African Sleeping Sickness: Tyrpanosome Invasion Mechanism: Difference between revisions
Lensmeyer1 (talk | contribs) |
Lensmeyer1 (talk | contribs) |
||
| Line 3: | Line 3: | ||
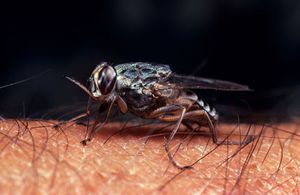
[[Image:GettyImages-654679151-59636a305f9b583f180e29eb.jpg|thumb|300px|right|The insect vector of the trypanosome microbe, the tsetse fly. These flies are found in tropical parts of Southern Africa and are the only known vector for African Sleeping Sickness [https://www.tripsavvy.com/avoiding-the-tsetse-fly-on-safari-1454081].]] | [[Image:GettyImages-654679151-59636a305f9b583f180e29eb.jpg|thumb|300px|right|The insect vector of the trypanosome microbe, the tsetse fly. These flies are found in tropical parts of Southern Africa and are the only known vector for African Sleeping Sickness [https://www.tripsavvy.com/avoiding-the-tsetse-fly-on-safari-1454081].]] | ||
<br>By Katie Lensmeyer<br> | <br>By Katie Lensmeyer<br> | ||
<br><br>Other examples: | <br><br>Other examples: | ||
<br><b>Bold</b> | <br><b>Bold</b> | ||
| Line 17: | Line 10: | ||
<br><b>Superscript:</b> Fe<sup>3+</sup> | <br><b>Superscript:</b> Fe<sup>3+</sup> | ||
<br><br> African Sleeping Sickness is a microbial vector driven disease that affects many parts of Africa. The disease takes action by first invading the peripheral nervous system of its host and soon after | <br><br> African Sleeping Sickness is a microbial vector driven disease that affects many parts of Africa. The disease takes action by first invading the peripheral nervous system of its host and soon after passes the blood brain barrier to damage neurons within the brain. The result of this infection is fatal to the mammalian host. From initial infection to death of the host system, this microbe needs only a few short months to become fatal. How is it that this disease can invade such secure parts of the human system so quickly? The human immune system seems to be far to advanced to let this microbe produce the affects it does. Recent research looks deeply into both the spread of the infection through the mammalian host as well as the growth of the microbe within its vector, the tsetse fly. | ||
<br> | |||
<br>Phylogenetic reconstruction of this disease has shown that this disease has existed for approximately 300 million years. The microbial coexistence with its insect parasites has existed for equally as long. This ancient coexistence between African mammals and their blood-sucking insect counterparts is assumed to be why most native Animalia are not affected by the disease. This immunity, however, does not stretch to the domesticated animals of the area. <i>Trypanosome breci</i>, the form of trypanosomes that causes sleeping sickness, is expected to be a recent development within history of trypanosomes. Most researchers suspect this because <i>Trypanosome breci</i> is the only trypanosome microbe that humans are not resistant to. <ref>https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2270819/=PDF Steverding, Dietmar. “The History of African Trypanosomiasis.” <i>Parasites & Vectors,</i> BioMed Central, 2008</ref> | |||
What is the disease mechanism for this harmful microbe? | |||
<br>Introduce the topic of your paper. What is your research question? What experiments have addressed your question? Applications for medicine and/or environment?<br> | <br>Introduce the topic of your paper. What is your research question? What experiments have addressed your question? Applications for medicine and/or environment?<br> | ||
Revision as of 18:46, 15 April 2018
Introduction
By Katie Lensmeyer
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
African Sleeping Sickness is a microbial vector driven disease that affects many parts of Africa. The disease takes action by first invading the peripheral nervous system of its host and soon after passes the blood brain barrier to damage neurons within the brain. The result of this infection is fatal to the mammalian host. From initial infection to death of the host system, this microbe needs only a few short months to become fatal. How is it that this disease can invade such secure parts of the human system so quickly? The human immune system seems to be far to advanced to let this microbe produce the affects it does. Recent research looks deeply into both the spread of the infection through the mammalian host as well as the growth of the microbe within its vector, the tsetse fly.
