Functional Amyloids: Difference between revisions
Cindy.liu92 (talk | contribs) No edit summary |
Cindy.liu92 (talk | contribs) No edit summary |
||
| Line 24: | Line 24: | ||
All genes for the eight chaplin proteins are expressed in aerial hyphae and the proteins are secreted to form a layer around the emerging aerial hyphae[[#References|[1]]]. Due to colony differentiation, cells on the colony surface give rise to reproductive aerial hyphae which emerge from the soil to septate into spores that are then dispersed by wind[[#References|[3]]]. Aerial hyphae emerged from soil are coated with two layers: the outside layer is comprised of rodlets composed of paired chaplin fibrils and the inner layer contains all eight chaplin proteins[[#References|[1]]]. This coating makes the surface of aerial hyphae hydrophobic, whereas the surface of submerged hyphae is hydrophilic[[#References|[1]]]. The change to hydrophobicity prevents the aerial hyphae from growing back into the aqueous substrate and facilities dispersion of spores[[#References|[1]]]. Through this mechanism in ''S. coelicolor'', it is evident that amyloid proteins are crucial in biological processes such as formation of reproductive hyphae. | All genes for the eight chaplin proteins are expressed in aerial hyphae and the proteins are secreted to form a layer around the emerging aerial hyphae[[#References|[1]]]. Due to colony differentiation, cells on the colony surface give rise to reproductive aerial hyphae which emerge from the soil to septate into spores that are then dispersed by wind[[#References|[3]]]. Aerial hyphae emerged from soil are coated with two layers: the outside layer is comprised of rodlets composed of paired chaplin fibrils and the inner layer contains all eight chaplin proteins[[#References|[1]]]. This coating makes the surface of aerial hyphae hydrophobic, whereas the surface of submerged hyphae is hydrophilic[[#References|[1]]]. The change to hydrophobicity prevents the aerial hyphae from growing back into the aqueous substrate and facilities dispersion of spores[[#References|[1]]]. Through this mechanism in ''S. coelicolor'', it is evident that amyloid proteins are crucial in biological processes such as formation of reproductive hyphae. | ||
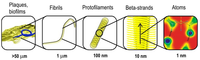
[[File:assembly.png|200px|thumb|right| Assembly of biofilms from different stages of the amyloid structure. http://www.grin.com/object/external_document.232917/7781a1bbae753a35434cbf68731004a4_LARGE.png.]] | |||
==Biofilm Formation== | ==Biofilm Formation== | ||
[[File:chaplin.jpg Assembly of amyloid proteins on medium-air interface to direct aerial hyphae growth.]] | |||
Biofilms formation is advantageous for a community of bacteria to adhere to medium or host surfaces and to protect from chemicals or antibiotics. A widespread of bacteria have the property to induce biofilm formation, some of which involve amyloids. These include representatives from the phyla ''Proteobacteria'' (''Alpha-, Beta-, Gamma-, and Deltaproteobacteria), Bacteriodetes, Chloroflexi, Actinobacteria''[[#References|[5]]]. | Biofilms formation is advantageous for a community of bacteria to adhere to medium or host surfaces and to protect from chemicals or antibiotics. A widespread of bacteria have the property to induce biofilm formation, some of which involve amyloids. These include representatives from the phyla ''Proteobacteria'' (''Alpha-, Beta-, Gamma-, and Deltaproteobacteria), Bacteriodetes, Chloroflexi, Actinobacteria''[[#References|[5]]]. | ||
| Line 34: | Line 38: | ||
TapA protein directs TasA amyloid fibre assembly and disassembly[[#References|[2]]]. TapA is localized on peptidoglycan and extracellular TasA elongates on this structure[[#References|[2]]]. However, it has been suggested that the sole interaction of TapA and TasA do not regulate disassembly, rather the high concentrations of D-amino acids (D-tyrosine, D-leucine, D-methionine, and D-tryptophan) and interactions with the peptidoglycan cause TasA amyloid fibres to be released from cells[[#References|[2]]]. Although biofilm formation may be directed by other proteins in different organisms, the example of ''B. subtilis'' demonstrates the importance of integral amyloid fibres. | TapA protein directs TasA amyloid fibre assembly and disassembly[[#References|[2]]]. TapA is localized on peptidoglycan and extracellular TasA elongates on this structure[[#References|[2]]]. However, it has been suggested that the sole interaction of TapA and TasA do not regulate disassembly, rather the high concentrations of D-amino acids (D-tyrosine, D-leucine, D-methionine, and D-tryptophan) and interactions with the peptidoglycan cause TasA amyloid fibres to be released from cells[[#References|[2]]]. Although biofilm formation may be directed by other proteins in different organisms, the example of ''B. subtilis'' demonstrates the importance of integral amyloid fibres. | ||
==Virulence Factors== | ==Virulence Factors== | ||
Revision as of 19:42, 14 December 2012
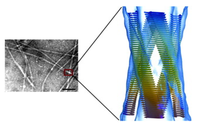
Functional amyloid are insoluble proteinaceous fibres which function in normal physiological processes in prions, fungi, and bacteria[3]. Until the past decade, amyloid production was only defined as pathogenic due to improperly folded protein aggregates and was associated with amyloidosis and chronic neurodegenerative diseases, such as alzeihmers[3] or parkinsons[9]. However, in gram positive and gram negative bacteria, these highly organized structures have nonhuman pathogenic properties crucial to reproduction regulation, biofilm production, and virulence[5].
