Zoonosis: Brucellosis in Animals and Humans: Difference between revisions
| Line 2: | Line 2: | ||
==Introduction== | ==Introduction== | ||
<br>By Hannah Wedig<br>< | <br>By Hannah Wedig<br> [[Image:BPPH.jpg|thumb|300px|right| <b>Figure 1.</b> The phylogeny of the genus <i>Brucella</i> aligned with the phylogeny of their respective, preferred host species. The width of the cones in the <i>Brucella</i> phylogeny is proportional to the number of strains analyzed for that species. The host phylogeny is represented in millions of years. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4026726/ <ref name = Moreno>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4026726/. Moreno, E. 2014. Retrospective and prospective perspectives on zoonotic brucellosis. <i>Front Microbiol.</i>, 5:1–18.]</ref>]]] | ||
Brucellosis is among the most common and highly contagious zoonoses. Zoonoses are diseases which can be transmitted from animals to humans. <ref name = “Seleem”>[http://www.sciencedirect.com/science/article/pii/S0378113509003058. Seleem, M. N., Boyle, S. M., Sriranganathan, N. 2010. Brucellosis: A re-emerging zoonosis. <i>Veterinary Microbiology</i> 140:392–398.]</ref> | Brucellosis is among the most common and highly contagious zoonoses. Zoonoses are diseases which can be transmitted from animals to humans. <ref name = “Seleem”>[http://www.sciencedirect.com/science/article/pii/S0378113509003058. Seleem, M. N., Boyle, S. M., Sriranganathan, N. 2010. Brucellosis: A re-emerging zoonosis. <i>Veterinary Microbiology</i> 140:392–398.]</ref> In the case of brucellosis, mammals – domestic, wild, terrestrial, and marine – are capable of transmitting the disease. <ref name = “Seleem”>[http://www.sciencedirect.com/science/article/pii/S0378113509003058. Seleem, M. N., Boyle, S. M., Sriranganathan, N. 2010. Brucellosis: A re-emerging zoonosis. <i>Veterinary Microbiology</i> 140:392–398.]</ref> <ref name = "Taleski">[http://www.sciencedirect.com/science/article/pii/S037811350200250X. Taleski, V., Zerva, L., Kantardijev, T., Cvetnic, Z., Erski-Biljic, M. et al. 2002. An overview of the epidemiology and epizootiology of brucellosis in selected countries of Central and Southeast Europe. <i>Veterinary Microbiology</i> 90:147–155.]</ref> Brucellosis is caused by certain species of bacteria in the genus <i>Brucella</i>, the most common and virulent of which are <i>B. melitensis, B. suis,</i> and <i>B. abortus</i> (<b>Figure 1</b>). <ref name = "Hagan">Hagan, W. A., Bruner, W. D. <i>Hagan and Bruner’s Microbiology and Infectious Diseases of Domestic Animals: With Reference to Etiology, Epizootiology, Pathogenesis, Immunity, Diagnosis, and Antimicrobial Susceptibility</i>. Ithaca: Comstock Publ., 1992. Print.</ref> <ref name = "Young">[http://www.antimicrobe.org/new/b87.asp. Young, E. J., M.D. “Brucella species (Brucellosis).” Infectious Disease and Antimicrobial Agents. Antimicrobe, 2014 Web.]</ref> The genus <i>Brucella</i> is within the class <i>α-Proteobacteria</i>, which includes many bacterial parasites of both plants and animals. <ref name = "Moreno and Moriyon">[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC117501/. Moreno, E. and Moriyon, I. 2002. <i>Brucella melitensis</i>: A nasty bug with hidden credentials for virulence. <i>Proc. Natl. Acad. Sci. USA.</i> 99:1–3.]</ref> | ||
All <i>Brucella</i> are miniscule (≈ 0.5µm-1.5µm in diameter), sessile, Gram-negative coccobacilli, and are facultative intracellular pathogens (<b>Figure 2</b>). <ref name = "Godfroid">[https://www.ncbi.nlm.nih.gov/pubmed/23837363. Godfroid, J., Garin-Bastuji, B., Saegerman, C., Blasco, J. M. 2013. Brucellosis in terrestrial wildlife. <i>Rev. sci. tech. Off. int. Epiz.</i>, 32:27–42.]</ref> <ref name = "Hagan">Hagan, W. A., Bruner, W. D. <i>Hagan and Bruner’s Microbiology and Infectious Diseases of Domestic Animals: With Reference to Etiology, Epizootiology, Pathogenesis, Immunity, Diagnosis, and Antimicrobial Susceptibility</i>. Ithaca: Comstock Publ., 1992. Print.</ref> When cultured, <i>Brucella</i> colonies appear either smooth or rough. Smooth colonies are more common in the virulent species of <i>Brucella</i> (e.g. <i>B. melitensis, suis</i>, and <i>abortus</i>), and rough colonies are associated with non-virulent species. The rough morphotype is attributed to a lipopolysaccharide molecule that elicits strong immune responses in animals, which may explain why it is associated with the non-virulent species. <ref name = "Schurig">[http://www.sciencedirect.com/science/article/pii/S0378113502002559. Schurig, G. G., Sriranganathan, N., Corbel, M. J. 2002. Brucellosis vaccines: past, present and future. <i>Veterinary Microbiology</i>, 90:479–496.]</ref> [[Image:BM.jpg|thumb| | All <i>Brucella</i> are miniscule (≈ 0.5µm-1.5µm in diameter), sessile, Gram-negative coccobacilli, and are facultative intracellular pathogens (<b>Figure 2</b>). <ref name = "Godfroid">[https://www.ncbi.nlm.nih.gov/pubmed/23837363. Godfroid, J., Garin-Bastuji, B., Saegerman, C., Blasco, J. M. 2013. Brucellosis in terrestrial wildlife. <i>Rev. sci. tech. Off. int. Epiz.</i>, 32:27–42.]</ref> <ref name = "Hagan">Hagan, W. A., Bruner, W. D. <i>Hagan and Bruner’s Microbiology and Infectious Diseases of Domestic Animals: With Reference to Etiology, Epizootiology, Pathogenesis, Immunity, Diagnosis, and Antimicrobial Susceptibility</i>. Ithaca: Comstock Publ., 1992. Print.