Pseudomonas chlororaphis: Difference between revisions
| Line 1: | Line 1: | ||
==Classification== | ==Classification== | ||
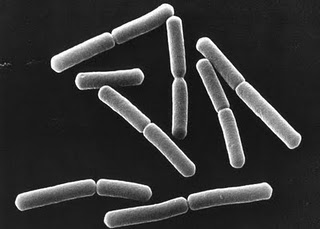
[[File:P. Cloroaphis.jpg| | [[File:P. Cloroaphis.jpg|4000px|right|frame|''Pseudomonas chlororaphis'']] | ||
Kingdom: Bacteria | Kingdom: Bacteria | ||
Revision as of 20:20, 27 April 2010
Classification
Kingdom: Bacteria
Phylum: Proteobacteria
Class: Gamma Proteobacteria
Order: Pseudomonadales
Family: Pseudomonadaceae
Species
Pseudomonas chloroaphis
Sub Groups: aurantiaca, aureofaciens, chlororaphis, fragi, lundensis, taetrolens
Description and Significance
In Greek, Pseudomonad literally means 'false unit', being derived from pseudo and monas (Applied, 2002). The term "monad" was used in the early history of microbiology to denote single-celled organisms (Applied, 2002).
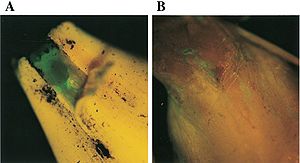
Characteristics of Pseudomonas chloroaphis includes secretion of pyoverdin (picture to the right), or fluorescent (Tombolini, 1999). This fluorescent is generally a yellow-green pigment and comes out when the organism is attached to the desired plant species, within the hystol section of soil, under iron limiting conditions (Meyer, 2002). Emission of these pigments allow for easy detection, using siderophore typing (Meyer, 2002).
In addition, Pseudomonas chlororaphis is an effective biocontrol agent against Pythium aphanidermatum, a casual damping affect of hot pepper seedlings in greenhouse vegetable production, and this microorganism reduces and often eliminates soft-rot in leaves of the tobacco plant that is caused by Erwinia carotovora (European, 2009).
Many applications of Pseudomonas chlororaphis are in agriculture and horticulture by acting against various fungal plant pathogens by creating phenazine (antibiotic to root rot of cucumbers, peppers, tomatoes, and many other agricultural crops) (Woeng, 2000).
Genome Structure
The genome of Pseudomonas chlororaphis has not been mapped at this current time.
Cell Structure, Metabolism and Life Cycle
Pseudomonas chlororaphis are rod-shaped, non-spore forming, gram-negative bacteria with one or more polar flagella (Todar, 2006). The microorganisms' flagella allow for motility (Asano et al, 1981).
The organism is typically an aerobic heterotroph, but has also been shown to perform denitrification within the rhizosphere of Glyceria maxima (Reed mannagrass). The end product of dentrification by P. chlororaphis tends to be N20 (Bodelier et al, 1997). Growth of P. chlororaphis is stimulated by the roots of G. maxima, not only in the direct rhizosphere, but also within the surrounding sediments (Bodelier and Laanbroek, 1997). It was also shown that P. chlororaphis competes with Nitrosomonas europaea for oxygen in the rhizosphere of plants, but competition is controlled by the oxidation kinetics of the electron donor ("instead of the oxygen uptake kinetics of the respective organisms." (Bodelier and Laanbroek, 1997)
As described above, The United States Environmental Protection Agency (EPA) states that some strains of P. chlororaphis act as effective fungicides when found in situ, as well as when used in soil inoculation. The bacteria can be applied directly to cereal seeds prior to sowing, which has been shown to act as an effective biocontrol agent in over 11 European countries (Tombolini et al, 1999). The organisms shield plant roots by producing antibiotics and by immobilizing iron that is needed for fungal growth (EPA, 2009). P. chlororaphis also produces cytokinin and other plant growth factors that help plants resist fungal infection (EPA, 2009). Efficacy of P. chlororaphis as an antifungal agent is dependent upon the characteristics of each strain, thus, several companies (such as Lantmännen BioAgri AB of Sweden) have performed extensive research comparing strains and method of inoculation (Tombolini et al, 1999).
