Cladosporium sphaerospermum: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{ | {{Talbot2014}} | ||
{| | {| | ||
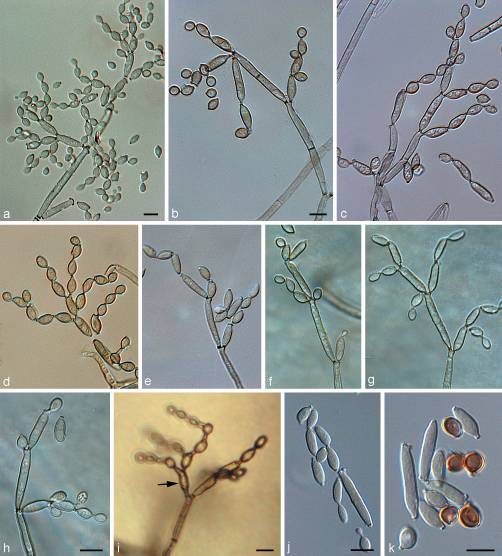
[[File:''C. spaerospermum''.jpg|500x500px|frame|thumb|''Cladosporium sphaerospermum''. Light micrographs of Cladosporium sphaerospermum NRRL 8131. a–h. Conidiophores at various stages of development, showing their characteristic branching patterns, ramoconidia, secondary ramoconidia, intercalary conidia, and small, terminal conidia (all on SNA); i. conidiophore with alternarioid secondary ramoconium (arrow), formed on MEA; j, k. secondary ramoconidia and intercalary conidia (note older intercalary conidia, which become dark brown and globose). — Scale bars = 10 μm. Reprinted with permission from Duban et al. 2008[[#References |[26]]]] | [[File:''C. spaerospermum''.jpg|500x500px|frame|thumb|''Cladosporium sphaerospermum''. Light micrographs of Cladosporium sphaerospermum NRRL 8131. a–h. Conidiophores at various stages of development, showing their characteristic branching patterns, ramoconidia, secondary ramoconidia, intercalary conidia, and small, terminal conidia (all on SNA); i. conidiophore with alternarioid secondary ramoconium (arrow), formed on MEA; j, k. secondary ramoconidia and intercalary conidia (note older intercalary conidia, which become dark brown and globose). — Scale bars = 10 μm. Reprinted with permission from Duban et al. 2008[[#References |[26]]]] | ||
Latest revision as of 15:21, 12 February 2016
Classification
| Kingdom | Fungi |
| Division | Ascomycota |
| Class | Dothideomycetes |
| Order | Capnodiales |
| Family | Cladosporiaceae |
| Genus | Cladosporium |
| Species | Cladosporium Sphaerospermum |
|
NCBI: [1] |
Introduction
Cladosporium Sphaerospermum is a cosmopolitan saprobic fungus that inhabits a variety of environments. Predominantly airborne, it is found in indoor and outdoor air and sampled from not only dwellings and plants but humans also [2]. As a halotolerant microorganism, C. sphaerospermum thrives in areas of high salinity. It can also proliferate in the areas of moderate and low salinity [3]. Phylogenetic analysis of RNA suggests that C. sphaerospermum is a complex fungal species encompassing a number of different strains. Recent studies show that C. sphaerospermum, an infectious and allergenic anthropologic fungus [4], can survive and thrive in the areas of high radioactivity and can reduce levels of radiation [5]. Moreover, industrial off-gas emissions, namely aromatic hydrocarbons, ketones and some organic acids can also be degraded by C. sphaerospermum [6], rendering the fungus a potential model to study natural biofiltration mechanisms. In addition, C. sphaerospermum can possibly become a substitute for chemical fertilizers due to its ability to produce gibberellins [7], plant growth hormones that are essential for plant growth and development [8].
