Racial Disparities in MRSA Infections: Difference between revisions
| Line 2: | Line 2: | ||
[[Image:mrsa_magn_lg.jpg|thumb|300px|right| Image taken from a colorized scanning electron micrograph (SEM) magnified at 20,000X depicting a grouping of methicillin-resistant Staphylococcus aureus (MRSA) bacteria. Photo credit belongs to Public Health Image Library. [http://phil.cdc.gov/Phil/home.asp/ CDC].]] | [[Image:mrsa_magn_lg.jpg|thumb|300px|right| Image taken from a colorized scanning electron micrograph (SEM) magnified at 20,000X depicting a grouping of methicillin-resistant Staphylococcus aureus (MRSA) bacteria. Photo credit belongs to Public Health Image Library. [http://phil.cdc.gov/Phil/home.asp/ CDC].]] | ||
<br> Methicillin-Resistant Staphylococcus aureus, or MRSA for short, is a Gram-positive cocci-shaped (spherical) bacterium that measures approximately 1μm in diameter and forms clusters that are popularly described as being grape-like. <ref name=sadef>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6148192/#B1 Lakhundi, S., & Zhang, K. https://doi.org/10.1128/CMR.00020-18 "Methicillin-Resistant <i>Staphylococcus aureus<i>: Molecular Characterization, Evolution, and Epidemiology." 2018. Clinical microbiology reviews, 31(4), e00020-18.]</ref> S. aureus is present on and within the bodies of many individuals asymptomatically, and as a result of this it often remains unnoticed. According to studies around 20% of people are persistent nasal carriers of S. aureus and around 30% are intermittent carriers, with the remaining 50% not carrying the bacterium. <ref name=sadef/> Other than within the nose, S. aureus can be commonly seen present on the skin, skin glands, guts, and a variety of mucous membranes. This presence within the body is referred to as colonization, and it significantly increases the chances of acquiring an infection by providing a reservoir of the pathogen. <ref name=sadef/> In most cases, the previously asymptomatic, commensal S. aureus that previously colonized | <br> Methicillin-Resistant Staphylococcus aureus, or MRSA for short, is a Gram-positive cocci-shaped (spherical) bacterium that measures approximately 1μm in diameter and forms clusters that are popularly described as being grape-like. <ref name=sadef>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6148192/#B1 Lakhundi, S., & Zhang, K. https://doi.org/10.1128/CMR.00020-18 "Methicillin-Resistant <i>Staphylococcus aureus<i>: Molecular Characterization, Evolution, and Epidemiology." 2018. Clinical microbiology reviews, 31(4), e00020-18.]</ref> S. aureus is present on and within the bodies of many individuals asymptomatically, and as a result of this it often remains unnoticed. According to studies around 20% of people are persistent nasal carriers of S. aureus and around 30% are intermittent carriers, with the remaining 50% not carrying the bacterium. <ref name=sadef/> Other than within the nose, S. aureus can be commonly seen present on the skin, skin glands, guts, and a variety of mucous membranes. This presence within the body is referred to as colonization, and it significantly increases the chances of acquiring an infection by providing a reservoir of the pathogen. <ref name=sadef/> | ||
<br> In most cases, the previously asymptomatic, commensal S. aureus that previously colonized different sections of individuals' microbiomes are responsible for their infection. <ref name=mrsaresevoir> [https://www.tandfonline.com/doi/abs/10.5172/conu.13.1.38 Rhonda Griffiths, Ritin Fernandez, Elizabeth Halcomb "Reservoirs of MRSA in the acute hospital setting: A systematic review" 2002. Contemporary Nurse, 13:1, 38-49] </ref> Within the world of public health and medicine, a hugely important factor associated with S. aureus is its significant level of acquisition of resistance against multiple antibiotic classes, which greatly complicates efforts to treat it clinically. <ref name=antibioticresistance> [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4378521 Ventola, C Lee. “The antibiotic resistance crisis: part 1: causes and threats.” 2015 P & T : a peer-reviewed journal for formulary management vol. 40,4: 277-83.] </ref> Methicilin is an antibiotic class of particular interest with regard to resistance acquisition among S. aureus. According to the latest CDC data released in 2019, Methicillin-Resistant Staphylococcus aureus and other, less prominent strains of S. aureus accounted for an estimated 119,247 bloodstream infections within the United States in 2017, while causing 19,832 of those infected to pass away from complications associated with infection. <ref name=staphdeaths> [https://www.cdc.gov/mmwr/volumes/68/wr/mm6809e1.htm Kourtis AP, Hatfield K, Baggs J, et al. "Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections — United States." 2019 MMWR Morb Mortal Wkly Rep 2019;68:214–219.] </ref> | |||
