’s genome. The Cry gene is inserted into common crops that are in high demand, such as corn, wheat, cotton, canola, soy, and potato crops. Once the Cry gene is inserted into the genome, the plant can express its own insecticidal properties. This genetic alteration results in plants that are continuously producing pesticides, which helps to cut down or even eliminate the usage of spray-on pesticides. This new technological advancement is so popular in agriculture that by 2014, a total of 93% of all corn crops in the United States contained the Cry gene [3]. There are many different strains of Bacillus thuringiensis, all of which can be used to genetically modify plants to be resistant to specific target organisms. Despite the many seeming advantages to using genetically modified crops, there are several ecological effects that could drastically change the success rate of Bt crops. Both target organisms and secondary pests have been observed to adapt or evolve to resist the toxic effects of Bt crops. The following page will further describe the integration of Bacillus thuringiensis into our modern day agricultural system along with the social and ecological implications associated with the usage of this microbe.
History
In 1901, a Japanese scientist by the name of Shigetane Ishiwata was the first to discover Bacillus thuringiensis. In the early twentieth century, Bacillus thuringiensis was the cause of a widespread disease that targeted silkworms. Ishiwata was able to isolate the microbe from samples of dead silkworm larvae. In 1927, a German scientist named Ernst Berliner isolated another strain of Bacillus thuringiensis and observed that the microbe developed endospores and parasporal crystals simultaneously. It was not until 1955 that Christopher Hannay finally determined that the parasporal crystals directly caused insect death. This discovery sparked research involving the potential uses for this naturally occurring insecticide. Finally in 1961, the first Bt-based insecticide was registered by the US Environmental Protection Agency to be used in the United States. However, the market for the Bacillus thuringiensis pesticide properties changed drastically in 1982 when the technology for the genetic modification of plants was developed. Scientists discovered that if they extracted the Cry gene from Bacillus thuringiensis, inserted it into a plasmid, and integrated that plasmid into a plants genome, the plant would express a resistance to insect activity. After several years of working with the plasmid, the first crop of transgenic plants was grown in 1996 [5].
Structure and Phylogeny
Bacillus thuringiensis is a rod-shaped microbe that appears in pairs or short chains. It is a gram-positive organism that is motile. Bacillus thuringiensis contains the hag gene, which encodes for the expression of flagellin. This protein is produced in large quantities and comes together to form the microbe’s flagellum. The presence of the hag gene is crucial for the process of H serotyping, which allows us to categorize different bacterial strains based on their antigens [9]. However, H serotyping sometimes is not able to differentiate all of the different strains of Bacillus thuringiensis. Additional technology for identifying the different Bacillus thuringiensis strains has been developed due to the importance of identifying novel strains.
Bacillus thuringiensis belongs to the phylum Firmicutes, which is known to include gram-positive bacteria that produce endospores. Bacillus thuringiensis is also in the genus Bacilli, which is characterized by rod-shaped bacteria that can be aerobic or facultative anaerobes. The closest relatives to Bacillus thuringiensis are Bacillus cereus, Bacillus anthracis, and Bacillus mycoides. Although they are all close relatives, Bacillus thuringiensis is unique due to its sporulation products and environmental habitats. During sporulation, Bacillus thuringiensis produces a crystalline protein byproduct. Additionally, Bacillus thuringiensis can live in the midgut of insects and in environments that are free of other gram-positive, spore-forming, bacillus bacteria.
Interestingly, a small number of strains of Bacillus thuringiensis are known to be pathogenic to humans. Genetic sequencing of these strains shows that the insecticidal Cry genes are not present. In fact, genetic analysis shows that the two pathogenic strains, Bt 97-27 and Bt Al Hakam, are more closely related to Bacillus anthracis and Bacillus cereus than other strains of Bacillus thuringiensis. Additional analysis of 16S and 23S rDNA nucleotide sequences show that Bacillus thuringiensis and Bacillus cereus must exchange genetic material and a fairly high frequency in their natural environment [9]. Overall, there are a total of five known Bacillus thuringiensis strains, but novel strains are still being discovered.