</ref> When cultured, <i>Brucella</i> colonies appear either smooth or rough. Smooth colonies are more common in the virulent species of <i>Brucella</i> (e.g. <i>B. melitensis, suis</i>, and <i>abortus</i>), and rough colonies are associated with non-virulent species. The rough morphotype is attributed to a lipopolysaccharide molecule that elicits strong immune responses in animals, which may explain why it is associated with the non-virulent species. <ref name = "Schurig">[http://www.sciencedirect.com/science/article/pii/S0378113502002559. Schurig, G. G., Sriranganathan, N., Corbel, M. J. 2002. Brucellosis vaccines: past, present and future. <i>Veterinary Microbiology</i>, 90:479–496.]</ref> [[Image:BM.jpg|thumb|250px|right| <b>Figure 2.</b> Colored scanning electron micrograph of <i>B. melitensis</i>. All <i>Brucella</i> species are non-flagellated, Gram-negative coccobacilli. [http://bioinfo.bisr.res.in/cgi-bin/project/dofpath/get_details.cgi?group=bacteria&&organism=Brucella%20melitensis <ref>http://bioinfo.bisr.res.in/cgi-bin/project/dofpath/get_details.cgi?group=bacteria&&organism=Brucella%20melitensis. "Brucella Melitensis." <i>Database of Bacterial Food Pathogen.</i> Birla Institute of Scientific Research, 2015.</ref>] | ||
<ref>[http://www.oie.int/doc/ged/D12397.PDF. Brucellosis in terrestrial wildlife. 2013. Godfroid, J., Garin-Bastuji, B., Saegerman, C., Blasco, J. M. <i>Rev. sci. tech. Off. int. Epiz.</i> 32 (1): 27-42]</ref>]] | <ref>[http://www.oie.int/doc/ged/D12397.PDF. Brucellosis in terrestrial wildlife. 2013. Godfroid, J., Garin-Bastuji, B., Saegerman, C., Blasco, J. M. <i>Rev. sci. tech. Off. int. Epiz.</i> 32 (1): 27-42]</ref>]] | ||
<br><i>Brucella</i> are capable of surviving, but rarely reproduce, in external environments for well over a year given the appropriate conditions. <ref name = "BM"> | <br><i>Brucella</i> are capable of surviving, but rarely reproduce, in external environments for well over a year given the appropriate conditions. <ref name = "BM"> | ||
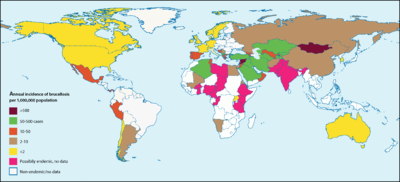
[http://wildpro.twycrosszoo.org/S/0zM_Gracilicutes/Brucella/Brucella_melitensis.htm. Bourne, D. “Brucella melitensis.” <i>Brucella melitensis</i> (Bacterial Type). Twycross Zoo, n.d. Web.]</ref> They fare best in environments with a pH greater than 5.5, high humidity, and, most especially, freezing temperatures <ref name = "BM">[http://wildpro.twycrosszoo.org/S/0zM_Gracilicutes/Brucella/Brucella_melitensis.htm. Bourne, D. “Brucella melitensis.” Brucella melitensis (Bacterial Type). Twycross Zoo, n.d. Web.]</ref> <ref name = "BA">[http://wildpro.twycrosszoo.org/S/0zM_Gracilicutes/Brucella/Brucella_abortus.htm. Bourne, D. “Brucella abortus.” <i>Brucella abortus</i> (Bacterial Type). Twycross Zoo, n.d. Web.]</ref> In animals, <i>Brucella</i> can be found all over the globe, with notably high occurrences in the Mediterranean, Middle East, Latin America, and Africa.<ref name = "BM">[http://wildpro.twycrosszoo.org/S/0zM_Gracilicutes/Brucella/Brucella_melitensis.htm. Bourne, D. “Brucella melitensis.” Brucella melitensis (Bacterial Type). Twycross Zoo, n.d. Web.]</ref> The highest incidences of <i>Brucella</i> in humans are recorded in Syria and Mongolia (<b>Figure 3</b>). <ref name = "Ariza">[http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.0040317#pmed-0040317-g001. Ariza, J., Bosilkovski, M., Cascio, A., Colmenero, J. D., Corbel, M. J., Falagas, M. E., <i>et al.</i> 2007. Perspectives for the Treatment of Brucellosis in the 21st Century: The Ioannina Recommendations. <i>PLoS Med</i> 4(12):317.]</ref> [[Image:bdist.png|thumb| | [http://wildpro.twycrosszoo.org/S/0zM_Gracilicutes/Brucella/Brucella_melitensis.htm. Bourne, D. “Brucella melitensis.” <i>Brucella melitensis</i> (Bacterial Type). Twycross Zoo, n.d. Web.]</ref> They fare best in environments with a pH greater than 5.5, high humidity, and, most especially, freezing temperatures <ref name = "BM">[http://wildpro.twycrosszoo.org/S/0zM_Gracilicutes/Brucella/Brucella_melitensis.htm. Bourne, D. “Brucella melitensis.” Brucella melitensis (Bacterial Type). Twycross Zoo, n.d. Web.]</ref> <ref name = "BA">[http://wildpro.twycrosszoo.org/S/0zM_Gracilicutes/Brucella/Brucella_abortus.htm. Bourne, D. “Brucella abortus.” <i>Brucella abortus</i> (Bacterial Type). Twycross Zoo, n.d. Web.]</ref> In animals, <i>Brucella</i> can be found all over the globe, with notably high occurrences in the Mediterranean, Middle East, Latin America, and Africa.<ref name = "BM">[http://wildpro.twycrosszoo.org/S/0zM_Gracilicutes/Brucella/Brucella_melitensis.htm. Bourne, D. “Brucella melitensis.” Brucella melitensis (Bacterial Type). Twycross Zoo, n.d. Web.]</ref> The highest incidences of <i>Brucella</i> in humans are recorded in Syria and Mongolia (<b>Figure 3</b>). <ref name = "Ariza">[http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.0040317#pmed-0040317-g001. Ariza, J., Bosilkovski, M., Cascio, A., Colmenero, J. D., Corbel, M. J., Falagas, M. E., <i>et al.</i> 2007. Perspectives for the Treatment of Brucellosis in the 21st Century: The Ioannina Recommendations. <i>PLoS Med</i> 4(12):317.]</ref> [[Image:bdist.png|thumb|400px|right|<b>Figure 3.