Gas gangrene: Difference between revisions
| Line 27: | Line 27: | ||
==Pathogenesis== | ==Pathogenesis== | ||
===Transmission=== | ===Transmission=== | ||
The transmission of gas gangrene causing bacteria is enabled through physical interaction or daily activity; the attainment of | The transmission of gas gangrene causing bacteria is enabled through physical interaction or daily activity; the attainment of <i>Clostridium</i> species may occur through ingesting feces or soil contaminated food or the infection of a wound [[#References|[6]]]. <i>Clostridium</i> species are a part of the normal gut flora [[#References|[5]]]. However, <i>Clostridium</i> bacteria can not be transmitted between people [[#References|[6]]].Gas gangrene infections most often occur as a result of an epithelial wound or injury that allows for the entry of bacterial species into the area of trauma; <i>Clostridium perfringens</i> is responsible for 80-95% of wound-induced gas gangrene [[#References|[5]]]. However, not all cases are the result of trauma. Spontaneous or nontraumatic gas gangrene may occur within the body, and this form of infection is almost always caused by <i>C. septicum</i>. Though it is the most rare form, nontraumatic gas gangrene is most deadly due to decreased detection. [[#References|[5]]] Nontraumatic gas gangrene is induced when blood flow to tissues is compromised. The tissues are then subject to infection of gas gangrene inducing bacteria. Primary illnesses such as peripheral arterial disease, diabetes, and atherosclerosis may allow for the development of gas gangrene within the tissues. [[#References|[6]]] | ||
<br> | <br> | ||
Revision as of 18:00, 29 July 2014

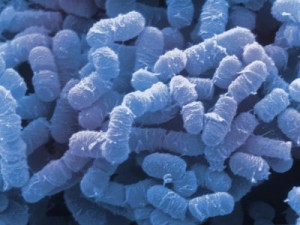
Etiology/Bacteriology
Taxonomy
| Domain = Bacteria
| Phylum = Firmicutes
| Class = Clostridia
| Order = Clostridiales
| Family = Clostridiaceae
| Genus = Clostridium
Description
Gas gangrene, or clostridial myonecrosis, is a bacterial disease caused by microorganisms belonging to the genusClostridium. Gas gangrene is a toxin-induced infection characterized by necrosis of the infected area [2]. Clostridium perfringens is the most common bacterium found in gas gangrene lesions; it appears in 80% of cases.[3] In addition to C. perfringens, the species C. histolyticum, C. fallax, C. novyi, C. septicum, and C. bifermentans are some of the other species responsible for and found within the lesions of gas gangrene [3]. Clostridium species are anaerobic, and deep tissues of the body provide this needed anaerobic environment. The gas production within gas gangrene infections may occur due to glucose fermentation; in past studies, hydrogen, nitrogen, oxygen and carbon dioxide have composed the produced gas, supporting the idea of glucose fermentation. [4] Gas gangrene is characterized by rapid development; it takes 6 to 48 hours from initial contamination of clostridial species for the onset of severe symptoms. If ignored, death may occur within the first 24 to 48 hours. [5]
Pathogenesis
Transmission
The transmission of gas gangrene causing bacteria is enabled through physical interaction or daily activity; the attainment of Clostridium species may occur through ingesting feces or soil contaminated food or the infection of a wound [6]. Clostridium species are a part of the normal gut flora [5]. However, Clostridium bacteria can not be transmitted between people [6].Gas gangrene infections most often occur as a result of an epithelial wound or injury that allows for the entry of bacterial species into the area of trauma; Clostridium perfringens is responsible for 80-95% of wound-induced gas gangrene [5]. However, not all cases are the result of trauma. Spontaneous or nontraumatic gas gangrene may occur within the body, and this form of infection is almost always caused by C. septicum. Though it is the most rare form, nontraumatic gas gangrene is most deadly due to decreased detection. [5] Nontraumatic gas gangrene is induced when blood flow to tissues is compromised. The tissues are then subject to infection of gas gangrene inducing bacteria. Primary illnesses such as peripheral arterial disease, diabetes, and atherosclerosis may allow for the development of gas gangrene within the tissues. [6]
Infectious Dose and Incubation Period
For Clostridium perfringens in particular, the infectious dose is at least 105 microorganisms per gram of food (7). Typically, it takes 6 to 48 hours for the onset of symptoms after the time of infection (4). Death may also occur quickly as many cases have resulted in death within 24 to 48 hours of infection (5).