Life Cycle

The life cycle of Bacillus thuringiensis occurs in two cycles: vegetative cell division and sporulation. During cell division, a septum forms midway along the plasma membrane. The original vegetative cell then divides into two identical daughter cells. Sporulation of Bacillus thuringiensis is a far more complicated process that involves asymmetric cell division and is completed after a total of seven stages. Stage 1 is when the axial filament forms and stage 2 is when the forespore septum begins to form. Next, during stage 3, the parasporal crystals and the forespore begin to form. Stages 4 through 6 involve the development of the spore nucleoid as well as the formation of the spore cortex and coat. In the final stage, the spore becomes fully mature. Figure 2 shows an endospore at the end of the seventh stage of sporulation; it has a fully developed spore and several parasporal crystals within the endospore. [9]. While sporulation is a common process for many microbes, the production of the parasporal crystals is unique to Bacillus thuringiensis. These toxic crystalline proteins are created due to the expression of Cry genes and are produced only during sporulation. These proteins constitute 20-30% of the cell dry weight. Once the spores are released into a soil environment, they are able to survive for between 6 months and a year. The germination speed of the spores greatly depends on the surrounding environment. There is little bacterial competition in an insect larvae gut, which is when there are slow rates of Bacillus thuringiensis spore germination. However, in the soil, the spores have a faster germination speed, which may enhance the microbe’s ability to survive. The type of soil, whether it is used for agricultural purposes or not, does not have an effect on the survival of Bacillus thuringiensis spores. Only intense heat-shock or extremely acidic soils are shown to kill off Bacillus thuringiensis spores [11].
Bt Toxins

Bacillus thuringiensis, in both the vegetative cell state and the sporulation stage produces a variety of different toxins. The different strains of bacteria within this species produce varying amounts of toxins that can have different toxicity levels. The most commonly produced toxins are the insecticidal proteins, called Cry (crystal) delta-endotoxins (figure 3). There are over 120 types of the Cry proteins, all of which have varying effects on different organisms. For example, Cry1 proteins target insects from the Lepidoptera species. Furthermore, Cry3 proteins specifically target insects from the Coleoptera species. If an insect from the Lepidoptera species were to consume Cry3 proteins, it is unlikely that the organism would be killed.
The structure of the Cry proteins varies depending on the strain of Bacillus thuringiensis. The most common Cry protein structure is a three dimensional structure that consists of three different domains. The first domain is the N-terminal domain, which consists of several alpha-helices. The second and third domains are made of beta-sheets. The beta sheets within the second and third domains are crucial for the binding properties of the Cry proteins to the insect intestinal receptors. The initial structure of the Cry protein is not the activated form of the toxin; it must undergo several conformational changes before it can bind to insect gut receptors.
In order for the Cry protein to have an effect on an insect, it must be consumed in its crystalline form. The activation of the Cry protein is dependent on whether or not the protein is solubilized. The consumed protoxins are activated when they react with the high pH of the insect gut. This produces another form of the protoxins that then reacts with enzymes that exist within the insect’s gut. This final reaction produces the activated toxin. The toxin then binds to specific receptors that are found along the epithelial cells of the insect gut. The toxin binds, inserts itself into the cells, and creates small pores throughout the epithelial cells. The newly formed pores in the cellular membrane throw off the ion concentrations within the cell and allows water to rush into the cells. The gut cells quickly undergo lysis, the effected insect stops eating, becomes paralyzed, and dies. While the effect of Cry proteins varies slightly depending on the toxin strain and host combination, the overall process of binding and cellular death is fairly uniform [5].
Bt Crops
Crops with the incorporated Bacillus thuringiensis Cry gene have quickly taken over the agricultural market in the last several decades. While the usage of simple Bacillus thuringiensis crystals as a topical pesticide is still effective, the toxin is unable to protect the crops from any pests that may attack the plant below the surface of the soil. Crops that have successfully transformed the Cry gene into their own genome can suddenly produce their own Cry proteins. The result is new strains of plants that can protect themselves at all times both above and below the soil. These self-sufficient plants can now produce their own pesticides. The built-in insect resistance allows farmers to save the money they would spend on spraying pesticides while still producing a high yield of crops that are harvested.
As previously explained, the Cry gene encodes for the expression of Cry proteins. A genetically modified plant steadily produces the crystalline proteins if the Cry gene has been incorporated into its genome. The genetically modified plants that contain the Cry gene are known as Bt plants. If a target organism begins to eat a Bt plant, it also is consuming Cry proteins. The proteins are solubilized once they are consumed and activated into toxins. These toxins then bind to specific receptors that are found on the epithelial cells of the insect gut. They insert themselves into the epithelial cells, which create large pores in the cells. These pores change the osmolar balance of the cells, which results in cell death (figure 4). This process occurs rapidly, resulting in the quick death of the target organism before it further damages the crop. The type of insecticide and target organism varies depending on the strain of Bacillus thuringiensis from which the Cry gene was extracted. For example, the strain of Bacillus thuringiensis that is used to make Bt176 corn targets the cell receptors in the guts of European corn borers [1]. Therefore, there are a large variety of different strains of Bt crops that are engineered to resist specific insect activity.
