Rickettsia rickettsii: Difference between revisions
| Line 42: | Line 42: | ||
==Pathology== | ==Pathology== | ||
[[Image:Rickettsia_rickettsii3.jpg|frame|right|A rash developed from late stage Rocky Mountain Spotted Fever on a patient's legs. Photo credit: [http://www.cdc.gov/ncidod/dvrd/rmsf/ Rocky Mountain Laboratories, NIAID, NIH, Hamilton, Montana.]]] | |||
Typical symptoms of RMSF can appear 2 to 14 days after exposure and include fever, headache, depression, nausea, vomiting, and gradually may develop a skin rash called purpura or petechiae. Sometimes the rash occurs 2 to 5 days after the onset of the fever. Serious cases of RMSF can include central nervous system, pulmonary, or hepatic injuries. Patients that have compromised immune systems often have an increased susceptibility to other infections. [2][9] If Rocky Mountain Spotted Fever is left untreated, there is a high mortality rate. [11]<i> Rickettsia rickettsii</i> can infect endothelial cells within the human body through the vascular smooth muscle cells and tissues. [5] In addition, it is an intracellular pathogen that can infect and multiply within the nucleus or cytosol of endothelial cells of the blood vessels. [9] | Typical symptoms of RMSF can appear 2 to 14 days after exposure and include fever, headache, depression, nausea, vomiting, and gradually may develop a skin rash called purpura or petechiae. Sometimes the rash occurs 2 to 5 days after the onset of the fever. Serious cases of RMSF can include central nervous system, pulmonary, or hepatic injuries. Patients that have compromised immune systems often have an increased susceptibility to other infections. [2][9] If Rocky Mountain Spotted Fever is left untreated, there is a high mortality rate. [11]<i> Rickettsia rickettsii</i> can infect endothelial cells within the human body through the vascular smooth muscle cells and tissues. [5] In addition, it is an intracellular pathogen that can infect and multiply within the nucleus or cytosol of endothelial cells of the blood vessels. [9] | ||
Revision as of 11:16, 29 August 2007
A Microbial Biorealm page on the genus Rickettsia rickettsii
Classification
Higher order taxa
Bacteria; Proteobacteria; Alpha Proteobacteria; Rickettsiales; Rickettsiaceae; Spotted fever group
Species
|
NCBI: Taxonomy |
Rickettsia rickettsii
Other Names
synonym: Dermacentroxenus rickettsii
synonym: "Dermacentroxenus rickettsii" Wolbach 1919
synonym: Rickettsia rickettsii (Wolbach 1919) Brumpt 1922 [1]
Description and significance
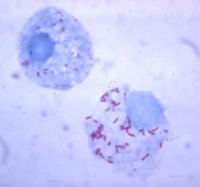
Rickettsia rickettsii is a small, rod-shaped bacterium known to cause Rocky Mountain spotted fever (RMSF). This disease can be transmitted to humans either from a tick bite with an incubation period of 1 week, or by contamination of a cut on the skin or a wound with ticks feces. Dr. Ricketts first isolated this microbe in Montana, 1906. Rickettsia need host cells to be able to grow. In addition, they also require arthropods as vectors; therefore, ticks are the vectors used for Rickettsia rickettsii. [2] [4] [6]
Genome structure
The unfinished genome sequencing of Rickettsia rickettsii is made up of 1,257,710 nucleotides long. There are 1311 genes, and all of them code for proteins. The guanine and cytosine content is 32% of its DNA. Rickettsia rickettsii has no pseudo genes, and it’s made up of DNA molecules. The topology of Rickettsia rickettsii is neither circular or linear chromosomes. In addition, no known plasmids have been found. [3]
Infection with Rickettsia rickettsii also triggers an active response that alters cellular phenotypes. There is an increase in the secretion of cytokines and proteins. During Rickettsia rickettsii infection, nuclear factor-kappa B (NF-KB) is activated. This is a group of transcription factors that regulate cell adhesion, proliferation, the immune system, inflammation, stress responses, and host pathogenic interactions. [10]
Rickettsia rickettsii regulates COX-2 expression, and the inhibition of COX-2 activity during an infection causes the release of prostaglandins. COX (cyclooxygenase) is the rate limiting enzyme for the breakdown of arachidonic acid (AA) into PGH2, an intermediate. The activation of COX-1, however, has no effect on Rickettsia rickettsii. The regulation of COX-2 expression in RMSF infection involves posttranscriptional control mechanisms. This organisms uses a signaling system through a p38 mitogen-activated protein kinase. Inhibition of the p38 kinase inhibits the activation of COX-2. It's still unclear whether COX-2 is responsible for rickettsia replication and triggering the RMSF infection through vascular inflammation. [15]
Cell structure and metabolism
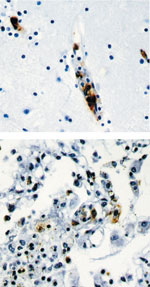
The Rickettsia rickettsii bacterium are intracellular organisms, and they live in the cytoplasms of host cells sometimes in the nucleus. They are adapted as intracellular organisms because they have transport systems and metabolic enzymes. These microbes divide by binary fission and take about 8 hours to double. [2] Rickettsia rickettsii undergo aerobic oxidation, and they live in a host-associated habitat. The optimal temperature they live in is 37 degrees Celsius, which is in the mesophilic range. [11]