As shown in the number of cases within 2017 alone given above, infections due to MRSA are a widespread phenomenon, however, there are stark differences in the incidence rates between different races in the US, particularly amongst Black and White people. <ref name=gualandiclass> [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6232852/figure/F1/ Gualandi, Nicole et al. “Racial Disparities in Invasive Methicillin-resistant Staphylococcus aureus Infections, 2005-2014.” Clinical infectious diseases: an official publication of the Infectious Diseases Society of America vol. 67,8 2018: 1175-1181. doi:10.1093/cid/ciy277] </ref> <ref name= atlanta> [http://doi.org/10.5334/egems.308 Ali F, Immergluck LC, Leong T, Waller L, Malhotra K, Jerris RC, et al.. A Spatial Analysis of Health Disparities Associated with Antibiotic Resistant Infections in Children Living in Atlanta (2002–2010). eGEMs (Generating Evidence & Methods to improve patient outcomes). 2019;7(1):50.] </ref> It is speculated that these disparities are the result of the combined effects of socioeconomic inequalities, socio-environmental inequalities, and broader disparities in the overall prevalence of certain health factors between races, for instance, diabetes. <ref name= gualandiclass/> | As shown in the number of cases within 2017 alone given above, infections due to MRSA are a widespread phenomenon, however, there are stark differences in the incidence rates between different races in the US, particularly amongst Black and White people. <ref name=gualandiclass> [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6232852/figure/F1/ Gualandi, Nicole et al. “Racial Disparities in Invasive Methicillin-resistant Staphylococcus aureus Infections, 2005-2014.” Clinical infectious diseases: an official publication of the Infectious Diseases Society of America vol. 67,8 2018: 1175-1181. doi:10.1093/cid/ciy277] </ref> <ref name= atlanta> [http://doi.org/10.5334/egems.308 Ali F, Immergluck LC, Leong T, Waller L, Malhotra K, Jerris RC, et al.. A Spatial Analysis of Health Disparities Associated with Antibiotic Resistant Infections in Children Living in Atlanta (2002–2010). eGEMs (Generating Evidence & Methods to improve patient outcomes). 2019;7(1):50.] </ref> It is speculated that these disparities are the result of the combined effects of socioeconomic inequalities, socio-environmental inequalities, and broader disparities in the overall prevalence of certain health factors between races, for instance, diabetes. <ref name= gualandiclass/> | ||
Revision as of 18:08, 9 April 2021
MRSA Overview
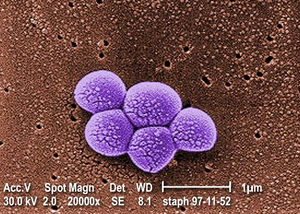
Methicillin-Resistant Staphylococcus aureus, or MRSA for short, is a Gram-positive cocci-shaped (spherical) bacterium that measures approximately 1μm in diameter and forms clusters that are popularly described as being grape-like. [1] S. aureus is present on and within the bodies of many individuals asymptomatically, and as a result of this it often remains unnoticed. According to studies around 20% of people are persistent nasal carriers of S. aureus and around 30% are intermittent carriers, with the remaining 50% not carrying the bacterium. [1] Other than within the nose, S. aureus can be commonly seen present on the skin, skin glands, guts, and a variety of mucous membranes. This presence within the body is referred to as colonization, and it significantly increases the chances of acquiring an infection by providing a reservoir of the pathogen. [1]
In most cases, the previously asymptomatic, commensal S. aureus that previously colonized different sections of individuals' microbiomes are responsible for their infection. [2] Within the world of public health and medicine, a hugely important factor associated with S. aureus is its significant level of acquisition of resistance against multiple antibiotic classes, which greatly complicates efforts to treat it clinically. [3] Methicilin is an antibiotic class of particular interest with regard to resistance acquisition among S. aureus. According to the latest CDC data released in 2019, Methicillin-Resistant Staphylococcus aureus and other, less prominent strains of S. aureus accounted for an estimated 119,247 bloodstream infections within the United States in 2017, while causing 19,832 of those infected to pass away from complications associated with infection. [4]