Structure
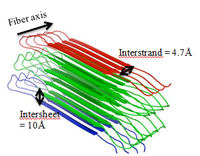
Amyloids have distinct unbranched protein fibers composed of β-sheet quaternary structures[3]. Thousands of highly arranged peptide or protein repeats consisting of β-strands that are oriented perpendicular to the fiber axis form what is known as a cross-β pattern, with diameters of 6-12 nanometers wide and 0.1-10 micrometers long[3]. These peptide strands appear to be “stacked” in either parallel or antiparallel directions[3]. X-ray fibre diffraction demonstrates that the interstrand and intersheet stacking distances of the cross-β core are 4.7Å and 10Å, respectively[7].
Amyloid fibrils are stabilized by non-covalent intermolecular interactions, such as non-polar Van der Waals and aromatic stacking, or hydrogen bonds[4]. Individual β-strands interact with adjacent sheets by two classes of β-sheet stacking interfaces called the dry or wet interfaces[4]. In the dry interface, the highly packed side chains interlink due to steric forces caused by close proximately and structural similarity[4]. This is termed the steric zipper[4]. In wet interfaces, hydrogen bonds exist between side chains[4].
Sequence conservation is limited between the amyloid domains of different protein. However, some shared characteristics are hydrophobic-rich sequences and the presence of glutamine and asparagine polypeptides[3]. This structure gives amyloid its resistant characteristic to environmental attacks, such as proteolysis, and inability to bind to certain dyes[9].
Common Functions and Mechanisms
Regulation in Reproduction
A prime model of the regulatory role in amyloid fibrils are eight hydrophobic chaplin proteins (ChpA, B, C, D, E, F, G, H) identified in gram positive soil-dwelling Streptomyces coelicolor[3]. Chaplin proteins have a rich β-sheet secondary structure and form cross-β amyloid fibrils with a diameter of 4-6 nanometers[1]. Chaplin proteins self-assemble into amyloid fibers which can be categorized into long proteins and short proteins[8]. ChpA, B, C are long proteins and have two hydrophilic N-terminal chaplin domains and a LAXTG sequence at its C-terminal tail[1]. The sorting signal sequence ensures covalent attachment to S. coelicolor peptidoglycan of aerial hyphae by sortase enzymes[1].
Analogous to fungi, S. coelicolor grows in filamentous colonies after spore germination by producing mycelium, a network of vegetative hyphae in search of nutrients[3]. During this growth phase, genes chpE and chpH are highly expressed[1]. These proteins diffuse out of the cell and form an insoluble layer of 4-6 nanometers wide fibrils between the air and soil interface[1]. This rigid film confers hydrophobicity and reduces the water surface tension, allowing aerial hyphae to form and penetrate the soil barrier[8].
All genes for the eight chaplin proteins are expressed in aerial hyphae and the proteins are secreted to form a layer around the emerging aerial hyphae[1]. Due to colony differentiation, cells on the colony surface give rise to reproductive aerial hyphae which emerge from the soil to septate into spores that are then dispersed by wind[3]. Aerial hyphae emerged from soil are coated with two layers: the outside layer is comprised of rodlets composed of paired chaplin fibrils and the inner layer contains all eight chaplin proteins[1]. This coating makes the surface of aerial hyphae hydrophobic, whereas the surface of submerged hyphae is hydrophilic[1]. The change to hydrophobicity prevents the aerial hyphae from growing back into the aqueous substrate and facilities dispersion of spores[1]. Through this mechanism in S. coelicolor, it is evident that amyloid proteins are crucial in biological processes such as formation of reproductive hyphae.
Biofilm Formation
Biofilms formation is advantageous for a community of bacteria to adhere to medium or host surfaces and to protect from chemicals or antibiotics. A widespread of bacteria have the property to induce biofilm formation, some of which involve amyloids. These include representatives from the phyla Proteobacteria (Alpha-, Beta-, Gamma-, and Deltaproteobacteria), Bacteriodetes, Chloroflexi, Actinobacteria[5].