Genome structure
Although a number of strains of C. sphaerospermum have been discovered, only one has been sequenced. C. sphaerospermum UM843 was isolated from human blood culture and the genome was sequenced in 2012. It is in the vicinity of 31.92Mb [8]. The genome consists of a total of 10,020 genes, with approximately 94% encoding for proteins of longer than 100 amino acids. The exon frequency in the proposed model was 2.26 exons per gene [8]. Among the genes detected in the gnome were genes associated with human allergens, the genes for asenolase, aldehyde dehydrogenase, and mannitol dehydrogenase. Some of the genes found in the genome of C. sphaerospermum are linked to the resistance to the antifungal drugs fluconazole, quinidine, and fluorocytosine. The genome also includes sequences encoding for the key enzymes in the melanin biosynthesis pathway [8].
Cell structure
Thick-walled flagellate cells of this fungus form a dikaryon in which, after cytoplasmic fusion of two cells (plasmogamy), the two nuclei cohabit without fusion. Dikaryotic cells are most common for the ascogenous hyphae and the ascocarp of the fungus rendering the rest of the mycelia monokaryotic. The spores of C. sphaerospermum have different shapes and are released through an apical pore [1]. Under magnification, the fungus appears to form tree-like structures principally assembled by branching chains of dark round conidia. Although conidia are 3-4.5 μm in diameter and are often single-celled, they frequently form chains by budding, leaving the youngest cell at the tip of the chain [1]. The older conidia might become oblong or shield-shaped and reach 15 μm in length. Upon budding, C. sphaerospermum conidia often undergo septation and consequently might have numerous constriction scars. At 30°C, Cladosporium sphaerospermum forms 1.0 cm in diameter powdered dark grey/green colored colonies that look like domes [1].
Metabolic processes
As a saccharaomycetae, C. sphaerospermum use various metabolic enzymes to convert glucose, sucrose and starch into carbon dioxide and alcohol [6]. Some of the C. sphaerospermum strains, however, use different types of metabolic adaptations to withstand extreme environments. Halotolerant C. sphaerospermum, for example, increases activity of extracellular invertase when grown in the environment of high salinity [9]. Other enzymes that increase activity in such environments are fructose 1,6-diphosphate aldolase, isocitrate lyase, and cytosolic malate dehydrogenase. Adaptive response of C. sphaerospermum extends to its ability to grow on toluene as a sole source of carbon and energy [10]. In fact, C. sphaerospermum is the first eukaryotic organism that was reported to catabolize toluene as the sole source of carbon and energy. C. sphaerospermum is also a producer of secondary metabolites. Among them are citrinin, quinolactacin A1 and A2, oxylipins [11], and melanin [12]. It is most likely that due to production of citrinin, which is a mycotoxin, some strains of C. sphaerospermum are considered plant pathogens because citrinin causes chromosome breakage, modification of amino acid uptake, inhibition of seed germination, and polyploidy in plants [13]. Quinolactacins are known tumor necrosis factor inhibitors [14]. However, the function of quinolactacins in C. sphaerospermum is not clear. Oxylipins, or oxidized fatty acids, include prostaglandins that are essential for fungal cell communication and viability [15].
Melanin metabolism.
Melanin is one of the secondary metabolites produced by C. sphaerospermum. It provides protection from ultraviolet light and oxidizing agents, as well as facilitates fungal proliferation in the areas with high radiation levels [16]. It is, however, unlikely that melanin is metabolized by C. sphaerospermum— solely for protection, as some microorganism can survive exposure to high radiation regardless of melanization. Mechanisms of melanin synthesis in C. sphaerospermum are chemically diverse and not yet well understood. However, there is evidence that this fungus produces melanin from an endogenous substrate via a 1,8-dihydroxynaphthalene (DHN) intermediate [16]. Recent microscopic studies show that granulated melanin is localized to the cell wall where it participates in cross-linking with polysaccharides. It is likely that the internal vesicles similar to mammalian melanosomes are the sites of melanin synthesis in C. sphaerospermum. Melanin is transported to the cell wall via these vesicles [17].
The use of ionizing radiation.