</b> Global occurrences of brucellosis in humans. The highest incidences are recorded in Syria (1603.4 incidences per 1M people per year), Mongolia (605.9 incidences per 1M people per year), and Kyrgyzstan (362.2 incidences per 1M people per year). The incidence of brucellosis in the United States is 0.4 per 1M people per year. [http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.0040317#pmed-0040317-g001 <ref>[http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.0040317#pmed-0040317-g001 Ariza, J., Bosilkovski, M., Cascio, A., Colmenero, J. D. <i>et al.</i> "Perspectives for the Treatment of Brucellosis in the 21st Century: The Ioannina Recommendations." 2008. PLoS Med 4(12): e317]</ref>]]] <br>The first concrete documentation of brucellosis symptoms in humans occurred in the 1850’s when a British Army surgeon, Dr. Jeffrey Marston, described a fever he had, the symptoms of which differed from any other known fever at the time. <ref name = Moreno>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4026726/. Moreno, E. 2014. Retrospective and prospective perspectives on zoonotic brucellosis. <i>Front Microbiol.</i>, 5:1–18.]</ref> <ref name = Wyatt3>[https://www.oie.int/doc/ged/D12396.PDF. Wyatt, H. V. 2013. Lessons from the history of brucellosis. <i>Rev. sci. tech. Off. int. Epiz.</i>, 32:17–25.]</ref> In 1887, the first species of <i>Brucella</i> (<i>B. melitensis</i>) was isolated from the spleens of Maltese soldiers who had come down with what was then called Malta fever (the earliest name for brucellosis). <ref name = Moreno>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4026726/. Moreno, E. 2014. Retrospective and prospective perspectives on zoonotic brucellosis. <i>Front Microbiol.</i>, 5:1–18.]</ref> <ref name = Nicoletti>[http://www.sciencedirect.com/science/article/pii/S0378113502002092. Nicoletti, P. 2002. A short history of brucellosis. <i>Veterinary Microbiology</i> 90:5–9.]</ref> For a couple of decades, the transmission of this disease was a mystery, and people generally attributed it to “bad air” and poor public sanitation systems. A couple of decades later, a Maltese physician named Themistocles Zammit cleared the mystery by performing a series of ethically questionable experiments on goats. He fed his goats cultures of <i>Brucella</i>, observed their symptoms, and tested their urine, blood, and milk for presence of the bacteria. <ref name = Wyatt3>[https://www.oie.int/doc/ged/D12396.PDF. Wyatt, H. V. 2013. Lessons from the history of brucellosis. <i>Rev. sci. tech. Off. int. Epiz.</i>, 32:17–25.]</ref> Based on the results, he concluded that the disease’s causative agent was transmittable to humans through the ingestion of contaminated goat milk <ref name = Wyatt5>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1240100/. Wyatt, H. V. 2005. How Themistocles Zammit found Malta Fever (brucellosis) to be transmitted by the milk of goats. <i>J. R. Soc. Med.</i> 98:451–454.]</ref> A decade later, the abortive effects of <i>Brucella</i> in animals were discovered. <ref name = Moreno>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4026726/. Moreno, E. 2014. Retrospective and prospective perspectives on zoonotic brucellosis. <i>Front Microbiol.</i>, 5:1–18.]</ref> | ||
==Transmission, Signs, and Symptoms== | ==Transmission, Signs, and Symptoms== | ||
Revision as of 23:00, 7 May 2017
Introduction
By Hannah Wedig
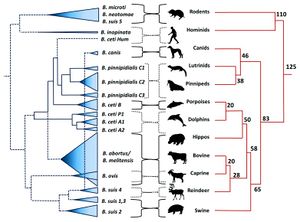
Brucellosis is among the most common and highly contagious zoonoses. Zoonoses are diseases which can be transmitted from animals to humans. [2] In the case of brucellosis, mammals – domestic, wild, terrestrial, and marine – are capable of transmitting the disease. [2] [3] Brucellosis is caused by certain species of bacteria in the genus Brucella, the most common and virulent of which are B. melitensis, B. suis, and B. abortus (Figure 1). [4] [5] The genus Brucella is within the class α-Proteobacteria, which includes many bacterial parasites of both plants and animals. [6]
All Brucella are miniscule (≈ 0.5µm-1.5µm in diameter), sessile, Gram-negative coccobacilli, and are facultative intracellular pathogens (Figure 2). [7] [4] When cultured, Brucella colonies appear either smooth or rough. Smooth colonies are more common in the virulent species of Brucella (e.g. B. melitensis, suis, and abortus), and rough colonies are associated with non-virulent species. The rough morphotype is attributed to a lipopolysaccharide molecule that elicits strong immune responses in animals, which may explain why it is associated with the non-virulent species. [8]
Brucella are capable of surviving, but rarely reproduce, in external environments for well over a year given the appropriate conditions. [11] They fare best in environments with a pH greater than 5.5, high humidity, and, most especially, freezing temperatures [11] [12] In animals, Brucella can be found all over the globe, with notably high occurrences in the Mediterranean, Middle East, Latin America, and Africa.[11] The highest incidences of Brucella in humans are recorded in Syria and Mongolia (Figure 3). [13]