As shown in the number of cases within 2017 alone given above, infections due to MRSA are a widespread phenomenon, however, there are stark differences in the incidence rates between different races in the US, particularly amongst Black and White people. [5] [6] It is speculated that these disparities are the result of the combined effects of socioeconomic inequalities, socio-environmental inequalities, and broader disparities in the overall prevalence of certain health factors between races, for instance, diabetes. [5]
Health Consequences of MRSA
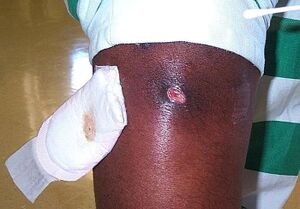
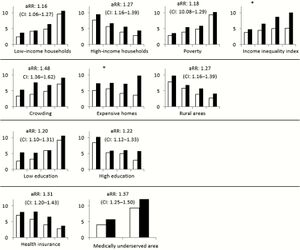
MRSA is responsible for causing a wide array of negative health outcomes. Among these outcomes are the likes of sepsis syndrome, toxic shock, skin and soft tissue infections (SSTIs),
bacteremia and endocarditis, pneumonia, bone and joint infections, CNS disease, and bacteremia. [9] Of note, bacteremia is present in roughly 75% of invasive MRSA cases. [9] These varied infections can result in health outcomes ranging from rashes to organ failure and, of course, death. [1] When considering the often dire health consequences associated with invasive MRSA infections, it becomes clear to see why the stark disparities in the rate of infection between races are a topic of burgeoning importance within the medical community. [10]
Hospital and Healthcare Acquired MRSA
Hospital Acquired MRSA Disparities
Methicillin-Resistant Staphylococcus aureus has been one of the most well-known and clinically relevant pathogens within hospitals and the broader healthcare industry for decades, and it has remained prevalent within them despite some oscillations. [11]
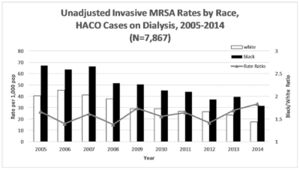
This relevance is why the study of racial health disparities is of the essence, and given such there have been studies and analyses conducted to sparse out just how significant these racial disparities with regard to MRSA infection are, and to what degree said racial disparities exist in clinically. According to a ten-year study conducted in the states of California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New York, Oregon, and Tennessee, the rate of hospital-associated decreased by numbers in excess of 65% for Black and White people from 2005 through 2013, declining from 18.16 cases per 100,000 Black people to 2.94 cases per 100,000 Black people. In White people a decline of 8.46 White people per 100,000 to 2.94 per 100,000. [5] These changes represent a small shift in the unadjusted rate ratios of Black MRSA infections from 2.15 in 2005 to 2.11 in 2013. [5] When adjusting the rate ratio to account for age, sex, and year, Black people's adjusted rate ratio was 3.20 instances of MRSA infection per 100,000 Black people. These results showcase that even though the raw number of cases throughout these 9 states decreased in excess of 65% over the course of the observational period, the rate of infection amongst Black people did not change by any significant amount, whereas in White people it did. [5]
This study plainly demonstrates how the Black race acquires more instances of MRSA infection than the White race. A now commonly proposed reason for this observed disparity is the fact that Black people within the US face heavily burdening socioeconomic challenges to a degree that White people do not. Within the United States, it has been discerned that Black people who are socioeconomically challenged, particularly those below the poverty line, as well as those living in urban areas, face many persistent challenges in gaining access to quality health care centers where they will not be discriminated against. [12]
Healthcare Acquired MRSA Disparities
Healthcare acquired MRSA [13] In the same 9 state study conducted to measure the rate change in Hospital-acquired MRSA along racial lines Black people were found to possess a rate ratio of 3.84 when accounting for age, sex, and year. [5] This adjusted rate ratio once again demonstrates a rate of MRSA acquisition among Black people that failed to fall in proportion to the smaller number of overall cases of MRSA acquisition over time, remaining roughly the same instead.