Pseudomonas chlororaphis has also been used in industrial acrylamide production. Specific strains actively produce nitrile hydrases that hydrate nitriles to amides (Yamada and Kobayashi, 1996). This technique of enzymatic acrylamide production consumes 30% less energy and produces 7% less CO2 than conventional acrylamide production (includes steam, electric power, and raw materials; OECD, 2001). Common uses of acrylamide include adhesives, coagulants, paints, paper, and soil ammendments (Yamada and Kobayashi, 1996).
Ecology and Pathogenesis
Pseudomonas chlororaphis is a heterotrophic, soil bacteria that can be found in the rhizosphere, phyllosphere and surrounding sediments (Bodelier and Laanbroek, 1997; Legard et al. 1994). It is mesophilic with an ideal growth temperature range between 20C-28C (Selin et al., 2010; Palmfeldt et al., 2003; Bardas et al., 2009; Bodelier et al., 1997) and growth best around a neutral pH between 6.3-7.5 (Rij ET, 2004; Bodelier and Laanbroek, 1997).
Pseudomonas chlororaphis has been observed to occupy the same site (the grooves along the junctions of epidermal cells) as Pseudomonas fluorescens when applied to tomato seedlings (Bloemberg et al., 2001); however, a clear symbiotic relationship has not been defined for P. chlororaphis. Additionally, many soil microorganisms are difficult to culture, and non-culturable microorganisms may account for a large part of the microflora, this does not so the possibility of a symbiosis being discovered as non-culturable techniques improve, should be considered. Biogeochemical significance.
Due to their ability to perform denitrification, P. chlororaphis have a biogeochemical significance of producing dinitrogen gas from nitrate (Palleroni et al., 2005)
Several strains of P. chlororaphis have been shown to be efficient root colonizers (Bloemberg et al., 2001); capable of producing a variety of antifungal substances including phenazine-1-carboxamide (PCN), hydrogen cyanide, chitinases and proteases (Bloemberg et al., 2001); The effectiveness of P. chlororaphis as a biocontrol agent has made it an organism of interest for controlling root rot in a variety of organisms, including tomatoes, peppers, canola, and others (Selin et al., 2010, Khan et al., 2003)
Although P. chlororaphis is an effective biocontroller of certain fungi, it is generally considered a nonpathongenic bacterium. The Environmental Protection Agency (EPA,2009) states that,”Pseudomonas chlororaphis has shown no toxicity or pathogenicity to humans, wildlife, or the environment(EPA, 2009)” However, there have been limited reports of P. chlororaphis acting as a fish toxin (Hatai K, 1975).
References
Asano, Y., Yasuda, T., Tani, Y., and Yamada, H. "A New Enzymatic Method of Acrylamide Production." Agricultural Biology and Chemistry. 1982. Volume 46 (5). p. 1183-1189.
Bardas G., LagopodiA., Kadoglidou K., Tzavella-Klonari K.“Biological control of three Colletotrichum lindemuthianum races using Pseudomonas chlororaphis PCL1391 and Pseudomonas fluorescens WCS365”. Biological Control. 2009. Volume 49:2. p.139-145
Bloemberg G., Lugtenberg B .“Molecular basis of plant growth promotion and biocontrol by rhizobacteria.” Current Opinion in Plant Biology. 2001. Volume 4: 4. p.343-350
Bodelier P. and Laanbroek H. “Oxygen uptake kinetics of Pseudomonas chlororaphis grown in glucose- or glutamate-limited continuous cultures.” Research Microbiology. 1997. Volume 167. p.392-395
Bodelier PL, Wijlhuizen, AG, Blom, CW and Laanbroek, HJ. “Effects of photoperiod on growth of and denitrification by Pseudomonas chlororaphis in the root zone of Glyceria maxima, studied in a gnotobiotic microcosm.” Plant and Soil. Volume 190:1. 1997. p.91–103.