The first concrete documentation of brucellosis symptoms in humans occurred in the 1850’s when a British Army surgeon, Dr. Jeffrey Marston, described a fever he had, the symptoms of which differed from any other known fever at the time. [1] [15] In 1887, the first species of Brucella (B. melitensis) was isolated from the spleens of Maltese soldiers who had come down with what was then called Malta fever (the earliest name for brucellosis). [1] [16] For a couple of decades, the transmission of this disease was a mystery, and people generally attributed it to “bad air” and poor public sanitation systems. A couple of decades later, a Maltese physician named Themistocles Zammit cleared the mystery by performing a series of ethically questionable experiments on goats. He fed his goats cultures of Brucella, observed their symptoms, and tested their urine, blood, and milk for presence of the bacteria. [15] Based on the results, he concluded that the disease’s causative agent was transmittable to humans through the ingestion of contaminated goat milk [17] A decade later, the abortive effects of Brucella in animals were discovered. [1]
Transmission, Signs, and Symptoms
Themistocles Zammit had discovered the most common means of Brucella infection: ingestion of contaminated animal products (such as dairy products for humans, and aborted fetuses or placenta for animals). However, Brucella can also enter via the respiratory tract, broken skin, or any mucous membranes that come into contact with infected fluids or tissues (such as feces, urine, milk, fetal fluids, vaginal discharge, semen, placenta, aborted fetuses, etc.). [11]
In humans, Brucella primarily inhabit parts of the phagocytic system including lymph nodes, liver, spleen, and bone marrow. The incubation period can range from one to three weeks, during which the cells remain in the phagocytic system and replicate.[1] Because of this, symptoms may be entirely absent up to several months after infection. The symptoms, once they arise, have been described as so bad “‘it often makes a patient wish he were dead”.[2] Human brucellosis caused by B. melitensis was first known as Malta fever (though it has an abundance of other names, such as Mediterranean fever, Corps disease, undulant fever, Cyprus fever, to name a few), an illness characterized by fatigue and back pain in addition to elevated body temperatures.[11] [1] Other species of Brucella can cause a range of complications including anorexia, myalgia, encephalitis, meningitis, endocarditis, and various forms of arthritis [18] [2] Although rarely fatal, if left untreated, brucellosis can become a chronic, debilitating disease. [19] It is also not uncommon for infected individuals to have a relapse as early as several months after recovering from the initial infection. [20]
In animals, Brucella can inhabit an array of organs, including the aforementioned ones in phagocytic system but also the kidneys and reproductive organs. If a pregnant female is infected, the bacteria can cause premature birth or spontaneous abortion of the fetus – this can also occur in female humans, but is much less common.[21] Most infected female animals will only abort once but, if untreated, their placenta can retain the bacteria and cause complications in subsequent birthings.[2]
Brucella have also been isolated from a range of arthropods including ticks, bedbugs, lice, and mites. Though these paratenic hosts do not support Brucella reproduction, they can function as brucellosis vectors.[11]
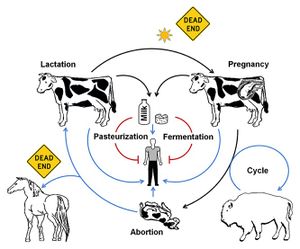
As previously mentioned, Brucella fare best in cooler conditions and are extremely susceptible to heat – a mere hour or two of exposure to direct sunlight will kill the bacteria.[1] [12] In addition to sunlight exposure, mammals that are resistant to the bacteria and or mammals (such as humans) that rarely pass on the bacteria are considered “dead ends” in terms of bacterial transmission (Figure 4).[1]
Major Brucella Species
Terrestrial Brucella
B. melitensis
Among the ten known species of Brucella, B. melitensis is the most common and most pathogenic for humans. [2] [7] [16] Sheep and goats are the predominant natural hosts of B. melitensis, but it has been isolated from a vast array of other mammals including humans, cattle, deer, squirrels, guinea pigs, mice, hedgehogs, fox, dogs, cats, chickens, and frogs.[11] [22] B. melitensis is aerobic, and does not require supplementary CO2 to grow (see B. abortus).[11]
B. suis
B. suis is most commonly found in domestic pigs and wild boars, but can also inhabit humans, peccary, cattle, moose, horses, bears, fox, voles, ravens, and turkeys. Like B. melitensis, B. suis is aerobic and does not rely on increased levels of CO2 for growth. [23] Unlike B. melitensis, certain subspecies of B. suis produce H2S during growth. The pathogenicity of B. suis is variable, but is typically less virulent than B. melitensis and more virulent than B. abortus.[23] [24]
B. abortus
B. abortus typically infects cattle, bison, yaks, buffalo, and sheep, but has also been discovered in humans, deer, horses, coyotes, fox, badgers, raccoons, and opossums.[12] B. abortus is the only one of the three main species that is aerobic and also typically requires an atmosphere with about 5-10% CO2 to grow.[12] [25] Though B. abortus causes spontaneous abortions in animals, the infection tends to be subclinical in humans. [12]
Aquatic Brucella
B. ceti and B. pinnipedialis