Phylogenetic reconstruction of this disease has shown that this disease has existed for approximately 300 million years. The microbial coexistence with its insect parasites has existed for equally as long. This ancient coexistence between African mammals and their blood-sucking insect counterparts is assumed to be why most native Animalia are not affected by the disease. This immunity, however, does not stretch to the domesticated animals of the area. Trypanosome breci, the form of trypanosomes that causes sleeping sickness, is expected to be a recent development within history of trypanosomes. Most researchers suspect this because Trypanosome breci is the only trypanosome microbe that humans are not resistant to. [1]
What is the disease mechanism for this harmful microbe?
Introduce the topic of your paper. What is your research question? What experiments have addressed your question? Applications for medicine and/or environment?
Sample citations: [2]
[3]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
What is African Sleeping Sickness?
Include some current research, with at least one figure showing data.
Every point of information REQUIRES CITATION using the citation tool shown above.
African Trypanosomiasis, or better known as African Sleeping Sickness, is a parasite driven infection of the human nervous system. The disease is caused by the microbial parasites of the species Trypanosoma brucei and than transmitted through the tsetse fly, found only in rural parts of Africa. Throughout history, this disease has been classified as a public health problem seen primarily in sub-saharan areas of Africa. About 10,000 cases of the disease are reported every year to the World Health organization, but unfortunately it is expected that most cases go unreported and/or undiagnosed.
[4]
Because this disease is vector borne, the microbe, trypanosome brucei, enters the human system by ways of the skin. An infected tsetse fly must bite the host, and through this wound the protozoan enters the system. After initial infection, the disease has two stages. The first of these stages is the time in which the parasite is found within the peripheral nervous system, but has not yet made its way into the central nervous system. The second stage begins when the infection has passed the blood brain barrier and resides within the central nervous system. The disease than acts quickly, leaving its host with symptoms of fever, tremors, swollen lymph nodes, sleep disturbances, and speech problems within the first two weeks of infection. Following weeks lead to neurological deterioration ending in coma and soon after death. An untreated case can expect the the disease to become fatal within a few months. [5]
Cell Structure and Function
Include some current research, with at least one figure showing data.
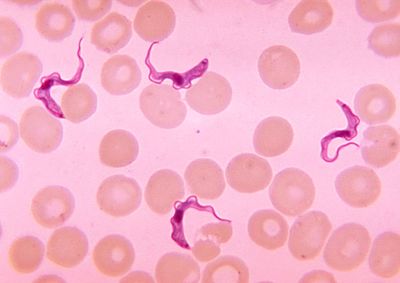
Trypanosoma cells are small (approxiamtely 50um) and heterotrophic, meaning they do not generate their own food source. The shape of the cell itself is long and oval with curved edges with a strong flagellum projecting off of the back end of the cell. The cell holds its struture through the presence of a highly polarized microtubule cytoskeleton. This cytoskeleton provides defiinite locations for the cell’s organelles (i.e. the flagellar pocket, flagellum, kinetoplast, mitochondrion and nucleus) within the center and posterior ends of the cell (end opposite of the flagella). [6] This cell expresses traits within its structure that are rather unique. Analogous to what is seen in its phylum Euglenozoa, the cell has a stiffening paraxial rod within its flagellum. Similarities with members of the cells order, Kinetoplastida, exist as well. The Trypansoma cell expresses a large cluster of DNA at the opposite end of the cell from the flagellum. This clump of DNA, otherwise known as the kinetoplast, extends from the cell’s unusually long mitochondrion and functions to determine its form within its human host. [7]
Upon initial entry into the host environment, the microbe finds itself floating within the bloodstream of the mammal in which it infected. This part of the human system is flowing with host defense mechanisms, both innate and adaptive immune responses, ready to attack any intruder. Trypanosoma has evolved to travel through this environment without detection through the presence of variant surface glycoproteins (VSG) that coat its cell wall. These VSG express one of ~1500 surface glycoprotein genes. The gene used to express these proteins changes with every 100th replication cycle to ensure the infections longevity. Upon detection, the host immune system will begin launching a complimentary protection response against trypanosoma. The change in transcription of the glycoprotein genes ensures that this complementary immune response is ineffective against the pathogen because the new VSG have developed and are undetectable. This characteristic is why patients with trypanosomiasis often experience symptoms of the disease followed by a period of latency. The disease has not dissipated but rather evaded the developed defenses of the host cell. These VSG properties are only found at certain times within the cells lifecycle: when the cell is developing within the saliva of the tsetse fly and when traveling through the host's bloodstream.
