The Relationship Between Ebola Virus and Host Factors: Difference between revisions
| Line 36: | Line 36: | ||
==STAT1 and VP24== | ==STAT1 and VP24== | ||
VP24 is one of the eight proteins that EBOV encodes in its genome. It has a number of different roles ranging from virus replication and assembly to binding to assisting in immune suppression [10]. Through X-ray crystallography, VP24 has been shown to bind directly to signal transducer and activator of transcription 1-α/β (STAT1). STAT1 is a transcription factor that upregulates genes when signaled by interferons [10]. In infected cells, EBOV VP24 binds to karyopherin α1, α5, and α6 helices, which inhibit ranslocation of phosphorylated-STAT1. | |||
==Conclusion== | ==Conclusion== | ||
Revision as of 01:30, 10 November 2014
By: Amanda He (currently being edited)
Introduction to Ebolavirus
Ebolaviruses (EBOV) are enveloped viruses that belong to the Filoviridae family. EBOV has received a significant amount of international attention because it causes severe hemorrhagic fevers in humans with a fatality rate of approximately 60% [1]. A minority of cases with infection results in flu-like symptoms with the addition of mild blood coagulopathy, blood loss, and increase in white blood cells, but these patients have a full recovery. The majority of cases develop severe illness with excessive hemorrhaging and blood clotting, which results in shock and death. However, it is important to note that a major factor of the fatality rate of Ebolavirus disease is the access to proper healthcare. EBOV is extremely infectious and can be transmitted through exposure to bodily fluids such as feces, saliva, urine, vomit, and semen from an infected individual and can enter the body through broken skin or unprotected mucous membrane. For a healthcare worker who may be interacting with a number of different patients, it is important to wear appropriate protective equipment and practice appropriate infection control and sterilization measures [2]. Potentially infected individuals must be isolated from other patients to prevent further outbreaks. Although an individual may be infected, the symptoms are not automatic as the virus has an incubation period ranging from 2 to 21 days [3]. The average incubation period is 7-10 days. Through isolation of a potentially infected individual, it can prevent the spread to others.
Several studies have characterized EBOV infection to disable the immune system and the vascular system. The disabling of the vascular system is what leads to the symptoms such as hemorrhage, hypotension, and blood pressure drop [Ansari]. Autopsy of EBOV infected patients found the viruses located primarily in the endothelial cells, mononuclear phagocytic cells, and several fibroblasts and hepatic sinusoids [3]. As a result, it is likely that the initial infection of EBOV targets macrophages and monocytes [4]. The infection of macrophages and monocytes leads to increased synthesis of tumor necrosis factor-α (TNF-α), which induces fever and lymphoid cell apoptosis [3]. Additionally, infection of macrophages and monocytes can induce the release of various pro-inflammatory proteins.
As of 2014, there are no approved therapeutic strategies to treat infection. As a result, there has been a significant emphasis on studying the host factors EBOV recruits [5]. Host factors are traits found in an individual that could have an effect on their susceptibility to disease. In recent studies, certain host factors such as cathepsin B, heat shock 70 kDa protein 5, and STAT1 have been identified to play a role in EBOV infection. Further investigation of host factors can help grasp a better understanding about EBOV and potentially lead to a treatment.
Ebola Genome and Replication Cycle
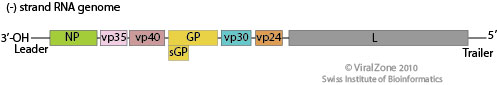
Image source: http://viralzone.expasy.org/all_by_species/207.html [6]
EBOV has a filamentous shaped virus with a lipid envelope, lipid bilayer coat, and genome. The lipid bilayer coats serves as a mechanism to protect the genome and assists with entry into the host cell [6]. The genome of EBOV consists of single stranded, nonsegmented, negative-sense RNA virus with approximately 19,000 bases [7]. The EBOV genome encodes for seven proteins: glycoprotein (GP), matrix protein viral protein 40 (VP40), nucleoprotein (NP), viral protein 24 (VP24), viral protein 30 (VP30), viral protein 35 (VP35), and large protein, an enzyme subunit (L) [1].
Each of these proteins play a significant role in EBOV infection. GP is found on the viral envelope and plays a role in viral attachment and entry into the host cell [7]. VP40 is found in the viral lipid coat and plays a role in virus structure and stability [1]. Researchers found that with the expression of only one EBOV protein, VP40, the virus is still able to form virus like particles in human cells. All the remaining proteins are used to compose the nucleocapsid. The nucleocapsid is the capsid surrounding the RNA, which plays an important role in viral transcription and replication.