Rickettsia rickettsii are similar to small, gram-negative nods. They are barely visible under a light microscope, and they stain poorly with Gram stains. The cell wall is made up of peptidoglycan, lipopolysaccharide, and a cell membrane. These microbes contain two outer membrane proteins, outer membrane protein A (OmpA) and outer membrane protein B (OmpB). OmpA sticks to the host cell wall, and it is a protective layer of about 190 kDa. It contains a region of 13 identical repeating units that include antigenic function. OmpB is an abundant outer membrane protein that contains sequences related to the typhus group of rickettsiae.[2][7]
Ecology
Rickettsia rickettsii can be found in the western hemisphere, more notably in North America and South America. In North America, it’s more well known in the southeast and southcentral states. In North America, Rickettsia rickettsii is transmitted by the American dog tick (Dermacentor variabilis) and the Rocky Mountain wood tick (Dermacentor andersoni). In Latin America. In Latin America, Rickettsia rickettsii is transmitted by Rhipicephalus sanguineus and the Cayenne tick Amblyomma cayenne. [9] The reason the various species of ticks is significant is that it allows the organism to adapt to new ecological niches. [15]
Pathology
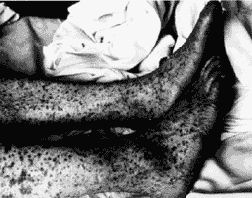
Typical symptoms of RMSF can appear 2 to 14 days after exposure and include fever, headache, depression, nausea, vomiting, and gradually may develop a skin rash called purpura or petechiae. Sometimes the rash occurs 2 to 5 days after the onset of the fever. Serious cases of RMSF can include central nervous system, pulmonary, or hepatic injuries. Patients that have compromised immune systems often have an increased susceptibility to other infections. [2][9] If Rocky Mountain Spotted Fever is left untreated, there is a high mortality rate. [11] Rickettsia rickettsii can infect endothelial cells within the human body through the vascular smooth muscle cells and tissues. [5] In addition, it is an intracellular pathogen that can infect and multiply within the nucleus or cytosol of endothelial cells of the blood vessels. [9]
Rocky Mountain Spotted Fever is prevalent more commonly among children in the southeast and southcentral United States. The most incidences of RMSF occurs in children among 5 to 9 years olds. Success in treatment of RMSF increases with initial diagnosis and treatment and reported tick bites or tick attachments prior to major symptoms. [12]
Application to Biotechnology
The organism Rickettsia rickettsii has no significant contribution in forming compounds or enzymes related to biotechnology. However, the only aspect is that it can be used as a form of a bioterrorism agent, especially using dogs to spread the infection of Rocky Mountain Spotted Fever. [14]
Current Research
Currently there is a major lack in controlling the RMSF organism. In addition, an effective diagnostic test to detect early symptoms is still not available. Doctors have a hard time detecting RMSF because the patients rarely have the Rickettsia rickettsii antibodies. Although PCR (Polymerase Chain Reaction) testing is available in a few laboratories, detection of Rickettsial DNA in the blood is still lacking, especially in the early symptoms. After diagnosis with RMSF, it is usually treated with Tetracycline or Doxycycline unless the CNS has already been affected thereby using Chloramphenicol instead.[8] [9]
Scientists currently are searching for an accurate PCR assay for detecting Rickettsia rickettsii. They designed the primers and TaqMan-MGB probes according to the ompB gene of Rickettsia rickettsii. A DNA fragment taken from the ompB gene was isolated and amplified by PCR in order to be used as a template. They found 5 copies of the ompB fragments, and the genomic DNA sequence of Rickettsia rickettsii was also detected by the PCR assay. The PCR assay is being used to detect Rickettsia rickettsii in DNA samples in order to diagnose patients infected with Rickettsia rickettsii as early as possible. [13]
Rocky Mountain spotted fever has mortality rates in both humans and dogs in the United States. The symptoms in the dogs can sometimes precede the illness in people. The Centers for Disease Control and Prevention (CDC) has classified Rickettsia rickettsii as a Category C priority pathogen due to the fact that the organism can be used as a bioterroism agent. In addition, dogs could serve as the foundation of bioterrorist attacks using Rickettsia rickettsii. After PCR testing in laboratories of the genes ompA, rrs, and gltA, scientists deduced that the Rickettsia rickettsii organisms that infected humans were similar to the ones that infected dogs with sequences that differed in only 1 base pair. The ompA gene was 100% similiar to the tick strain (Bitterroot) of Rickettsia rickettsii. The rrs gene was 99.8% similar to the tick strain (Sawtooth) of Rickettsia rickettsii. Currently the sequencing of the gltA gene is still taking place. [14]
References
1) NCBI Taxonomy Browser "Rickettsia rickettsii" Retrieved 26 August, 2007.