In light of recent accidents on nuclear power plants, particularly the one on Chernobyl power plant in 1986, it has been discovered that C. sphaerospermum can withstand high levels of radiation and use it to its advantage. Production of melanin by the fungus is linked to its ability to colonize areas of high radioactive contamination [16]. Moreover, in presence of radiation, C. sphaerospermum can thrive on high nutrient media as well as on the minimal nutrition media. Studies conducted with samples from Chernobyl indicate that change in electronic properties of melanin induces fungal proliferation [16]. After exposure to radiation the electronic structure of melanin changes. They also demonstrated that the ability of melanin to transfer electrons in the NADH oxidation/reduction reaction increased 4-fold [16]. The stable free radicals in melanin can interact with high-energy electrons that can damage fungal DNA. The interaction of the free radicals created by gamma radiation with the stable radicals in melanin protects DNA from radiation damage because the free radicals are prevented from entering the cell since melatonin is localized to the cell wall and extracellular space [16]. Moreover, the melanised fungal cells that were exposed to radiation levels 500 fold higher than background level grew considerably faster than nonmelanised fungus or the cells that received background level of radiation. Additional studies about the effects of radiation on C. sphaerospermum show directional growth of the fungus toward the source of radiation [18]. Thus, it is possible that C. sphaerospermum, facilitated by melanin, can capture ionizing radiation and use it for metabolic energy [16].
Degradation of volatile organic compounds.
Volatile organic compounds (VOC’s) degraded by melanised fungi include aromatic hydrocarbons, ketones, and organic acids. It has been discovered that C. sphaerospermum can use its metabolic machinery to degrade nine different VOC [5]. For example, toluene, which is toxic for central nervous system in humans and animals, can be degraded by the fungus and used as a single source of carbon and energy. In this fungus, methyl group of toluene is initially attacked to form benzoate via hydroxylation. By using NADPH and O2 to oxidize toluene, glycerol, EDTA, DTT, and PMSF, toluene monooxygenase catalases assimilation of toluene by the fungus [19]. Further hydroxylation of benzoate to 4-hydrozybenzoate leads to formation of protocatechuate as the ring fission substrate [10]. Benzene, ethylbenzene, styrene, methyl ethyl ketone methyl isobutyl ketone, and methyl propyl ketone, along with n-butyl acetate and ethyl 3-ethoxypropionate can be used by C. sphaerospermum as a sole carbon and energy source [5].
Gibberellins production.
One of the strains of C. sphaerospermum can potentially become a substitute for chemical fertilizers due to its ability to produce gibberellins, plant growth hormones that are essential for plant growth and development [7]. It has been shown that newly identified based on 18s rDNA sequence MH-6 strain of C. sphaerospermum is an endophytic fungus that produces nine different gibberellins inducing those responsible for maximal shoot elongation in plants. The mechanism by which gibberellins are produces by this fungus has yet to be elucidated. However, Hamayun et all determined that biosynthesis pathway of gibberellins in C. sphaerospermum is similar to that of F. fujicori, a known producer of gibberellins [21].
Ecology
C. sphaerospermum is a complex species that can grow in extreme and polar environments. This psychrotolerant, UV resistant, and halotolerant fungus can survive in Antarctica. It can also survive at 25-30°C in the areas where some of the strains were isolated in low salinity environments [3]. Saprobe, C. sphaerospermum also lives in symbiotic relationships with live plants. Some strains of C .sphaerospermum are able to adopt and thrive in areas exposed to high levels of ionizing radiation [4].
Pathology
C. sphaerospermum is one of the most commonly isolated airborne contaminants. Some of the strains of the fungus are not pathogenic to humans and animal; they are, however, detrimental to plants. Some of the species, can cause cerebral and cutaneous phaehyphomycoses, sinusitis, and peritonitis in humans [3]. In 2003 a case was reported of a woman who developed intrabronchial lesion due to C. sphaerospermum [22]. In animals, skin and lungs are the most affected by the fungus organs. For example, exposed to the fungus mice showed systemic and subcutaneous infections and even death in immunocompromised mice [21]. C. sphaerospermum can also cause erratic behavior in red snappers following infection of the bladder and kidney [20]. Eledone cirrhosa, the lesser octopus, is also not immune to infection by this fungus [8].
