User:S4288871: Difference between revisions
No edit summary |
No edit summary |
||
| Line 4: | Line 4: | ||
<ref>MICR3004</ref> | <ref>MICR3004</ref> | ||
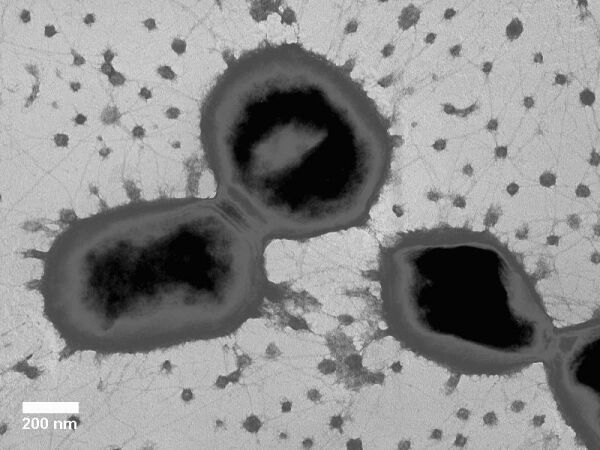
[[File:PorphyromonasGingavalisMorphology.jpeg| | [[File:PorphyromonasGingavalisMorphology.jpeg|type|border|right|middle|upright|link=Link|alt=Alt|Electron micrograph of the budding vesicles and fimbriae of strain ATCC 33277]] | ||
==Classification== | ==Classification== | ||
Revision as of 11:03, 18 September 2016
Louise Chan Bench E 31082016 [1]
Classification
Higher order taxa
Bacteria – Bacteroidetes – Bacteroidia– Bacteroidales – Porphyromonadaceae – Porphyromonas
Species
Species: P. gingivalis
Type Strain: Strain 2561 = ATCC 33277= CCUG 25893 = CCUG 25928 = CIP 103683 = DSM 20709 = JCM 12257 = NCTC 11834 [1].
Description and significance
P. gingivalis is a gram negative, non-sporeforming bacteria that forms convex black colonies when cultured on blood agar medium [2]. It was first isolated in 1977 from the gingival sulcus of patients with advanced periodontitis [3]. These bacteria appear as non-motile long rods or coccobacilli that are approximately 0.5 by 1-2µm in size [2]. It is most commonly found in subgingival plaque on tooth surfaces and gingival sulcular epithelial cells, and has also been occasionally been found in the healthy gingival margin [4,5]. P. gingivalis is an opportunistic pathogen that has co-evolved with Homo sapiens over a period of at least 10,000 years [6,7].
P. gingivalis is considered a 'keystone' biofilm species that is part of the "Red Complex", a group of bacteria that are categorised by their association with severe forms of periodontitis [8]. It causes the resorption of alveolar bone at the surface of the tooth root and leads to tooth loss . This is mediated through several virulence factors including lipopolysaccharide (LPS), fimbriae, hemagglutinins, capsular proteins, and proteolytic enzymes [9]. In addition to this, it has been suggested that P. gingivalis has a significant role in increasing virulence of interacting bacteria [10]. Furthermore, its ability to evade and subvert the host immune surveillance following the invasion of epithelial cells leads to the persistence of several other oral pathogens [11].
Genome structure
Strain ATCC 33277 is the type strain of P. gingivalis. It has a single circular chromosome of 2,354,886 bp, an average G + C content of 48.4% and does not contain any plasmids. 2090 coding sequences have been identified in the genome of ATCC 33277, making up 86.1% of the genome. The genome has 53 tRNA genes that provide the specificity for producing all necessary amino acids and 4 RNA operons [12].
ATCC 33277 has a total of 93 Insertion Sequence (IS) elements that have the ability to independently transpose, and 48 miniature inverted-repeat transposable elements (MITES) that are dependent on IS element transposase. The genome also has an abundance of other mobile genetic elements including novel conjugative transposons (CTns) encoding Na+-driven multi-drug efflux pumps and composite transposons (Tns) encoding tetR family transcriptional regulators, proteases and ABC transporter ATP-binding proteins. It has previously been proposed that P. gingivalis is assaccharolytic due to a nonsense mutation in the glucose kinase-encoding gene (glk). An insertion of MITE239 was identified in the glk gene of ATCC 33277, however, no nonsense mutation was identified. Nonsense mutations were not identified in the glk gene of five other strains of P. gingivalis, suggesting that glk may not be the only determinant of assaccharolysis [12].