2) Whitney, Jamie MD. "Rocky Mountain Spotted Fever". Primary Care Update for OB/GYNS. Volume 9. Issue 1. January-February 2002. Pages 28-32.
3) NCBI Genome "Rickettsia rickettsii" Retrieved 28 August, 2007.
4) Joshi, S., Kovács, A. "Rickettsia rickettsii infection causes apoptotic death of cultured cerebellar granule neurons". Journal of Medical Microbiology. Volume 56. 2007. Pg.138-141.
5) Li, H., Walker, D. "rOmpA is a critical protein for the adhesion of Rickettsia rickettsii to host cells". Microbial Pathogenesis. Volume 24. 1998. Pg.289-298.
6) Roux, V., Raoult, D. "Phylogenetic analysis of the genus Rickettsia by 16S rDNA sequencing". Research in Microbiology. Volume 146. Issue 5. June 1995. Pg.385-396.
7) Gilmore, R.D. "Comparison of the rompA gene repeat regions of Rickettsiae reveals species-specific arrangements of individual repeating units". Gene. Volume 125. Issue 1. 15 March, 1993. Pages 97-102.
8) Fatal Human Infection with Rickettsia rickettsii, Yucatan, Mexico Retrieved 28 August, 2007.
9) Leiby, D., Gill, J. "Transfusion-transmitted tick-borne infections: A cornucopia of threats". Transfusion Medicine Reviews. Volume 18. Issue 4. October 2004. Pg.293-306.
10) Joshi, S., Francis, C., Silverman, D., Sahni, S. "NF-KB activation suppresses host cell apoptosis during Rickettsia rickettsii infection via regulatory effects on intracellular localization or levels of apoptogenic and anti-apoptotic proteins". FEMS Microbiology Letters. Volume 234. Issue 2. 15 May 2004. Pg.333-341.
11) NCBI Genome Project "Rickettsia rickettsii" Retrieved 28 August, 2007.
12) Buckingham, S., Marshall, G., Schutze, G., Woods, C., Jackson, M., Patterson, L., Jacobs, R. "Clinical and Laboratory Features, Hospital Course, and Outcome of Rocky Mountain Spotted Fever in Children". The Journal of Pediatrics. Volume 150. Issue 2. February 2007. Pg.180-184. E1.
13) Niu, D., Chen, M., Wen, B., Li, Q., Qiu, L., Zhang, J. PubMed - "Study on the development of a real-time quantitative polymerase chain reaction assay to detect Rickettsia" June 2006. Volume 27. Issue 6. Pg.529-529. Retrieved 28 August, 2007.
14) Kidd L., Hegarty B., Sexton D., Breitschwerdt E. PubMed - "Molecular characterization of Rickettsia rickettsii infecting dogs and people in North Carolina" October 2006. Volume 1078. Pg.400-409. Retrieved 28 August, 2007.
15) Rydkina, E., Sahni, A., Baggs, R., Silverman, D., Sahni. "Infection of Human Endothelial Cells with Spotted Fever Group Rickettsiae Stimulates Cyclooxygenase 2 Expression and Release of Vasoactive Prostaglandins". Infect Immun. September 2006. Volume 74. Issue 9. Pg.5067-5074.
Edited by Amber Chou; student of Rachel Larsen