Future research avenues
- Since quinolactacin Al and quinolactacin A2 isolated from Pinicillium citrinum can inhibit acetylcholinesterase activity which is linked to the senile dementia in Alzheimer patients (24), it is possible that quinolactacin Al and quinolactacin A2 isolated from C. sphaerospermum also can be used in studies of patients with Alzheimer disease. C. sphaerospermum strains that produce quinolactacin Al and quinolactacin A2 could be gown in radioactive environment to induce proliferation of the fungus in order to obtain high yield of the metabolites. Determining what effects such metabolites would have on mammalian cells could be an avenue for further search of cure for Alzheimer’s disease.
- Prostaglandin E2 (PGE2), a hormone-like compound, is linked to smooth muscle functioning and immune response in humans (25). Since C. sphaerospermum can produce oxylipins, some of its strains cloud be synthesizing PGE2. This could explain why some individuals have allergic reaction to the fungus. The ability of the fungus to produce PGE2 is yet to be determined.
- Production of gibberellins by C. sphaerospermum renders it as a potential fertilizer. However, it is unknown if such use can induce adverse or beneficial reaction in mammals.
- Considering the ability of C. sphaerospermum to grow and capture ionizing radiation along with its metabolism of volatile organic compounds, this fungus could be used as a universal bioradioremediator.
References
[1] [Campbell, C., Johnson, E., & Warnock, D. W. (Eds.). (2013). Identification of Pathogenic Fungi (2nd Edition). Somerset, NJ, USA: John Wiley & Sons. Accessed 29 Oct. 2014, http://www.ebrary.com.] [2] [Zalar, P., De Hoog, G., Schroers, H., Crous, P., Groenewald, J. and Gunde-Cimerman, N. (2007). Phylogeny and ecology of the ubiquitous saprobe Cladosporium sphaerospermum, with descriptions of seven new species from hypersaline environments. Studies in Mycology, 58, pp.157--183.] [3] [Tasic S. and N. Miladinovic. 2007. Cladosporium spp. Cause of opportunistic Mycoses. ACTA FAC MED NAISS, 24 (1), pp. 15-19.] [4] [Dadachova, E. and Casadevall, A. (2008). Ionizing radiation: how fungi cope, adapt, and exploit with the help of melanin. Curr. Opin. Microbiol. 11: 525–531. [5] [Qi B., Moe W. M. and • K.A. Kinney. (2002). Biodegradation of volatile organic compounds by five fungal species. Appl Microbiol Biotechnol, 58:684–689.] [6] [Gottlieb, D. (1963). Carbohydrate catabolism by fungi. Pure and Applied Chemistry. 7(4).] [7] [Brian, P. W. (1959). Effects of Gibberellins on Plant Growth and Development. Biological Reviews, 34: 37–77.] [8] [Polglase, J., Dix, N. and Bullock, A. (1984). Infection of skin wounds in the lesser octopus, Eledone cirrhosa, by Cladosporium sphaerospermum. Transactions of the British Mycological Society, 82(3), pp.577--580.] [9] [Kalpana K., Τ. V. Parekh and H. S. Chhatpar. (1985). Salt mediated changes in some enzymes of carbohydrate metabolism in halotolerant Cladosporium sphaerospermum. J, Biosci. 