Solenopsis invicta virus 1: Difference between revisions
Engelbrechte (talk | contribs) |
Engelbrechte (talk | contribs) |
||
| Line 15: | Line 15: | ||
[[Image:ant ball.jpg|thumb|200px|left|<i>S. invicta</i>, Imported red fire ants adapt to inhospitable conditions. Here they have coagulated in response to increased water levels to form a raft [7]. [http://www.ars.usda.gov/IS/pr/2007/071119.htm].]] | [[Image:ant ball.jpg|thumb|200px|left|<i>S. invicta</i>, Imported red fire ants adapt to inhospitable conditions. Here they have coagulated in response to increased water levels to form a raft [7]. [http://www.ars.usda.gov/IS/pr/2007/071119.htm].]] | ||
<br>Introduced into the U.S. in the early 1900s from South America, <i>Solenopsis invicta</i>, or the red imported fire ant, is one of the most successful and invasive ant species in North America [2]. These ants exhibit uninterrupted-foraging activity, aggressive behavior, and are capable of adapting to disturbed habitats – each contributing to their ecological dominance [3]. Damage attributed to <i>S. invicta</i> includes physical damage to livestock, agricultural and electrical equipment, and human health (2, 3 (New England Journal article). It has become apparent that the most effective way to control <i>S. invicta</i> population densities is through a biological mechanism. SINV-1, SINV-2, and SINV-3 are under investigation as biological control agents of the imported red fire ant [1, 2, 3, 5]. | <br>Introduced into the U.S. in the early 1900s from South America, <i>Solenopsis invicta</i>, or the red imported fire ant, is one of the most successful and invasive ant species in North America [2]. These ants exhibit uninterrupted-foraging activity, aggressive behavior, and are capable of adapting to disturbed habitats – each contributing to their ecological dominance [3]. Damage attributed to <i>S. invicta</i> includes physical damage to livestock, agricultural and electrical equipment, and human health (2, 3 (New England Journal article). It has become apparent that the most effective way to control <i>S. invicta</i> population densities is through a biological mechanism. SINV-1, SINV-2, and SINV-3 are under investigation as biological control agents of the imported red fire ant [1, 2, 3, 5]. | ||
[[Image:ant graph.jpg|thumb|200px|left|<i>S. invicta</i>, Efforts to control <i>S. invicta</i> growth in the U.S. have been unsuccessful [2]. | [[Image:ant graph.jpg|thumb|200px|left|<i>S. invicta</i>, Efforts to control <i>S. invicta</i> growth in the U.S. have been unsuccessful [2]. ]] | ||
<br>While a number of effective insecticides are available to control <i>S. invicta</i> population growth, they must be used regularly because of the fire ant’s ability to rapidly re-inhabit treated areas [3]. Compared to a biological mechanism of control, insecticide use is impractical from both an environmental and economic standpoint [3]. | <br>While a number of effective insecticides are available to control <i>S. invicta</i> population growth, they must be used regularly because of the fire ant’s ability to rapidly re-inhabit treated areas [3]. Compared to a biological mechanism of control, insecticide use is impractical from both an environmental and economic standpoint [3]. | ||
Revision as of 00:24, 24 September 2012
The imported red fire ant (Solenopsis invicta) has emerged as one of the most successful and destructive ant species in North America, causing an annual average of $6 billion in damages [2]. Efforts to thwart population density growth of S. invicta with insecticides since the 1960s have proven largely unsuccessful. In 2004, S. invicta virus 1 was sequenced and identified as the only known viral pathogen of the imported red fire ant.
A Viral Biorealm page on the family Solenopsis invicta virus 1
Virus Classification
<Viruses; Group IV (+) ssRNA virus, Dicistroviridae>
Description and Significance

Introduced into the U.S. in the early 1900s from South America, Solenopsis invicta, or the red imported fire ant, is one of the most successful and invasive ant species in North America [2]. These ants exhibit uninterrupted-foraging activity, aggressive behavior, and are capable of adapting to disturbed habitats – each contributing to their ecological dominance [3]. Damage attributed to S. invicta includes physical damage to livestock, agricultural and electrical equipment, and human health (2, 3 (New England Journal article). It has become apparent that the most effective way to control S. invicta population densities is through a biological mechanism. SINV-1, SINV-2, and SINV-3 are under investigation as biological control agents of the imported red fire ant [1, 2, 3, 5].