Chin-A-Woeng Tf. Mol Plant Microbe Interact 13. 2002. 12: 1340–5.
EPA. "Pseudomonas chlororaphis strain 63-28 (006478) Fact Sheet." 2009. http://www.epa.gov/oppbppd1/biopesticides/ingredients/factsheets/factsheet_006478.htm
European Food Safety Authority. Scientific Opinion on the maintenance of the list of QPS microorganisms intentionally added to food or feed. EFSA Journal 2009, 7(12): 1431.
Hatai K. Pseudomonas chlororaphis as a Fish Pathogen. Bulletin of the Japenese Society of Scientific Fisheries. 1975. Volume 41:11. P.1203
Khan A., Sutton J. , Grodzinski B. "Effects of Pseudomonas chlororaphis on Pythium aphanidermatum and Root Rot in Peppers Grown in Small-scale Hydroponic Troughs". Biocontrol Science and Technology. 2003. Volume 13:6
Legard D., McQuilken M., Whipps J., Fenlon J., Fermor T., Thompson I., Bailey M., Lynch J. “Studies of seasonal changes in the microbial populations on the phyllosphere of spring wheat as a prelude to the release of a genetically modified microorganism.” Agriculture, Ecosystems & Environment. 1994. Volume 50:2. p.87-101
Meyer JM, and Geoffroy VA. (2002). Applied Environmental Microbiology 68 (6): 2745–53.
OECD. Application of Biotechnology to Industrial Sustainability. Google Books (Online). 2001. p 71-75. Accessed 20 April 2010.
Selin C., Habibian R., Poritsanos N., Athukorala S., Fernando D., de Kievit T. “Phenazines are not essential for Pseudomonas chlororaphis PA23 biocontrol of Sclerotinia sclerotiorum, but do play a role in biofilm formation.” FEMS Microbiology Ecology. 2010. Volume 71:1. p. 73-83.
Palleroni N. Genus I. Pseudomonas. In Bergey’s Manual of Systematic Bacteriology, 2nd edition. 2005. Volume 2. p. 323-379
Palmfeldt J., Radstrom P., Hahn-Hagerdal B.”Optimisation of initial cell concentration enhances freeze-drying tolerance of Pseudomonas chlororaphis.” Cryobiology. 2003. Volume 47:1. p.21-29
Rij ET, Wesselink M, Chin-A-Woeng TF, Bloemberg GV, Lugtenberg BJ. “Influence of Environmental Conditions on the Production of Phenazine-1-Carboxamide by Pseudomonas chlororaphis PCL1391.” Molecular Plant-Microbe Interactions. 2004. Volume 17:5. p.557-566
Todar, Kenneth. Pseudomonas. 16 Aug. 2006. http://microbewiki.kenyon.edu/index.php/Pseudomonas accessed 19 Apr. 2010
Tombolini Riccardo, Gaag Dirk Jan Gerhardson, Berndt and Jansson Janet. Colonization Pattern of the Biocontrol Strain Pseudomonas chlororaphis MA 342 on Barley Seeds Visualized by Using Green Fluorescent Protein. Applied and Environmental Microbiology. Aug. 1999. 3674-3680.
Yamada, Hideaki and Michihiko Kobayashi. "Nitrile Hydratase and Its Application to Industrial Production of Acrylamide." Bioscience, Biotechnology, and Biochemistry. 1996. Volume 60 (9).
http://aem.asm.org/cgi/reprint/65/8/3674
Author
Page authored by Brad J. Wardynski, Michael Wandersee, and Erika White, students of Prof. Jay Lennon at Michigan State University.