Ethical Issues Surrounding Bt Crops
In the late 1990s and early 2000s, there was a high level of controversy associated with the usage of Bt crops. The increase in the world population and rapid globalization has resulted to a high demand of food. However, much to the dismay of many people, farmers began to turn to genetically modified food (GMF) to meet this high demand. The proponents of GMF argue that this biotechnology will help mankind in a variety of ways. GMF can increase the crop output and overall increase the world’s food supply, improve the self-life and quality of the products, enhance the nutritional content, and help the economy by increasing job opportunities. Additionally, some genetically modified crops could be engineered to recycle toxins in the soil [12]. Despite all the potential benefits for the human race, many people are against the usage of GMF. The public feared that the newly developed techniques used during the process of genetically modifying a plant were unreliable. Additionally, previous research conducted by Hansen Jesse et. al., indicated that the pollen produced by a commonly grown Bt corn strain, known as Bt176, had insecticidal effects on Monarch butterfly larvae. Their research showed a significant decrease in Monarch butterfly larval viability when populations were exposed to known environmental concentrations of the Bt176 corn pollen [8]. While this research was conducted over a decade ago, the potential ecological implications for the effect of genetically modified crop byproducts on non-target organisms is upsetting for many people. The unknown potential health consequences, along with the lenient product labeling regulations by the FDA and unknown ecological consequences, are some of the many reasons for the public unrest. Although the integration of the Cry gene into crops was an influential step in the field of biotechnology, the public backlash has inhibited genetically modified crops from being utilized in many parts of the world [6].
Evolved Resistance and Secondary Pests
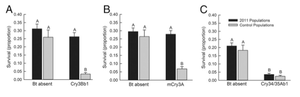
While the genetic modification of plants has helped the agricultural system flourish, insects are beginning to evolve a resistance to the Bt crops. Cry3Bb1, mCry3A, and Cry34/35Ab1 are three strains of Bt corn that are known to express a resistance to the western corn rootworm. However, in 2009, farmers began to observe severe damage to their Cry3Bb1 corn crops due to the presence of western rootworm larvae. Additionally, in 2010 there was evidence of western corn rootworm damage in mCry3A corn crops. Gassmann et. al., studied the possible evolved resistance of the western rootworm larvae to the Bt corn crops (2014). They tested the survival rate of western rootworm larvae when exposed to Bt and non-Bt corn crops. Additionally, they also tested the survival of western rootworm larvae that had been isolated in the US Department of Agriculture’s North Central Agricultural Research Laboratory (NCARL) since 2003. Their results determined that the control group of isolated western rootworm larvae was still killed off by the toxins released by Cry3Bb1, mCry3A, and Cry34/35Ab1. However, their results showed that wild western rootworm larvae inflicted the same amount of damage on Cry3Bb1 and mCry3A crops compared to non-BT crops. There was no apparent evolved resistance to the Cry34/35Ab1 strain (figure 5). This phenomenon can be explained due to the great phylogenetic similarity between the Cry3Bb1 and mCry3A strains. The toxins produced by Cry3Bb1 and mCry3A corn may be similar in structure and function, therefore, the western rootworm larva were able to evolve a resistance to both strains [7]. The reports for the evolved resistance to these strains are currently isolated to Iowa, however these results indicate that target organisms are able to evolve a resistance to the Bt crops.
An additional issue that has arisen since the usage of Bt crops is the emergence of secondary pests. Since the development of Bt crops, many farmers that had previously relied on the usage of spray-on pesticides have switched to genetically modified crops. The switch from topical pesticides to crops that naturally produce their own insecticide saves farmers a great deal of time and money. However, this change has allowed populations of insects that have been previously suppressed by the constant pesticide sprayings to reemerge. The new Bt crops are developed to effect target organisms, which means that the Bt toxins do not affect many of the previously suppressed insects species. For example, the cotton industry in China has been reestablished due to the introduction of Bt cotton strains that protect against bollworms. The usage of Bt cotton strains has meant that the farmers have greatly reduced their applications of pesticides during their growing season. Their crops are now protected from the bollworm, however the pests that were suppressed due to the frequent pesticide sprayings have began to flourish in the cotton crops. Due to the high level of damage from secondary pests, there seems to be no difference in productivity between the cotton farms that use Bt seeds and the cotton farmers that spray on their insecticides. The emergence of these secondary pests has been gradual and has gone unnoticed by most genetic engineers. Therefore, the companies developing the genetically engineered plants have not considered the effects of secondary pests [2]. The overall effects of the secondary pests on crop survival seem to negate all of the beneficial developments that have been made since the development of the Bt crops.