B. ceti and B. pinnipedialis preferentially infect cetaceans (i.e. whales, dolphins, etc.) and pinnipeds (i.e. seals), respectively. These two aquatic species of Brucella have been isolated from various tissues of cetaceans and pinnipeds that appeared perfectly healthy, suggesting that their virulence is much milder compared to the terrestrial species. [26] When it does cause disease, B. ceti is especially diverse in its pathogenic effects, as it can cause blubber abscesses, lesions, organ inflammation, pneumonia, and meningitis, to name a few. Strains of B. ceti and B. pinnipedialis are variable in their zoonotic capabilities, but in general they are considered non-zoonotic.[1] One strain (B. ceti Hum) can infect humans in the same fashion as the terrestrial Brucella, but other strains enter the human macrophage and are killed off almost immediately, and others still are incapable of entering the human macrophage cells altogether.[26][1] B. ceti and B. pinnipedialis differ slightly in their metabolic requirements – B. pinnipedialis, like B. abortus, requires supplementary CO2 to grow, and B. ceti, like B. melitensis and B. suis, does not.[26]
Brucellosis in Humans
Though brucellosis was officially identified in the mid 19th century, the earliest record of brucellosis in humans dates back to nearly 2 million years ago, where the bacteria was discovered in the bone of an Australopithecine (one of the earliest hominids).[1] Currently, over 500,000 cases of human brucellosis are reported annually worldwide, but the number of unreported or undetected cases is hypothesized to be significantly greater. [27] It is rare for individuals outside of an animal-handling profession – e.g. veterinarians, farmers, and zoo technicians – to contract brucellosis from animals, but it was recently determined that human-to-human transmission can occur (see next paragraph).[1][24] Not only does brucellosis present itself as a health concern, it can also have detrimental economic impacts since livestock are the preferred hosts for the most virulent Brucella species. [3] Furthermore, the potential manipulation of Brucella strains as biological weapons has also added to the urgency of eradicating the disease.[6]
As previously mentioned, humans are generally considered a dead end for the transmission of Brucella. Previously, brucellosis was thought to only be able to be transmitted between humans via operations such as blood transfusions or bone marrow transplants, but cases of infected individuals’ spouses or partners contracting the disease invalidated that contention. In 1991, a detailed case study revealed that brucellosis was indeed transmittable from humans to other humans, most likely via unprotected intercourse.[28] Cases of infected mothers transmitting the disease to their infants via breast-feeding have also been documented.[2]
Disease Management: Diagnostics, Treatment, and Prevention
Diagnostics
There is a diverse array of methods to diagnose brucellosis, the most common of which include observing the clinical symptoms and serologic methods, i.e., by testing bodily fluids for presence of Brucella.[24][1] Examples of common serologic methods include agglutination reactions (AR), complement fixation tests (CFT), and a test called BrucellaCapt (BCAP).[24][29]
AR: Individuals infected with Brucella produce anti-Brucella agglutinins, which are antibodies that cause agglutination (i.e. clumping) of cells. The patient’s blood sample is deposited into a test tube, to which a solution of Brucella cells are added. If the antibodies are present, they bind to the bacterial cells and induce physiological changes which cause the cells to become hydrophobic and thus agglutinate at the bottom of the test tube and indicate Brucella infection.[24] However, this diagnostic method is not entirely reliable, as the agglutinins can cause the agglutination of other pathogenic bacteria including Francisella tularensis (which causes tularemia), Rickettsia prowazekii (which causes epidemic typhus), and Mycobacterium tuberculosis (which causes tuberculosis).[30] [31] [32][24] In addition, AR is unable to detect brucellosis in immunocompromised individuals, newly-infected individuals whose bodies have not yet produced the agglutinins, and individuals who have had brucellosis for longer than several weeks.[24][27] Despite these drawbacks, AR is still used as the reference standard to which other serological tests are compared.[24]
CFT: This test is more efficient than AR in terms of sensitivity (it is able to detect brucellosis in individuals who have been infected for over a year) and specificity, although the general concept is very much the same – testing for the presence of anti-Brucella antibodies in patients’ bodily fluids.[24][27] The fluid sample is first heated to eliminate any existing antibody complements, after which a known amount of complements and antigens are added to the serum. If antibodies are present, they will bind (i.e. fix) the complements and antigens; if not, the complements and antigens will remain dispersed in the serum. Red blood cells from a different animal species and their respective antibodies are then added to the serum. If the previously added complements are fixed by anti-Brucella antibodies, the animal antibodies will fix the red blood cells and aggregate at the bottom of the test tube. If the complements are not fixed (that is, anti-Brucella antibodies are not present), the complements react with the animal red blood cells and induce lysis, causing the solution to turn pink.[33]