While a number of effective insecticides are available to control S. invicta population growth, they must be used regularly because of the fire ant’s ability to rapidly re-inhabit treated areas [3]. Compared to a biological mechanism of control, insecticide use is impractical from both an environmental and economic standpoint [3].
In South America, the fire ant is exposed to more natural enemies and is rendered less invasive. These natural enemies include bacterium Kneallhazia solenopsae, parasitoids such as Pseudacteon flies and eucharitid wasps, and a parasite Solenopsis daguerrei. In an effort to control S. invicta growth, five populations of Pseudacteon were released in the US in the past decade [3]. Female Pseudacteon flies launch an aerial assault down the thorax of a worker ant where they insert their eggs. 2-6 wk after oviposition, the larva migrates into the ant’s head and digests the tissue, ultimately decapitating the host ant [3]. Despite this lethal mechanism of parasitism, significant reductions of S. invicta population densities have not been observed yet [3].
Genome Structure
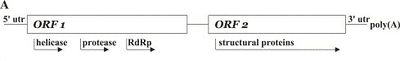
The genome of SINV-1 is 8,026 nucleotides long and consists of a single (+) ssRNA (accession number AY634314) and includes a 5' UTR, two ORFs, a 3' UTR and poly A tail (as determined by Rapid Amplification of cDNA Ends) [1]. ORF1 encodes helicase, cysteine protease, and RNA-dependent RNA polymerase (RdRp). ORF2 encodes capsid proteins. SINV-1 RdRp is exhibits homology with KBV (Kashmir bee virus), Acute bee paralysis virus (ABPV), and Taura syndrome virus. The viral genome was discovered as researchers were analyzing the sequences of an expression library from an S. invicta colony and noticed several genes with significant homology to genes from ssRNA viruses like the Picornaviridae [Valles 2004].
It has been reported that integration of (+) ssRNA viral genomes into the host genome may afford "protection to the host by the corresponding virus." Because researchers want to exploit SINV-1 as a lethal S. invicta pathogen, it was important to determine if the viral genome is incorporated into that of the host. In a 2004 study using 32 S. invicta genomic DNA samples, researchers did not detect integration of SINV-1 into the host genome, thus providing evidence of SINV-1 as a potential biopesticide.
Virion Structure of SINV-1
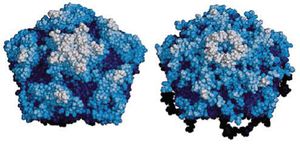
The SINV-1 virion is an icosahedral, non-enveloped particle that encloses (+) ssRNA. Dicistroviruses enter their host cell via endocytosis, but it is unclear how the SINV-1 interacts with a receptor. The capsid is 30-31 nm in diameter and consists of four repeating viral protein (VP) subunits, VP1, VP2, VP3, and VP4 (in order of decreasing MW).
Reproductive Cycle of SINV-1 in a Host Cell
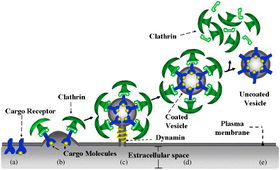
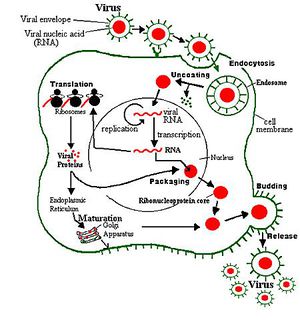
Unlike the ubiquitously replicating SINV-2 and -3, SINV-1 localizes in the gut of S. invicta. While the surface protein(s) that SINV-1 binds are unknown, SINV-1 likely enters its host cell via clathrin-mediated endocytosis. Once inside the cell, virions uncoat and release genomic RNA into the cytoplasm. Consistent with replication mechanisms of picornaviridae, the SINV-1 replication complex associates with virus-induced vesicles for RNA replication as a result of infection processes that remodel the Golgi apparatus [5, 9].