Conclusion
Bacillus thuringiensis has helped to shape our current agricultural system. In the mid-1990s, we discovered genetic engineering techniques that allowed us to alter crops. We isolated the Cry gene from Bacillus thuringiensis and incorporated it into the crops that we depend heavily on as a species. The result was plants that produce their own pesticides that selectively work on the primary pests that also feed on our highly valued crops. The usage of Bt crops is highly controversial around the world, but even the opponents cannot deny that these crops have much higher yields of food, and therefore increase the food availability for the exponentially growing human population. Although Bt crops have helped to increase food production, the effectiveness of this agricultural strategy may be short-lived. Evidence shows that target organisms are beginning to evolve a resistance to our Bt crops. Additionally, even successful Bt crops are beginning to experience damage due to the emergence of secondary pests that are no longer suppressed by spray-on pesticides. In order to maintain the high level of productivity that is expected from Bt crops, we may need to discover a variety of novel Bacillus thuringiensis strains that also target secondary pests. However, the overall effect on the environment may be too severe if we begin to eliminate all insect activity within the millions of acres that are dedicated to farming throughout this country. For now, it seems that the combination of spray-on pesticides and Bt crops may be our only way of maintain the high yield of food that our species relies on.
References
[1] Aeschbacher, K., Messikommer, R., Meile, L. & Wenk, C. (2005). Bt176 Corn in Poultry Nutrition: Physiological Characteristics and Fate of Recombinant Plant DNA in Chickens. Poultry Science Association, 84, 385 – 394.
[2] Catarino, R., G. Ceddia, F.J. Areal, and J. Park. (2015). The impact of secondary pests on Bacillus thuringiensis (Bt) crops. Plant Biotechnology Journal, 1-12.
[3] Cheeke, T.E., U.M. Schütte, C.M. Hemmerich, M.B. Cruzan, T.N. Rosenstiel, and J.D. Bever. (2015). Spatial soil heterogeneity has a greater effect on symbiotic aburscular mycorrhizal fungal communities and plant growth than genetic modification with Bacillus thuringiensis toxin genes. Molecular Ecology, 1-33.
[4] Cranshaw, W.S. Bacillus thuringiensis: Fact Sheet No. 5.556. Insect Series, Home and Garden. Colorado State Univserity.
[5] de Maagd, R.A. 2015. Chapter 20: Bacillus thuringiensis- Based Products for Insect Pest Control. Principles of Plant-Microbe Interactions. Springer International Switzerland, 2015. 185-192.
[6] Finucane, M.L. & J.L. Holup. (2005). Psychosocial and cultural factors affecting the perceived risk of genetically modified food: an overview of the literature. Social Science & Medicine, 60, 1603 – 1612.
[7] Gassmann, A.J. J.L., Petzold-Maxwell, E.H. Clifton, M.W. Dunbar, A.M. Hoffmann, D.A. Ingber, and R.S. Keweshan. (2014). Field-evolved resistance by western corn rootworm to multiple Bacillus thuringiensis toxins in transgenic maize. PNAS, 111(14), 5141-5146.
[8]Hansen Jesse, L.C. & J.J. Obrlycki. (2000). Field deposition of Bt transgenic corn pollen: lethal effects on the monarch butterfly. Oecologia, 125, 241 – 248.
[9] Ibrahim, M.A., N. Griko, M. Junker, and L.A. Bulla. 2010. Bacillus thuringiensis: A genomic and proteomics perspective. Bioengineered Bugs 1:1, 31-50.
[10] Jurat-Fuentes, J.L. "What Is Bacillus Thuringiensis (Bt)?" Bt Mode of Action. The University of Tennessee Institute of Agriculture. Web. <http://web.utk.edu/~jurat/Btresearchtable.html>.
[11] Petras, S.F. and L.E. Casida. (1985). Survival of Bacillus thuringiensis Spores in Soil. Applied and Environmental Microbiology, 59, 1496 – 1501.
[12] Uzogara, S.G. (2000). The impact of genetic modification of human foods in the 21st century: A review. Biotechnology Advances, 18, 179 – 206.