BrucellaCapt: The BrucellaCapt method was devised at the turn of the century. It is based on immunocapture-agglutination of anti-Brucella antibodies. In a BrucellaCapt test, the patient’s blood sample is exposed to anti-total human immunoglobulin and antigen suspensions. If anti-Brucella agglutinins are present, agglutination occurs and if not, a pellet forms. Out of the three methods introduced here, BrucellaCapt is the most sensitive, capable of detecting brucellosis in both newly infected and relapsing individuals.[29]
PCR is also a viable diagnostic method for brucellosis. It involves amplifying one of two genetic targets: BrucellaBCSP31 or 16S rRNA.[34] Using genus-specific PCR makes this method more specific than any serological test, preventing false-negative tests in individuals infected with uncommon species or subspecies of Brucella.[27] Because of this specificity, the PCR test is entirely reliable, unlike serological tests which typically require a secondary diagnosis to substantiate the results. However, PCR is complex, expensive, and requires more specialized equipment compared to the serological tests and thus is the least practical when considering the fact that brucellosis primarily affects developing countries.[34]
Treatment
Antibiotics are a common way to treat humans with brucellosis. As with all antibiotics, some strains of Brucella have developed resistance against them, but the most epidemiologically relevant strains have thankfully remained susceptible to the classic antibiotic treatment.[1] So far, an antibiotic regimen of six weeks of doxycycline supplemented by two or three weeks of streptomycin is the best option in terms of effectiveness, low relapse frequency, and mildest side effects.[35][2] Doxycycline, a common antibiotic for treating a range of infections, is administered daily orally or via intramuscular injection, so access to appropriate equipment and care is of some concern in the poorer countries where brucellosis is most prevalent. A TMP-SMX (trimethoprim-sulfamethoxazole) with rifampin regimen is a cheaper but significantly less effective alternative.[35] It tends to be a last resort treatment in countries that can afford other options, but in many third world countries it is the only economically feasible one.[13]
The use of antibiotics in animals, especially those raised for food, is typically discouraged. This is because overuse of antibiotics to treat livestock has negative economic, epidemiological, and public health implications. Widespread use of antibiotics is expensive, selects for antibiotic-resistance in the bacteria, and could potentially pose a biological threat to humans who consume the treated livestock. Unfortunately, there is no recommended treatment for animal (domestic or wild) brucellosis.[36] This is why it is especially important to implement adequate preventative measures (in both humans and animals) in areas where Brucella infection is common.[37]
Prevention
One of the most common ways that humans contract brucellosis is by consuming contaminated dairy products. Fortunately, there is a simple solution to prevent this form of transmission: pasteurization. As previously mentioned, Brucella is extremely sensitive to heat so heating infected milk is a quick and reliable way to eliminate the bacteria from the product.[1]
Another way to prevent the spread of Brucella, one commonly implemented in countries where livestock are infected, is to slaughter and properly dispose of all infected animals, preventing further transmission. Of course, this practice is costly and impractical in areas of high prevalence.[1]
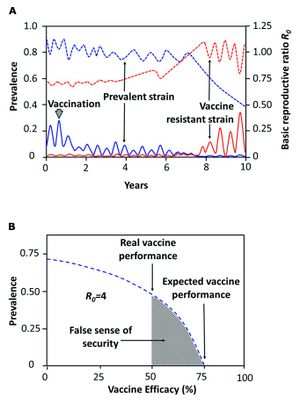
The third and most widely practiced preventative method is prophylactic vaccination.[1] One of the earliest forms of vaccination against brucellosis manipulated live, differentially virulent strains of B. abortus and B. melitensis. Female cattle and goats were subcutaneously vaccinated with one of the strains and artificially inseminated between two and four months after vaccination. [38][39] Success was determined based on prevention of udder infection and abortion. The success rate was about 86% for the least virulent strain, and nearly 100% for the intermediate and most virulent strains.[38] Studies have shown that the most effective means of preventing transmission and decreasing the bacteria’s abundance is by performing consistent vaccinations and quickly removing all infected animals from the area. When these two preventative methods are used in conjunction with each other, the result is described as the herd immunity theory. The theory proposes that increasing the overall tolerance of the existing herd also increases the resistance of subsequent, unvaccinated generations. Unfortunately, improper administration of vaccines and other preventative methods can have detrimental effects on a host population. In 2014, a group of researchers conducted a study regarding low efficacy vaccines. They concluded that the administration of weak, short-term vaccines induces what they referred to as a “false sense of security” in host populations. Low efficacy vaccines are not only rather useless for preventing the transmission of the disease, but they can have deleterious long-term effects by facilitating the evolution of new subspecies of virulent Brucella, selecting for stronger pathogenicity and better transmissibility (Figure 5). Furthermore, people are less likely to take appropriate preventative measures if they are told the animals have been vaccinated, making them more vulnerable to infection. Especially in developing countries where brucellosis is most prevalent, it is more difficult to educate the general public about this false sense of security, which has resulted in several violent outbreaks of brucellosis in animals and humans in recent years.