9 (3,4), 12: 197–201.] [10] [Weber, F., Hage, K. and De Bont, J. (1995). Growth of the fungus Cladosporium sphaerospermum with toluene as the sole carbon and energy source. Applied and environmental microbiology, 61(10), pp.3562--3566.] [11] [Kjer J. New Natural Products from Endophytic Fungi from Mangrove Plants – Structure Elucidation and Biological Screening. (2009). Aus dem Institut für Pharmazeutische Biologie und Biotechnologie der Heinrich-Heine Universität Düsseldorf. Accessed 29 Oct. 2014] [12] [Ng, K., Yew, S., Chan, C., Soo-Hoo, T., Na, S., Hassan, H., Ngeow, Y., Hoh, C., Lee, K. and Yee, W. (2012). Sequencing of Cladosporium sphaerospermum, a Dematiaceous Fungus isolated from blood culture. Eukaryotic cell, 11(5), pp.705--706.] [13] [Abd-Allah, E. E. and Ezzat, S. M. (2005). Natural Occurrence of Citrinin in Rice Grains and ItsBiocontrol by Trichoderma hamatum. Phytoparasitica 33(1):73-84.] [14] [Kakinuma N., Iwai H., Takahashi S., Hamano K., Yanagisawa T., Nagai K., Tanaka K., Suzuki K., Kirikae F., Kirikae T., Nakagawa A. (2000). Quinolactacins A, B and C: novel quinolone compounds from Penicillium sp. EPF-6. I. Taxonomy, production, isolation and biological properties. J Antibiot (Tokyo). 53(11), pp.1247-51.] [15] [Andreou, A., Brodhun, F. and Feussner, I. (2009). Biosynthesis of oxylipins in non-mammals. Progress in Lipid Research, 48(3-4), pp.148-170.] [16] [Dadachova, E., Bryan, R., Huang, X., Moadel, T., Schweitzer, A., Aisen, P., Nosanchuk, J. and Casadevall, A. (2007). Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PloS one, 2(5), p.457.] [17] [Eisenman, H.C. and Casadevall, A. (2012).Synthesis and assembly of fungal melanin. Appl Microbiol Biotechnol. 93(3):931-40.] [18] [Zhdanova, N., Tugay, T., Dighton, J., Zheltonozhsky, V. and Mcdermott, P. (2004). Ionizing radiation attracts soil fungi. Mycological research, 108(09), pp.1089--1096.] [19] [Luykx, D., Prenafeta-Bold'u, F. and de Bont, J. (2003). Toluene monooxygenase from the fungus Cladosporium sphaerospermum. Biochemical and biophysical research communications, 312(2), pp.373--379.] [20] [Blaylock, R., Overstreet, R. and Klich, M. (2001). Mycoses in red snapper (Lutjanus campechanus) caused by two deuteromycete fungi (Penicillium corylophilum and Cladosporium sphaerospermum)*. Hydrobiologia, 460(1-3), pp.221--228.] [21] [Hamayun M, Khan SA, Khan AL, Rehman G, Kim YH, Iqbal I, Hussain J, Sohn E.Y. and Lee I. J. (2010) Gibberellin production and plant growth promotion from pure cultures of Cladosporium sp. MH-6 isolated from Cucumber (Cucumis sativus. L). Mycologia 102:989–995.] [22] [Yano, S., Koyabashi, K. and Kato, K. (2003). Intrabronchial lesion due to Cladosporium sphaerospermum in a healthy, non-asthmatic woman. Mycoses, 46(8), pp.330--332. ] [23] [Huyan, X., Yang, Y., Fan, Y., Huang, W., Li, W. and Zhou, Y. (2012). Cutaneous and systemic pathogenicity of a clinical isolate of Cladosporium sphaerospermum in a murine model. Journal of comparative pathology, 147(2), pp.354--359.] [24] [Won-Gon K., Nan-Kyu S. and Ick-Dong, Y. (2001). Quinolactacins Al and A2, new Acetylcholinesterase Inhibitors from Penicillium centrinum. The Journal of Antibiotics. 54(10), pp.831-835.] [25] [Kalinski, P. (2011). Regulation of Immune Responses by Prostaglandin E2. The Journal of Immunology, 188(1), pp.21-28.] [26] [Dugan, F. M, Braun, U., Groenewald J.Z. and Crous P.W. (2008). Morphological plasticity in Cladosporium sphaerospermum. Persoonia 21: 9 –16.]
Edited by [Sviatlana Rose], student of Jennifer Talbot for BI 311 General Microbiology, 2014, Boston University.