Inoculation Within the Insect Vector
The only known vector for the disease is the tsetse fly, which can be found all over Sub-Saharan Africa. Strangely, the tsetse fly acquire the trypanosoma microbe from biting infected animals or humans who conceal pathogenic parasites. [8] Once an individual fly gains the pathogenic cell, it modifies itself into a form that can be delivered and infect human hosts. In order to transform into the correct version of itself, the cell undergoes a series of developemental cycles including cell division to prepare themselves for infection. This augmentation within the insect can be sectioned into three different stages: the procyclic form, the epimastigote form and the metacyclic form. The procyclic and epimastigote forms exist for multiplication purposes, whereas the metacyclic form functions as an adaptation period inorder to effectively integrate itself within a human host. [9]
Upon entrance into the insect from ingesting an infected human’s blood, the so called “stumpy” form of T. breci enters and must adjust itself to survive within the environemnt of the fly. This intial transformation is composed of a varitey of reactions that are not yet fully understood. Many suspect that proteases within the insect’s midgut ignite a trigger response to the new environment. Beyond this idea, some believe that the microbe posses a cold-shock response that appears as the microbe travels from the warm-blooded human to the exothermic fly. Despite the controversy of this initial transformation, the “stumpy” form of the trypanosome remodels into the first form, the procyclic form, which than quickly attaches to the cells of the ectoperitrophic space and begin their growth into a more motile form. This form is also called the trypomastigote. The trypomastigote moves rather rapidly up the midgut of the insect and into the salivary glands. By connection to the kinetoplast, the cell enters the second, epimastigote form. This long form resides and begins replication within the salivary gland cell membranes. The cell must soon divide to form the short epimastigote form. This developmental form is currently considered to be responsible for the growth of the metacyclic trypanosomes that arre found prior to the cellular infection of a mammalian host. This cell division process is currently a popular research topic. The specifics of this differntiation is not fully understood. However, research has proven that relocation of the kinetoplast is vital to the growth of long epimastigote cells.
Section 3
Include some current research, with at least one figure showing data.
Section 4
Conclusion
References
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2270819/=PDF Steverding, Dietmar. “The History of African Trypanosomiasis.” Parasites & Vectors, BioMed Central, 2008
- ↑ Hodgkin, J. and Partridge, F.A. "Caenorhabditis elegans meets microsporidia: the nematode killers from Paris." 2008. PLoS Biology 6:2634-2637.
- ↑ Bartlett et al.: Oncolytic viruses as therapeutic cancer vaccines. Molecular Cancer 2013 12:103.
- ↑ https://www.cdc.gov/parasites/sleepingsickness/=PDF “Disease.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 29 Aug. 2012, www.cdc.gov/parasites/sleepingsickness/disease.html.
- ↑ https://www.cdc.gov/parasites/sleepingsickness/disease.html=PDF “Disease.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 29 Aug. 2012
- ↑ [http://jcs.biologists.org/content/118/2/283=PDF Matthews, Keith R. “The Developmental Cell Biology of Trypanosoma Brucei.” Journal of Cell Science, The Company of Biologists Ltd, 15 Jan. 2005, jcs.biologists.org/content/118/2/283.
- ↑ https://microbewiki.kenyon.edu/index.php/Trypanosoma=PDF “Disease.” “Trypanosoma.” Trypanosoma - Microbewiki, 7 Aug. 2010
- ↑ http://www.who.int/mediacentre/factsheets/fs259/en/=PDF WHO. “Trypanosomiasis, Human African (Sleeping Sickness).” World Health Organization, World Health Organization, 21 Mar. 2017
- ↑ https://microbewiki.kenyon.edu/index.php/Trypanosome_Life_Cycle=PDF “Trypanosome Life Cycle.” Trypanosome Life Cycle - Microbewiki, 10 Aug. 2010
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2018, Kenyon College.