[1] Not only that, the use of certain strains of live Brucella species in vaccines for food animals has resulted in the transmission of brucellosis to humans who consumed the vaccinated animals’ products. Research is still ongoing with regards to developing a harmless, live Brucella vaccine. [8] The combined implementation of these three preventative measures has successfully eradicated brucellosis from practically all livestock herds and human populations in many countries, but it is still prevalent and problematic in developing countries where equipment, money, and care are less accessible.[1]
Current Conundrums
Though brucellosis has been around even longer than modern humans have, there are still many unanswered questions and unresolved problems surrounding the genus Brucella, especially with respect to the pathogenic species. Current research is primarily directed towards developing more effective, accessible, and cheaper treatments and prevention methods. Of course, in order to achieve these goals, researchers must start at the root of understanding Brucella as a pathogen. This in itself has proven to be quite the ordeal for a couple of reasons. First, within the monophyletic genus Brucella, the different species have very different host preferences and levels of pathogenicity. Also the pathogenic Brucella species have presented themselves as genetically anomalous pathogens. The gene sequences of pathogenic Brucella species are void of the “classic” virulence markers found in most pathogens. These include genes that code for factors such as exoenzymes, motility structures (e.g. fimbriae and flagella), toxins, lysogenic phages, and resistance forms. The details of exactly why Brucella has evolved in such a way are yet to be determined, and this discovery has led researchers to reevaluate the way they define pathogens.[6] Indeed, the development of new treatments and prevention methods may be out of reach until this mystery is resolved.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 Moreno, E. 2014. Retrospective and prospective perspectives on zoonotic brucellosis. Front Microbiol., 5:1–18.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Seleem, M. N., Boyle, S. M., Sriranganathan, N. 2010. Brucellosis: A re-emerging zoonosis. Veterinary Microbiology 140:392–398.
- ↑ 3.0 3.1 Taleski, V., Zerva, L., Kantardijev, T., Cvetnic, Z., Erski-Biljic, M. et al. 2002. An overview of the epidemiology and epizootiology of brucellosis in selected countries of Central and Southeast Europe. Veterinary Microbiology 90:147–155.
- ↑ 4.0 4.1 Hagan, W. A., Bruner, W. D. Hagan and Bruner’s Microbiology and Infectious Diseases of Domestic Animals: With Reference to Etiology, Epizootiology, Pathogenesis, Immunity, Diagnosis, and Antimicrobial Susceptibility. Ithaca: Comstock Publ., 1992. Print.
- ↑ Young, E. J., M.D. “Brucella species (Brucellosis).” Infectious Disease and Antimicrobial Agents. Antimicrobe, 2014 Web.
- ↑ 6.0 6.1 6.2 Moreno, E. and Moriyon, I. 2002. Brucella melitensis: A nasty bug with hidden credentials for virulence. Proc. Natl. Acad. Sci. USA. 99:1–3.
- ↑ 7.0 7.1 Godfroid, J., Garin-Bastuji, B., Saegerman, C., Blasco, J. M. 2013. Brucellosis in terrestrial wildlife. Rev. sci. tech. Off. int. Epiz., 32:27–42.
- ↑ 8.0 8.1 Schurig, G. G., Sriranganathan, N., Corbel, M. J. 2002. Brucellosis vaccines: past, present and future. Veterinary Microbiology, 90:479–496. Cite error: Invalid
<ref>tag; name "Schurig" defined multiple times with different content - ↑ http://bioinfo.bisr.res.in/cgi-bin/project/dofpath/get_details.cgi?group=bacteria&&organism=Brucella%20melitensis. "Brucella Melitensis." Database of Bacterial Food Pathogen. Birla Institute of Scientific Research, 2015.
- ↑ Brucellosis in terrestrial wildlife. 2013. Godfroid, J., Garin-Bastuji, B., Saegerman, C., Blasco, J. M. Rev. sci. tech. Off. int. Epiz. 32 (1): 27-42
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7
Bourne, D. “Brucella melitensis.” Brucella melitensis (Bacterial Type). Twycross Zoo, n.d. Web. Cite error: Invalid
<ref>tag; name "BM" defined multiple times with different content Cite error: Invalid<ref>tag; name "BM" defined multiple times with different content Cite error: Invalid<ref>tag; name "BM" defined multiple times with different content Cite error: Invalid<ref>tag; name "BM" defined multiple times with different content Cite error: Invalid<ref>tag; name "BM" defined multiple times with different content Cite error: Invalid<ref>tag; name "BM" defined multiple times with different content Cite error: Invalid<ref>tag; name "BM" defined multiple times with different content - ↑ 12.0 12.1 12.2 12.3 12.4 Bourne, D. “Brucella abortus.” Brucella abortus (Bacterial Type). Twycross Zoo, n.d. Web.