Although curli in Escherichia coli has been extensively studied, a recently researched amyloid protein associated with TasA protein in gram positive Bacillus subtilis[6] reveals novel information of the biofilm mechanism. The gene operon encodes three proteins which regulate biofilm formation in the following sequential order: sipW, tapA, and tasA[6]. Biofilm in B. subtilis is mainly comprised of polysaccharide and TasA, which form a network of 10-15 nanometer wide extracellular amyloid fibre appendages that surround the cell surface[6]. TasA serves as a backbone to provide structural support and cohesion to the more flexible extracellular polymeric substance[2].
TapA protein directs TasA amyloid fibre assembly and disassembly[2]. TapA is localized on peptidoglycan and extracellular TasA elongates on this structure[2]. However, it has been suggested that the sole interaction of TapA and TasA do not regulate disassembly, rather the high concentrations of D-amino acids (D-tyrosine, D-leucine, D-methionine, and D-tryptophan) and interactions with the peptidoglycan cause TasA amyloid fibres to be released from cells[2]. Although biofilm formation may be directed by other proteins in different organisms, the example of B. subtilis demonstrates the importance of integral amyloid fibres.
Virulence Factors
Amyloidogenic proteins can also be used as virulent intermediates produced by plant pathogenic species of Xanthomonas, Erwinia amylovora, and Pseudomonas syringae[10]. Specific to Xanthomonas and Pseudomonas species, toxic properties of amyloid fibrils are adopted into hairpin proteins in vitro[10]. Hairpin proteins are encoded in the hrp (hypersensitive response and pathogenicity) genes[5].
In gram negative Xanthomonas species, the hairpin structure composed of HpaG protein polymerization are secreted as virulence factors and induce the hypersensitive response in plants[5]. The hypersensitive response in plants is a defense mechanism in which infected cells undergo mediated cell death to prevent the spread of an intracellular pathogen[10]. The activation of hypersensitive response by hairpins is unknown, but experimental results demonstrate an increase in cation permeability during the interaction of hairpins with plant cell walls[10]. This suggests that the mechanism is caused by the disruption of the membrane[10]. The ability for parasitic bacteria to utilize amyloid fibrins as toxic substances is advantageous and crucial for its survival in plant hosts.
References
1. Claessen, D., Rink, R., Jong, W. D., Siebring, J., de Vreugd, P., Boersma, F. G. H., Dijkhuizen, L., and Wosten, H. A. B. “A Novel Class of Secreted Hydrophobic Proteins is Involved in Aerial Hyphae Formation in Streptomyces coelicolor by Forming Amyloid-Like Fibrils.” Genes & Development, 2003, DOI: 10.1101/gad.264303.
2. Driks, A. “Tapping into the Biofilm: Insights into Assembly and Disassembly of a Novel Amyloid Fibre in Bacillus subtilis.” Molecular Microbiology, 2011, DOI:10.1111/j.1365-2958.2011.07666.x.
3. Gebbink, M. F. B. G., Claessen, D., Bouma, B., Dijkhuuizen, L., and Wosten, A. B. “Amyloids – A Functional Coat for Microorganisms.” Nature Reviews, 2005, DOI:10.1038/nrmicro1127.
4. Greenwald, J., Riek, R. “Biology of Amyloid: Structure, Function, and Regulation.” Cell Press, 2010, DOI 10.1016/j.str.2010.08.009.
5. Otzen, D., Nielsen, P. H. “We Find Them Here, We Find Them There: Functional Bacterial Amyloid.” Cellular and Molecular Life Sciences, 2008, DOI: 10.1007/s00018-007-7404-4.
6. Romero, D., Aguilar, C., Losick, R., Kolter, R. “Amyloid Fibers Provide Structural Integrity to Bacillus subtilis Biofilms.” PNAS, 2010, DOI: 10.1073/pnas.0910560107.
7. Sawyer, E. B., Claessen, D., Gras, S. L, Perrett, S. “Exploiting Amyloid: How and Why Bacteria Use Cross-β Fibrils.” Biochemical Society Transactions, 2012, DOI:10.1042/BST20120013.
8. Sawyer EB, Claessen D, Haas M, Hurgobin B, Gras SL (2011). The Assembly of Individual Chaplin Peptides from Streptomyces coelicolor into Functional Amyloid Fibrils. PLoS ONE 6(4): e18839. DOI:10.1371/journal.pone.0018839
9. Shewmaker, F., McGlinchey, R. P., Wickner, R. B. “Structural Insights into Functional and Pathological Amyloid.” Journal of Biological Chemistry, 2011, DOI: 10.1074/jbc.R111.22 7108.
10. Wang, X. “The Determinants of Bacterial Amyloid Nucleation and Polymerization.” (Michigan, United States, 2008), 8-9.