Epidemiology
Historically, gas gangrene has made appearances under wartime conditions. This infection occured among injured soldiers during World War I, World War II, and the Vietnam War. Mortality rates from this infection were ~11%, ~0.9%, and 0.016%, respectively. (1) Due to the ubiquity and high counts of clostridia within soil, wound induced gas gangrene occurs most frequently. In the United States, approximately 3,000 cases of gas gangrene are reported each year. (8) Internationally, self inflicted injuries, surgeries, and natural disasters have contributed to gas gangrene infection. Records show that cases of gas gangrene in Ireland, Scotland, and England have been induced from the injection of heroine into muscular tissues. Indonesia experienced an outbreak of gas gangrene due to a tsunami in 2004; the combination of injuries and contaminated water allowed for the infection to occur. Similarly, 0.9% of those injured in the 2008 Sichuan earthquake in China developed gas gangrene infection. (8)
Virulence Factors
α-toxin
Clostridium perfringens, the most common causative agent for gas gangrene, possesses a gene that encodes for α-toxin. α-toxin contains zinc and is used as an enzyme—phospholipase C enzyme—to act on phosphatidylcholine and sphingomyelin. The importance of this α-toxin in virulent activity is because of three reasons:
1) α-toxin induces the constriction of blood vessels and clustering of platelets, restricting blood flow to the infection site.
2) α-toxin may interfere with the recruitment of neutrophils to the site of infection.
3) α-toxin will activate protein kinas C and the arachidonic acid cascade within a host cell, essentially taking control of the cells metabolism. (9)
Θ-toxin
C. perfringens uses an additional toxin called Θ-toxin. This toxin is responsible for cell lysis. Θ-toxin binds cholesterols in the host cell membrane then forms pores in the surface of the cell by oligomerizing Θ-toxin monomers. This does not directly contribute to the lethality of gas gangrene, but it does contribute to the disease’s ability to control inflammatory responses within the host system. (10)
Endospore
Clostridium perfringens, as well as other Clostridium species, are able to form spores as a survival mechanism. α/β-type small, acid-soluble proteins are essential in the formation of spores that allow for UV radiation, moist heat, and some chemical resistance. Because C. perfringens and other Clostridium species are able to withstand various extreme conditions, it allows for the bacteria to survive and reside in unfavorable environments until the spores come in contact with a host and colonization can begin. (11)
Clinical Features
The release of toxins by Clostridium species first induces anxious and sick feelings within the host. Symptoms then become more severe, but are focused around the site of infection. The site of infection becomes inflamed, may become a bronze or blue-black color, and an odorous fluid may seep from the wound. The fluid may look fizzy or frothy due to the presence gas produced from fermentation of glucose. (12) Mortality due to traumatic gas gangrene occurs in approximately 25% of cases. In nontraumatic cases, mortality occurs 67-100% of the time. (13)
Diagnosis
Diagnosis for gas gangrene begins with the analysis of symptoms. For nontraumatic gas gangrene, necrosis of the muscles and gas production may be viewed through MRI and CT scans. In traumatic cases, and if the infected muscle is visible, it will appear gray, green and purple in color. Fluids from the wound may be cultured to determine if both anaerobic Clostridium and other aerobic species are present. However, in some open wound gas gangrene, both pathogenic and non-pathogenic Clostridium species may inhabit the wound. When determining the significance of the Clostridium species within the wound, three points may be checked:
1) Large numbers of Clostridium species present in a Gram stain.