Community-Associated MRSA
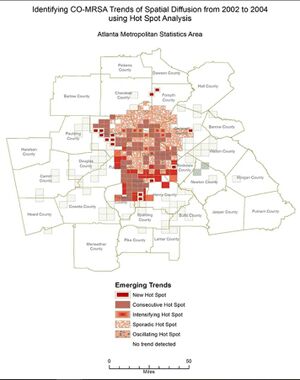
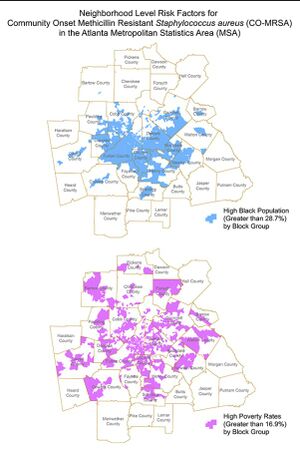
The similarity in the area covered by the three map models of Atlanta should be noted, as they demonstrate how Black people are disproportionately concentrated together whilst living in poverty and being at a higher risk of acquiring community-associated MRSA. [7]
Conclusion
References
- ↑ 1.0 1.1 1.2 1.3 Lakhundi, S., & Zhang, K. https://doi.org/10.1128/CMR.00020-18 "Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology." 2018. Clinical microbiology reviews, 31(4), e00020-18.
- ↑ Rhonda Griffiths, Ritin Fernandez, Elizabeth Halcomb "Reservoirs of MRSA in the acute hospital setting: A systematic review" 2002. Contemporary Nurse, 13:1, 38-49
- ↑ Ventola, C Lee. “The antibiotic resistance crisis: part 1: causes and threats.” 2015 P & T : a peer-reviewed journal for formulary management vol. 40,4: 277-83.
- ↑ Kourtis AP, Hatfield K, Baggs J, et al. "Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections — United States." 2019 MMWR Morb Mortal Wkly Rep 2019;68:214–219.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Gualandi, Nicole et al. “Racial Disparities in Invasive Methicillin-resistant Staphylococcus aureus Infections, 2005-2014.” Clinical infectious diseases: an official publication of the Infectious Diseases Society of America vol. 67,8 2018: 1175-1181. doi:10.1093/cid/ciy277
- ↑ Ali F, Immergluck LC, Leong T, Waller L, Malhotra K, Jerris RC, et al.. A Spatial Analysis of Health Disparities Associated with Antibiotic Resistant Infections in Children Living in Atlanta (2002–2010). eGEMs (Generating Evidence & Methods to improve patient outcomes). 2019;7(1):50.
- ↑ 7.0 7.1 7.2 Ali, F., Immergluck, L. C., Leong, T., Waller, L., Malhotra, K., Jerris, R. C., … Rust, G. S. (2019.) A Spatial Analysis of Health Disparities Associated with Antibiotic Resistant Infections in Children Living in Atlanta (2002–2010). Egems (generating Evidence & Methods to Improve Patient Outcomes), 7(1), 50.
- ↑ Isaac See, Paul Wesson, Nicole Gualandi, Ghinwa Dumyati, Lee H. Harrison, Lindsey Lesher, Joelle Nadle, Susan Petit, Claire Reisenauer, William Schaffner, Amy Tunali, Yi Mu, Jennifer Ahern "Socioeconomic Factors Explain Racial Disparities in Invasive Community-Associated Methicillin-Resistant Staphylococcus aureus Disease Rates" 2017. Clinical Infectious Diseases, Volume 64, Issue 5, 1 March 2017, Pages 597–604.
- ↑ 9.0 9.1 Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF; Infectious Diseases Society of America. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011 Feb 1;52(3):e18-55.
- ↑ Bakullari A, Metersky ML, Wang Y, Eldridge N, Eckenrode S, Pandolfi MM, Jaser L, Galusha D, Moy E. Racial and ethnic disparities in healthcare-associated infections in the United States, 2009-2011. Infect Control Hosp Epidemiol. 2014 Oct;35 Suppl 3:S10-6.
- ↑ [doi:10.1001/jamainternmed.2013.10423 Dantes R, Mu Y, Belflower R, et al. National Burden of Invasive Methicillin-Resistant Staphylococcus aureus Infections, United States, 2011. JAMA Intern Med. 2013;173(21):1970–1978.]
- ↑ D.‐L., Commodore‐Mensah, Y., Alexander, K.A., Jacques, K., Wilson, P.R., Akomah, J., Sharps, P. and Cooper, L.A. (2020), COVID‐19: Shedding light on racial and health inequities in the USA. J Clin Nurs, 29: 2734-2736.
- ↑ Linde, H., Wagenlehner, F., Strommenger, B. et al. Healthcare-associated outbreaks and community-acquired infections due to MRSA carrying the Panton-Valentine leucocidin gene in southeastern Germany. Eur J Clin Microbiol Infect Dis 24, 2005 419–422