- ↑ 13.0 13.1 Ariza, J., Bosilkovski, M., Cascio, A., Colmenero, J. D., Corbel, M. J., Falagas, M. E., et al. 2007. Perspectives for the Treatment of Brucellosis in the 21st Century: The Ioannina Recommendations. PLoS Med 4(12):317.
- ↑ Ariza, J., Bosilkovski, M., Cascio, A., Colmenero, J. D. et al. "Perspectives for the Treatment of Brucellosis in the 21st Century: The Ioannina Recommendations." 2008. PLoS Med 4(12): e317
- ↑ 15.0 15.1 Wyatt, H. V. 2013. Lessons from the history of brucellosis. Rev. sci. tech. Off. int. Epiz., 32:17–25.
- ↑ 16.0 16.1 Nicoletti, P. 2002. A short history of brucellosis. Veterinary Microbiology 90:5–9.
- ↑ Wyatt, H. V. 2005. How Themistocles Zammit found Malta Fever (brucellosis) to be transmitted by the milk of goats. J. R. Soc. Med. 98:451–454.
- ↑ Wong, K. “Brucella melitensis.” MicrobeWiki. Kenyon College. 7 July 2011. Web.
- ↑ Corbel, M. J. Brucellosis in Humans and Animals. World Health Organization. 2006. Print.
- ↑ Al Dahouk, S., Sprague, L. D., Neubauer, H. 2013 New developments in the diagnostic procedures for zoonotic brucellosis in humans. Rev. sci. tech. Off. int. Epiz., 32:177–188.
- ↑ Corbel, M. J. Brucellosis in Humans and Animals. World Health Organization. 2006. Print.
- ↑ “Zoonoses.” Health Concerns to be Aware of When Working With Wildlife. Wildlife Rehab Info. n.d. Web.
- ↑ 23.0 23.1 Bourne, D. “Brucella suis.” Brucella suis (Bacterial Type). Twycross Zoo, n. d. Web.
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 24.6 24.7 24.8 Galinska, E. M., Zagorski, J. 2013 Brucellosis in humans – etiology, diagnostics, clinical forms. Annals of Agri. Env. Med. 20:233–238.
- ↑ Costa, E. “Brucella abortus.” MicrobeWiki. Kenyon College, 25 July 2013. Web.
- ↑ 26.0 26.1 26.2 Nymo, I. H., Tryland, M., Godfroid, J. 2011. A review of Brucella infection in marine mammals, with special emphasis on Brucella pinnipedialis in the hooded seal (Cystophora cristata). Veterinary Research, 42–93.
- ↑ 27.0 27.1 27.2 27.3 Al Dahouk, S., Sprague, L. D., Neubauer, H. 2013 New developments in the diagnostic procedures for zoonotic brucellosis in humans. Rev. sci. tech. Off. int. Epiz., 32:177–188.
- ↑ [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3327908/. Vigeant, P., Mendelson, J., Miller, M. A. 1995. <Human to human transmission of Brucella melitensis. Can. J. Infect. Dis. 61:53–155.]
- ↑ 29.0 29.1 Casanova, A., Ariza, J., Rubio, M., Masuet, C., Diaz, R. 2009. BrucellaCapt versus Classical Tests in the Serological Diagnosis and Management of Human Brucellosis. Clin. Vaccine Immunol. 16:844–851.
- ↑ Su, D. “Francisella tularensis.” MicrobeWiki. Kenyon College. 29 April 2011. Web.
- ↑ Noble, M. “Rickettsia prowazekii.” MicrobeWiki. Kenyon College. 4 July 2011. Web.
- ↑ Liu, Y. “Mycobacterium tuberculosis.” MicrobeWiki. Kenyon College. 22 September 2014. Web.
- ↑ [https://www.youtube.com/watch?v=q3K_rH652Yk. Alam, T. “Complement Fixation Test HD Animation HIGH.” مكتبة وحيد طب بشري لخدمة طلاب طب طنطا علم ينتفع بة (Library of a single human medicine to serve the students of medicine). Youtube. Web.]
- ↑ 34.0 34.1 Bricker, B. J. 2002. PCR as a diagnostic tool for brucellosis. Veterinary Micrbiology 90:435–446.
- ↑ 35.0 35.1 Yousefi‐Nooraie, R., Mortaz-Hejri, S., Mehrani, M., Sadeghipour, P. 2012. Antibiotics for treating human brucellosis. Cochrane Database of Systematic Reviews 10.
- ↑ Landers, T. F., Cohen, B., Wittum, T. E., Larson, E. L. 2012. A Review of Antibiotic Use in Food Animals: Perspective, Policy, and Potential. Public Health Rep. 127:4–22.
- ↑ “Brucellosis.” Brucellosis. Department of Animal Sciences, University of Wisconsin-Madison, n.d. Web.
- ↑ 38.0 38.1 Cotton, W. E., Buck, J. M., Smith, H. E. 1933. Efficacy and Safety of Abortion Vaccines Prepared from Brucella abortus Strains of Different Degrees of Virulence. Journal of Agricultural Research 46:291–314.
- ↑ Elberg, S. S., Meyer, K. F. 1958. Caprine immunization against brucellosis. Bull World Health Organ. 19:71–724.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.