2) Small amount of granulocytes present in the obtained sample.
3) The presence of fat globules with Sudan stain as a result of the breakdown of tissues from gas production. (14)
Treatment
Treatment of gas gangrene should occur quickly and as soon as diagnosis is confirmed. Treatment of the infection begins with draining the wound, removal of infected tissues to prevent the spreading of infection, and implementation of antibiotics. Penicillin G is the most common drug that is immediately given. If the patient is allergic to penicillin and the infection is life threatening, he or she will receive desensitization to penicillin. In addition, clindamycin and broad-spectrum antibiotics—i.e. ampicillin plus sulbactam, ticarcillin plus clavulanate, piperacillin plus tazobactam—will be given to patient to rid the body of infection. (14)
Prevention
There is no vaccine to prevent gas gangrene infection. Prevention of infection during surgical procedures is carried out through the cleaving of wounds, the removal of foreign objects and dead tissues within the wounds, and the use of antibiotics before, during and after surgery. (15) The risk of non-traumatic gas gangrene infections reduces when there are no necrosis causing diseases within a host.
Host Immune Response
The immune response triggered by gas gangrene causing bacteria is not certain; however, analysis of gas gangrene virulence factors may show that an expected immune response to an invading pathogen will occur. The Θ-toxin possessed by C. perfringens counters this immune response when it displays power over the regular inflammatory response.
References
1. NCBI [3]
2. Pailler, J.L., F. Labeeu. Gas gangrene: a military disease? 1986. 86(2):63-71
[4]
3.Weinstein M.D., L., M.A. Barza M.D. Gas Gangrene. The New England Journal of Medicine. 1973. 289:1129-1131. [5]
4. Chi, C.H., K.W. Chen, J.J. Huang, Y.C. Chuang, M.H. Wu. Gas composition in Clostridium septicum gas gangrene. 1995. 94(12):757-9.
5. Lu M.D., Jun, X.Wu M.D., X. Kong M.D., W. Tang M.D., J. Cheng M.D., H. Wang M.D. Gas gangrene without wound: both lower extremities affected simultaneously. The American Journal of Emergency Medicine. 2008. 26(8):970.
6. WebMD. Gangrene. [6]
7. MSDS. Clostridium perfringens. 2001. [7]
8. Ho M.D., H., J.B. Figueroa-Casas M.D., D.G Maxfield, L.B Aragon M.D., E. Kanlic M.D. Gas Gangrene. 2013. [8]
9. Titball, R.W., C.E. Naylor, A.K. Basak. The Clostridium perfringens α-toxin. Anaerobe. 1999. 5(2): 51-64.
10. Bryant, A.E., D.L. Stevents. Clostridial Myonecrosis: New Insights in Pathogenesis and Management. Current Infectious Disease Reports. 2010. 12(5):383-91.
11.Paredes-Sabja, D., N. Sarker, B. Setlow, P. Setlow, M.R. Sarker. Roles of DacB and Spm Proteins in Clostridium perfringens Spore Resistance to Moist Heat, Chemicals, and UV Radiation. American Society for Microbiology: Applied and Environmental Microbiology. 2008. Online.
12. Rothwell, Ann. Clostridium perfringens: a flesh-eating bacterium living in your garden. Journal of Perioperative Practice. 2010. 20(10):376-8.
13. Jacobson M.D. PhD, L.S., A. Shukla M.D., J.K. Wong M.D., C.L. Rosen M.D. Emergent Treatment of Gas Gangrene. Medscape. 2014. [9]
14. The Merck Manual for Health Car Professionals. Clostridial Soft-Tissue Infections. 2013. [10]
15. The Merck Manual Home Health Handbook for Patients and Caregivers. Gas Gangrene. 2008. [11]