SINV-1 (+) RNA may be translated upon entry into the cytoplasm by host translational machinery (ribosomes) as a single polypeptide which is cleaved by viral proteases to produce about 7 viral proteins, including a 5’ viral protein genome (VPg, a peptide covalently linked at the 5’ terminus) which serves as a primer for viral RNA replication [2]. (+) RNA is also transcribed by SINV-1 RNA-dependent RNA polymerase to (-) RNA, which is then transcribed to (+) RNA for subsequent translation or packing into a capsid. Fully assembled SINV-1 virions spread after lysing the host cell, however it is unclear how frequently this happens as SINV-1 infected ants are typically asymptomatic [1, 2].
Viral Ecology & Pathology
Late-instar S. invicta digest all solid food for the colony and redistribute it as liquid to nestmates [2]. In this way, larvae serve as both a replication site and passage fir SINV-1 colonial propagation [2]. Experiments using Real-time PCR demonstrate that SINV-1 exists primarily in the midgut of S. invicta, evidence of the horizontal fecal-oral route of viral infection [2]. SINV-1 detection in "all developmental stages of S. invicta including eggs and queen" indicates vertical transmission of the virus [2]. SINV-1 infected S. invicta populations are asymptomatic, but their ability to compete with uninfected ants of their own and other species is reduced [2]. Some environmental stresses induce symptoms or even death in SINV-1 infected ants [2]. Researchers observed that SINV-1 internal ribosome entry site within the intergenic region (IGR IRES) exhibits increased activity at higher temperatures [10]. This data correlates with an increased incidence of S. invicta SINV-1 infection at higher temperatures in field studies [2]. In the laboratory, SINV-1 infection was associated with larval mortality [2]. These observations highlight the complexity of microscopic mutualisms in nature.
The goal of SINV research is to exploit these viruses so they can effectively wipe out populations of the imported red fire ant. Proposed methods include using the virus to deliver a toxin or an RNAi sequence to kill its host.
References
[1] Valles, Steven M., Charles A. Strong, et al. "A licorna-like virus from the red imported fire ant, Solenopsis invicta: initial discovery, genome sequence, and characterization." Virology. (2004): 151-157.
[2] Valles, Steven M. "Review Article: Positive-strand RNA viruses infecting the red imported fire ant, Solenopsis invicta ." Psyche. (2012).
[3] Briano, Juan, Luis Calcaterra, and Laura Varone. "Review Article: Fire ants (Solenopsis spp.) and their natural enemies in southern South America." Psyche. (2011).
[4] Valles, Steven M., and Yoshifumi Hashimoto. "Isolation and characterization of Solenopsis invicta virus 3, a new positive-strand RNA virus infecting the red imported fire ant, Solenopsis invicta." Virology. 388. (2009): 354-361.
[5] Bonning, Bryony C., and W. Allen Miller. "Dicistroviruses."Annu. Rev. Entomol.. (2010): 129-150.
[6] Porter, Sanford D. "Biology and behavior of pseudacteon decapitating flies (Diptera:Phoridae) that parasitize Solenopsis fire ants (Hymenoptera: Formicidai)."Florida Entomologist. 81. (1998): 292-309.
[7] United States. National Park Service. Congaree National Park. Print. <http://www.nps.gov/cong/siteindex.htm>.
[8] Sebastian, R., E. Diaz, et al. "Studying endocytosis in space and time by means of temporal Boolean models." Pattern Recognition. 39.11 (2006): 2175–2185.
[9] Belov, George A., Nihal Altan-Bonnet, et al. "Hijacking components of the cellular secretory pathway for replication of poliovirus RNA." Journal of Virology. 81.2 (2006): 558-565.
[10] M. I. Hertz and S. R. Thompson, “In vivo functional analysis of the Dicistroviridae intergenic region internal ribosome entry sites,” Nucleic Acids Research, vol. 39, no. 16, pp. 7276-7288, 2011.
Page authored for BIOL 375 Virology, September 2010