HOPS and NPC1
HSPA5
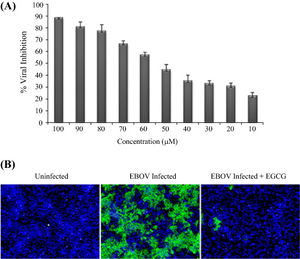
Image source: http://www.sciencedirect.com/science/article/pii/S0166354214002034 [6]
An endoplasmic reticulum (ER) chaperone called heat shock 70 kDa protein 5 (HSPA5) has been identified as a EBOV-associated host factor [9]. HSPA5 is a highly conserved ER protein that assists with protein folding and assembly. It has additional roles in regulation of ER stress responses. However, various viruses are able to utilize HSPA5 for their own purposes such as assistance in development of mature viral envelope proteins and viral entry [5]. It is likely that HSPA5 may be utilized by EBOV assist in infection.
In Reid, et al, 2014, the laboratory members investigated how inhibition of HSPA5 affects EBOV infection. First, they determined whether or not HSPA5 had an essential role in EBOV infection by inhibiting HSPA5 activity at the ATP-binding site. Epigallocatechin gallate (EGCG) is a small molecule that is able to bind to the ATP-binding site of HSPA5 and hinder its functions. In the experiment, EGCG was introduced into HeLa cells with at varying concentrations and incubated for two hours prior to infection with EBOV. Two days after EBOV infection, the cells were fixed and stained for with Hoechst 33342 [Fig 4]. Using immunofluroscence imaging, they identified the identified the significant decrease in viral infection. Infected cell nuclei were fluoresced a green color, while uninfected cell nuclei were stained blue [Fig 4B]. Quantifying the amount of cells that fluoresced green in proportion to the blue uninfected cells, there was a direct correlation between the levels of EGCG to the percentage of EBOV inhibition. There was a consistent pattern of increased EBOV inhibition with increased concentration of EGCG [Fig 4A].
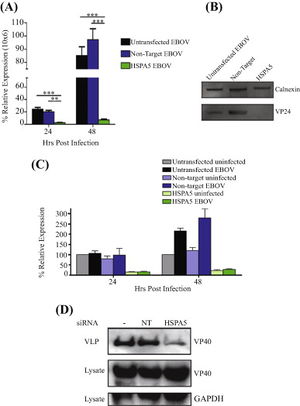
Image source: http://www.sciencedirect.com/science/article/pii/S0166354214002034 [6]
As there are known viruses that utilizes HSPA5 for viral protein maturation and viral entry, further experiments were ran to identify the protein production when HSPA5 is inhibited. 293T cells were treated with HSPA5 target siRNA and then infected with EBOV for 24 or 48 hours. RNA was isolated and quantified for presence of viral and HSPA5 mRNA [Fig 5A,C]. Only the 293T cells that had HSPA5 knocked out had significantly lower relative expression of EBOV mRNA, while the untransfected control and non-target siRNA control both had similar high levels of expressed EBOV mRNA [Fig 5A]. When used in a Western blot, they were able to identify that without the expression of HSPA5, there was VP24 formation and reduced formation of VP40 [Fig 5B,D]. The lack of HSPA5 preventing the formation of VP24, a protein involved in suppressing type I interferon production, and VP40, a protein involved in viral budding, shows that it is utilized by EBOV in a similar fashion to other viruses [5].
A takeaway from the Reid, et al study is the possibility of targeting HSPA5 to inhibit viral infection of EBOV instead of the EBOV genome. In both in vitro and in vivo experiments targeting HSPA5 resulted in significant reduction in virus replication and higher survival of mice against the lethal dosage of EBOV. By targeting a host factor instead of the viral genome, there would be greater difficulty for the virus to make escape mutants [5].
STAT1 and VP24
VP24 is one of the eight proteins that EBOV encodes in its genome. It has a number of different roles ranging from virus replication and assembly to binding to assisting in immune suppression [10]. Through X-ray crystallography, VP24 has been shown to bind directly to signal transducer and activator of transcription 1-α/β (STAT1). STAT1 is a transcription factor that upregulates genes when signaled by interferons [10]. In infected cells, EBOV VP24 binds to karyopherin α1, α5, and α6 helices, which inhibit ranslocation of phosphorylated-STAT1.
Conclusion
References
[2] Centers for Disease Control and Prevention. 2014. Ebola Virus Disease Prevention.
[4] Slonczewski, J.L. and Foster, J.W. Microbiology: An Evolving Science. "W.W. Norton & Company, Inc." 2013. Third Ed.
Edited by Amanda He of Joan Slonczewski for BIOL 375 Virology, 2014, Kenyon College.