ATCC 33277 is a less virulent strain of P. gingivalis and has been useful in genomic comparative studies for identifying virulence genes in other strains. Many of these virulence genes are located on pathogenicity islands and have been introduced through lateral gene transfer. A comparison of the ATCC 33277 genome to the virulent strain W83 revealed the absence of a cluster of ORFS in ATCC 332277 that are involved in the synthesis of capsular polysaccharide [12].
Cell structure and metabolism
P. gingivalis is a gram-negative bacteria that contains many virulence factors in its cell wall, including lipopolysaccharide (LPS), proteases, hemogglutinin, lipoproteins, and fimbriae [13]. LPS mediates a cellular proinflammatory response by activating nuclear factor-κB (NF-κB) ,Toll-like receptor 4 (TLR4) and TLR2, inducing the production of cytokines such as interleukin 1 beta (IL1β), interleukin 6 (IL-6) and tumour necrosis factor alpha (TNF-α) [14]. Several proteases are associated with the outer membrane of the cell wall including Arg- and Lys- gingipain cysteine proteinases (Rgp and Kgp), collagenase and aminopeptidases. Aminopeptidase enzymes provide peptides for growth of P. gingivalis. Enzymes such as gingipains and collagenases neutralise the innate immune system by hydrolysing serum and tissue proteins as well as proenzymes and T-cell receptors [15]. Hemogglutinin is another protein inbedded within the cell wall. This protein enables P. gingivalis to agglutinate erythrocytes. Hemogglutinin B (HagB) has also been found to have a role in the adhesion of P. gingivalis to host tissue in a similar mechanism to adhesins such as fimbriae [16]. The fimbriae of P. gingivalis are primarily used for adhesion and colonisation. Polymerized fimbrillin (FimA) interacts with extracellular matrix, bacteria and immune cells through modulation by its regulatory protein (FimB) and accessory proteins (FimCDE) [17,18]. Fimbriae facilitate in the invasion of P. gingivalis into host cells through the binding of β1 integrin on the host cell surface [19]. Subsequently, P. gingivalis is actively internalised into the cell and can remain dormant or replicate within epithelial cells without detection by the host immune system. Other cell wall associated proteins include OmpA-like proteins, porins, and lipoproteins such as RagB and RagA that form a receptor complex for the active transportation of hydrolysed proteins[13].
These virulence factors facilitate the colonisation of P. gingivalis in the oral environment and biolfilm formation. P. gingivalis is known as a late coloniser of the periodontal biofilm. Gram positive bacteria such as Streptococci are early colonisers that initially adhere to the salivary pellicles of the teeth through the use of adhesins. Intermediate colonisers such as Fusobacterium nucleatum, contain multiple adhesins and these act as bridging bacteria for P. gingivalis. It’s recruitment to the biofilm can alter the biofilm structure by increasing the overall bacterial load and increase the virulence of other bacteria within the community [11].
P. gingivalis is an assaccharolytic organism that requires the presence of amino acids as an energy source. Extracellular proteins are hydrolysed by cysteine proteinases, Rgp and Kgp, into oligopeptides which are further hydrolysed into di- and tri- peptides by aminopeptidases. These peptides are transported into the cell and further hydrolysed by specific exopeptidases within the periplasmic space [20]. The amino acids are metabolised, forming important substrates pyruvate, succinate and α-ketoglutamate. These are further metabolised and short chain fatty acids propionate, butyrate and acetate are excreted. It has been found that P. gingivalis preferentially metabolises glutamate/glutamine and asparate/asparagine for the production of ATP, although other amino acids such as serine are also metabolised [21]. P.gingivalis also has an absolute requirement for iron in its growth and for its virulence [22].
Ecology
P. gingivalis is an obligate anaerobe that is most commonly associated with the human oral cavity. Although present at low levels in the healthy human oral microbiome, it is most prevalent within subjects with periodontal disease. As such, its main niche within the oral cavity is on and within the periodontum (tissues damaged by periodontal disease) [6]. P. gingivalis has been identified in other areas of the body, however it does not naturally inhabit these regions. It has also been shown to survive and replicate within the environmental amoeba Acanthamoeba castellani, suggesting a possible environmental reservoir[23].
To aid in its survival within the oral cavity, P. gingivalis can invade the epithelial cells lining the host gingival tissues [24]. Gingival epithelial cells are typically the first cells to be colonised. These cells are an important first line of defence in the gingival innate immune system. They exhibit the purinergic receptor P2X7, which is responsive to ATP-induced apoptosis. However, P. gingivalis has evolved to circumvent this by consuming the ATP signal, preventing cell apoptosis [25]. Furthermore, it can replicate and disseminate to surrounding cells without rupturing the cell [19]. These features enable P. gingivalis to effectively shield itself from the host immune system and thus, secure its long term survival.
Pathology
P. gingivatis is one the key-stone organisms in the pathogenesis of periodontits, one of the most common chronic diseases which affects millions of people each year. It plays a dual role in the disease, firstly, in the development of a virulent biofilm and then secondly, in the progression of disease. Dental plaque can be associated with both healthy and diseased host tissue, and it is this plaque which consists of a poly-microbial biofilm [26]. P. gingivalis is considered a late-coloniser of dental biofilms. It binds to 'bridging' organisms such as Fusobacterium nucleatum and signals a change in the biofilm, elevating the virulence of the community and initiating disease [27]. Periodontal health is characterised by a balance between pro- and anti-inflammatory mediators [28]. P. gingivalis is capable of inhibiting normal host defence functions, such as IL-8, which disrupts the migration of neutrophils into the gingival crevice allowing for a more amenable environment for microbial colonisation [29]. All of this functions to increase the concentration of inflammatory mediators, and subsequently leads to the resorption of alveolar bone - a hallmark of periodontitis [26].
Application to biotechnology
Several different candidate vaccines for P. gingivalis have been developed and theses have focused on using the surface antigens which include capsule, LPS, fimbriae, outer-membrane proteins (OMPs), ginigpains and hemagglutinin [13]. Most of these methods have been shown to successfully attenuate the severity of disease. However, the majority of these antigens have several different serotypes and therefore limit the effectiveness of the vaccine to provide complete or adequate protection against all P. gingivalis strains. Therefore, a change in strategy will be needed to combat this issue. A recent study showed that outer membrane vesicles, which display a wide range of surface antigens, were effective at inhibiting gingipain proteolytic activity in the vaccine strain as well as heterologous strains [30]. Although promising, at present, there has been little if any vaccines trialed with humans and therefore a vaccine against P. gingivalis is still some time off.
Current research
Summarise some of the most recent discoveries regarding this species.
Recent research has highlighted the link between chronic inflammation and systemic disease. Periodontitis is the most common form of chronic inflammation and has been associated with Rheumatoid Arthritis (RA), a disease characterised by inflammation at the joints. Several studies have shown elevated rates of teeth loss and inflammation in RA patients compared to controls [31]. In a recent study,P. gingivalis was found to encode a unique enzyme, Peptidyl-arginine deiminase, which citrullinates host proteins [32]. RA is characterised by an autoimmune response to proteins with this modification [33].
Periodontal disease is recognised as a risk factor for the development of coronary heart disease, however, the mechanism by which this occurs is still an active field of research. Animal models have shown that disseminated P. gingivalis is capable of causing damage consistent with the histology of cardiovascular disease [34]. Liu et al. showed that cholesterol metabolism of macrophages is affected when challenged with P. gingivalis lipopolysaccharide (LPS) [35]. Macrophage transformation into lipid-loaded foam cells is an early process in the formation of atherosclerosis (thickening of the arterial walls) [36]. Another study used a mouse model to show that P. gingivalis enhanced cardiac hypertrophy by increasing the production of reactive oxygen species TLR2 and Nox4 [37].
An emerging theme in oncology is the role that inflammation, and more specifically, microorganism-induced inflammation, plays in the pathogenesis of cancer. At present, Helicobacter pylori is the only bacteria implicated in the development of cancer, with viruses making up the majority (e.g. Human papillomavirus, Hepatitis B and C etc.). However, the link between inflammation and cancer development is driving more research into the role of organisms such as P. gingivalis due to its association with chronic disease. Gao et al. found an association between infection with P gingivalis and the progression of esophageal squamous cell carcinoma [38], and a more recent study has shown that exposing oral squamous cell carcinoma cells to P. gingivalis increased the invasive ability of the oral cancer cells [39].
References
1. Parte A. 2016. Porphyromonas. Bacterio.net.
- ↑ MICR3004
This page is written by Louise Chan for the MICR3004 course, Semester 2, 2016